Indicators of dementia disease progression in primary care: An electronic health record cohort study
Funding information
This study was funded by The Dunhill Medical Trust (RPGF 1711/11) as part of the MEDDIP study. K.P.J. and C.A.C.-G. are also supported by matched funding awarded to the NIHR Applied Research Collaboration (West Midlands). The views and opinions expressed are those of the authors and not necessarily the views of the Dunhill Medical Trust, NHS, NIHR, or Department of Health and Social Care.
Abstract
Background and purpose
The objectives were to assess the feasibility and validity of using markers of dementia-related health as indicators of dementia progression in primary care, by assessing the frequency with which they are recorded and by testing the hypothesis that they are associated with recognised outcomes of dementia. The markers, in 13 domains, were derived previously through literature review, expert consensus, and analysis of regional primary care records.
Methods
The study population consisted of patients with a recorded dementia diagnosis in the Clinical Practice Research Datalink, a UK primary care database linked to secondary care records. Incidence of recorded domains in the 36 months after diagnosis was determined. Associations of recording of domains with future hospital admission, palliative care, and mortality were derived.
Results
There were 30,463 people with diagnosed dementia. Incidence of domains ranged from 469/1000 person-years (Increased Multimorbidity) to 11/1000 (Home Pressures). An increasing number of domains in which a new marker was recorded in the first year after diagnosis was associated with hospital admission (hazard ratio for ≥4 domains vs. no domains = 1.24; 95% confidence interval = 1.15–1.33), palliative care (1.87; 1.62–2.15), and mortality (1.57; 1.47–1.67). Individual domains were associated with outcomes with varying strengths of association.
Conclusions
Feasibility and validity of potential indicators of progression of dementia derived from primary care records are supported by their frequency of recording and associations with recognised outcomes. Further research should assess whether these markers can help identify patients with poorer prognosis to improve outcomes through stratified care and targeted support.
Abbreviations
-
- CI
-
- confidence interval
-
- CPRD
-
- Clinical Practice Research Datalink
-
- EHR
-
- electronic health records
-
- GP
-
- general practitioner
-
- HES
-
- Hospital Episode Statistics
-
- HR
-
- hazard ratio
-
- MEDDIP
-
- Measurement of Dementia Disease Progression in Primary Care
-
- ONS
-
- Office for National Statistics
-
- py
-
- person-years
INTRODUCTION
Over 850,000 people in the UK are estimated to live with dementia, projected to rise to 1.6 million by 2040 [1]. Dementia has a substantial impact on the lives of individuals. The UK government has prioritised early recognition and treatment, to prolong independence, delay and reduce admissions to nursing homes and hospitals, and prolong survival [2-4]. Primary care has a pivotal role in achieving these aims [5-7], particularly in the UK, where most people are registered with a general practitioner (GP) and receive care for long-term conditions.
There have been primary care studies on “case-finding” and identifying risk factors for dementia onset [8, 9], but little research on the course and prognosis of patients with dementia in primary care. One potential source of information on the course of dementia is primary care electronic health records (EHR) that contain coded reasons (morbidities, symptoms) for consultation and management (for example, prescriptions, referrals, tests, investigations). Although EHR can provide data on “hard” long-term outcomes such as hospital admissions and mortality, it is not known whether indicators of disease progression can be identified from primary care EHR.
A set of indicators in primary care could support case management decisions, contribute to understanding of prognosis, improve communication with patients and caregivers, and inform planning and monitoring of care at a population level. They could improve the efficiency of intervention studies, which currently rely on intensive and costly follow-up assessments and long-term outcomes such as mortality. In the first part of the Measurement of Dementia Disease Progression in Primary Care (MEDDIP) study, we established a set of markers of dementia-related health that may be recorded in UK primary care EHR [10], drawing on a rapid literature review, expert consensus, and scrutiny of a regional primary care EHR database. The set has 63 markers in 13 domains (Table 1).
| Domain | Marker | Examples |
|---|---|---|
| Care | Additional help | Home help, day care |
| Care | Carer | Evidence has a carer in records |
| Care | Shared decision-making | Shared decision-making |
| Care | Advanced directive | Advanced care planning |
| Home Pressures | Home pressures | Marital problems, family bereavement/row |
| Severe Neuropsychiatric | Severe mental illness – (a) coded | Psychosis, schizophrenia |
| Severe Neuropsychiatric | Severe mental illness – (b) medication | Antipsychotic drug |
| Severe Neuropsychiatric | Sectioned | Sectioned form completed/fee paid |
| Severe Neuropsychiatric | Crisis | Mental crisis plan, referral to crisis team |
| Severe Neuropsychiatric | Suicidal | Suicidal, high/medium suicide risk |
| Neuropsychiatric | Depression, anxiety, stress – (a) coded | Depression, anxiety, stress |
| Neuropsychiatric | Depression, anxiety, stress – (b) medication | Antidepressant drug |
| Neuropsychiatric | Aggressive behaviour | Aggressive/abusive behaviour |
| Neuropsychiatric | Sleep problems – (a) coded | Insomnia, nightmares |
| Neuropsychiatric | Sleep problems – (b) medication | Hypnotic/anxiolytic drug |
| Neuropsychiatric | Behavioural issues | Behavioural problem, disinhibited behaviour |
| Neuropsychiatric | Low mood | Low mood, tearful, worried, lack of concentration |
| Neuropsychiatric | Wandering | Wanders during day/night |
| Cognitive Function | Cognition | Cognitive decline, mentally vague |
| Cognitive Function | Memory loss | Memory loss, amnesia, poor memory |
| Cognitive Function | Confusion | Confusion, delirium, disorientated |
| Cognitive Function | Aphasia | Aphasia, speech therapy/defect, stammer |
| Daily Functioning | Bedbound | Bedbound, bed-ridden |
| Daily Functioning | Wheelchair | Provision of/independent in wheelchair |
| Daily Functioning | Severe mobility limitation | Housebound, chairbound, Zimmer frame |
| Daily Functioning | Mobility – less severe limitation | Mobility poor, walking stick, gait abnormality |
| Daily Functioning | Pressure sore | Pressure sore, decubitus ulcer |
| Daily Functioning | Driving | Unfit to drive, advised about driving |
| Daily Functioning | Difficulty in eating | Eating problem, dependent for eating |
| Daily Functioning | Difficulty handling finance | Needs help handling financial affairs |
| Daily Functioning | Personal care limitation | Dependent for dressing/toilet/bathing |
| Daily Functioning | Stairs limitation | Difficulty managing stairs, need help on stairs |
| Safety | Fall | Recorded fall |
| Safety | Fracture | Recorded fracture (excluding skull) |
| Safety | Intracranial injury | Skull fracture, concussion |
| Safety | Safety assessment | Falls risk assessment, home safety advice |
| Comorbidity | Cardiovascular | Myocardial infarction, ischaemic heart disease |
| Comorbidity | Stroke | Stroke, cerebral infarction |
| Comorbidity | Parkinson's disease | Parkinson's disease |
| Comorbidity | Motor neurone disease | Motor neurone disease |
| Comorbidity | Diabetes | Diabetes mellitus (type I or II) |
| Comorbidity | Epilepsy | Epilepsy, grand mal/petit mal, fit frequency |
| Comorbidity | Asthma/COPD | Asthma, COPD, chronic bronchitis |
| Comorbidity | Musculoskeletal pain | Osteoarthritis, regional pain, rheumatoid arthritis |
| Comorbidity | Anaemia | Iron deficiency anaemia, vitamin B12 deficiency |
| Comorbidity | Ocular | Cataract, retinopathy, glaucoma, blindness |
| Comorbidity | Hypertension | Essential hypertension, hypertensive disease |
| Comorbidity | Candidiasis | Candidiasis, thrush |
| Symptoms | Dizziness | Dizziness, vertigo, hypotension, giddiness |
| Symptoms | Incontinence | Incontinent of urine/faeces, urgency micturition |
| Symptoms | Constipation/IBS | Constipation, IBS |
| Symptoms | Diarrhoea | Diarrhoea, loose stools |
| Symptoms | Urinary | Retention of urine, haematuria, dysuria |
| Symptoms | Neurological | Fit (no epilepsy record), blackout |
| Symptoms | Chest pain (noncardiovascular) | Costochondritis, unspecified chest pain |
| Symptoms | Oral health | Stomatitis, poor oral hygiene, sore mouth |
| Symptoms | Swallowing | Difficulty swallowing liquids/solids, dysphagia |
| Symptoms | Hearing loss | Deafness, hearing loss/impairment |
| Symptoms | “Feels unwell” | Recorded "feels unwell" |
| Diet/Nutrition | Poor diet | Advice re diet, high fat diet, dietician referral |
| Diet/Nutrition | Nutrition | Vitamin/iron deficiency, osteomalacia |
| Diet/Nutrition | Weight loss | Weight decreasing/loss, underweight |
| Diet/Nutrition | Dietary supplement | Dietary supplement |
| Imaging | Imaging | X-ray, MRI, ECG, DXA, angiogram, CAT scan |
| Increased Multimorbidity | Increase in polypharmacy | Increase in count of different drugs prescribed |
| Dementia-Related Drug | Change in dementia-related drug | New or changed dementia drug prescribed |
Note
- Reproduced with permission from Campbell et al. [10]. © 2020 Taylor & Francis Group.
- Abbreviations: CAT, computer-assisted tomography; COPD, chronic obstructive pulmonary disease; DXA, dual-energy X-ray absorptiometry; ECG, electrocardiogram; IBS, irritable bowel syndrome; MRI, magnetic resonance imaging.
The objectives of this part of the MEDDIP study were to assess the feasibility and validity of these markers and domains as indicators of progression for people with dementia. Feasibility was assessed by estimating the prevalence and incidence of recorded markers and domains within primary care EHR at the time of, and early after, diagnosis of dementia. Validity was assessed by estimating the association of recording of these domains with established longer-term outcomes (hospital admission, palliative care, early mortality) under the hypothesis (construct validity) that patients with new recorded markers/domains early on in their dementia course will have a higher risk of poor outcomes.
METHODS
Setting
This was a cohort study set within the Clinical Practice Research Datalink (CPRD) GOLD, a database of longitudinal pseudonymised primary care data from 17 million patients across a network of UK general practices. The study was approved by the CPRD Independent Scientific Advisory Committee (ref 19_002).
Study population
Patients with a recorded diagnosis of Alzheimer's disease, vascular dementia, Lewy body dementia, Parkinson's dementia, frontotemporal dementia, and mixed and unspecified dementia between 1998 and 2017 were included. Diagnosis of dementia was based on a Read code list developed previously through consensus of GP and EHR researchers [11], and code lists used in other studies [12-14]. Read codes are the main method of recording morbidity and processes of care within UK primary care. Coding of dementia in UK primary care EHR has been validated previously [15]. Patients with a Read code suggesting a history of any type of dementia (for example, “H/O: (History of) dementia”, “Dementia annual review”) prior to the date of first dementia diagnosis (index date) or a recorded prescription for an antidementia drug (donepezil, galantamine, memantine, rivastigmine) prior to the index date were excluded. Read code lists are available at www.keele.ac.uk/mrr.
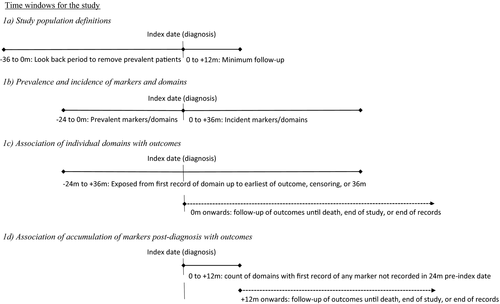
Patients had to have 3 years of up-to-standard data in CPRD prior to the index date and 1 year after index date as a minimum follow-up time to capture progression of dementia (Figure 1a). All patients were required to have linked Hospital Episode Statistics (HES) admissions, neighbourhood deprivation (Index of Multiple Deprivation 2015 [16]), and mortality (via the Office for National Statistics [ONS]) data. This encompasses around 50% of patients in CPRD GOLD (all in England).
Markers and domains
Code lists for markers utilised previous UK-based EHR research studies [17-24], existing databases of Read code lists [25, 26], and searches of the UK Clinical Terminology Read Code Browser. The markers and associated domains are shown in Table 1. The first recording of each marker in the period from 2 years before the index date to 3 years after was identified. The exception was for the Increased Multimorbidity domain, which was measured for each 12-month period within those 5 years and is a general measure of multimorbidity (increase in number of different drugs prescribed, based on British National Formulary sections, compared to the previous 12-month period). The Dementia-Related Drug domain was based on a new prescribed drug specific to dementia (donepezil, galantamine, memantine, rivastigmine) or a drug that may be used in dementia as well as other conditions (levodopa, clonazepam, rotigotine, selegiline, rasagiline, apomorphine).
Outcomes
The outcomes were first all-cause hospital admission, palliative care, and all-cause mortality measured until the end of follow-up, defined as the earliest of date of death, end of study (31 December 2018), and date of end of up-to-standard records in CPRD. Hospital admission from any cause was based on recorded date of admission in the linked HES data after diagnosis. Palliative care was defined based on Read coded indication of palliative care or coded indication of being within 12 months of end of life [27]. Patients with such a Read code prior to the index date were excluded from analysis of this outcome. All-cause mortality was defined as the earliest record of death from CPRD or ONS mortality data.
Baseline covariates
Covariates were age at index date, sex, year of diagnosis, geographical region, deprivation, and lifestyle factors. Deprivation was measured by the Index of Multiple Deprivation 2015, a composite measure of neighbourhood deprivation [16]. Lifestyle factors (body mass index, smoking status, alcohol consumption) were based on the most recent measurement in the 5 years prior to the index date.
Analysis
Time periods for each analysis are shown in Figure 1. When analysing associations with outcomes, markers and domains were measured prior to occurrence of that outcome.
Feasibility: prevalence and incidence of markers and domains
The prevalence of recorded markers at time of diagnosis, defined as the proportion of all people in the study population with a record of the marker in the 24 months prior to the index date, was determined. Incidence per 1000 person-years (py) at risk of recorded markers in the 36 months after the index date in those with no record of the marker in the 24 months prior to the index date was determined (Figure 1b). Prevalences and incidences of domains were also calculated. Incidence was defined for 12 domains as a record of any marker in the domain after the index date with no previous record of any marker from that domain. For the remaining domain, a first increase in the number of different drugs prescribed in a 12-month period compared to the previous 12 months indicated increased multimorbidity.
Validity: associations of domains with outcomes
Associations of domains with outcomes were assessed in two ways. First, we examined the associations of individual domains recorded up to 3 years after diagnosis (but before outcome). The incidence after the index date of each of the outcomes (hospital admission, palliative care, mortality) per 1000 py at risk was calculated. Cox proportional hazards models estimated the association of having any recorded marker in a domain with each outcome, adjusted for age and gender. The proportional hazards assumption was visually assessed using (log–log transformed) survival curves. Domain (exposure) was included as a time-varying covariate. Follow-up started at the index date, baseline exposure was defined as a record of any marker within the domain in the 24 months prior to the index date, and exposure status could change from unexposed at baseline to exposed over the first 3 years of follow-up (Figure 1c). It was assumed that once the patient was exposed (i.e., had a record of any marker from that domain), they were exposed for the remainder of follow-up (until censored or outcome occurred). As Increased Multimorbidity was defined over a 12-month period, date of changed exposure was the end of the 12-month period, that is, 1, 2, or 3 years after the index date.
Validity: accumulation of markers and association with outcomes
Our second approach to examine associations of domains with outcomes assessed the relationship of accumulation of new markers early after diagnosis with outcomes. For this analysis, for most domains, a record of any previously unrecorded marker in a domain indicated progression even if another marker within that domain had previously been recorded. The Daily Functioning domain has a hierarchical subset of markers (Mobility-Limited, Mobility-Severe, Wheelchair, Bedbound), and a marker was counted only if a more restricted mobility marker had not previously been used. In the Safety domain, any fall was counted regardless of previously recorded falls. For each patient, we determined the cumulative number of domains with at least one marker that was first recorded in the 12 months after the index date (i.e., no record of that marker in the 24 months prior to the index date, even if other markers in that domain had been recorded). Follow-up of outcomes started at 12 months after the index date (Figure 1d). Cox proportional hazards models determined the association of cumulative number of domains in that first 12 months with the outcomes adjusted for baseline covariates. Analysis was repeated for cumulative number of domains over the 36 months after the index date in those with at least 36 months of follow-up, with follow-up of outcomes starting at 36 months. Robustness of estimates was assessed by repeating the analysis using flexible parametric models.
Sensitivity analyses assessed whether any improvement in completeness of recording of markers over time influenced results by restricting the analysis to patients diagnosed with dementia from 2010 onward.
All analyses were performed in Stata v15.
RESULTS
A total of 30,463 patients with dementia met our inclusion criteria. Baseline patient characteristics are given in Table 2. Mean age was 81.6 (SD = 7.86) years, and 63% were female. Median follow-up was 2.73 (interquartile range = 1.75–4.24) years. The majority (96% of those with a type recorded) of dementia patients were diagnosed with Alzheimer's or vascular dementia.
| Characteristic | n (%)a |
|---|---|
| Age | |
| Mean years (SD) | 81.6 (7.86) |
| Gender | |
| Male | 11,132 (36.5) |
| Female | 19,331 (63.5) |
| Dementia type | |
| Alzheimer's | 10,399 (34.1) |
| Vascular | 7536 (24.7) |
| Lewy body | 478 (1.6) |
| Parkinson's | 262 (0.9) |
| Frontotemporal | 48 (0.2) |
| Not recorded or multiple types recorded | 11,740 (38.5) |
| Body mass index | |
| Normal | 11,044 (36.3) |
| Overweight | 7100 (23.3) |
| Obese | 3373 (11.1) |
| Not recorded | 8946 (29.4) |
| Smoking status | |
| Nonsmoker | 13,888 (45.6) |
| Current smoker | 3042 (10.0) |
| Ex-smoker | 8493 (27.9) |
| Not recorded | 5040 (16.5) |
| Alcohol consumption | |
| Nondrinker | 4743 (15.6) |
| Current drinker | 11,712 (38.5) |
| Ex-drinker | 987 (3.2) |
| Not recorded | 13,021 (42.7) |
| Year of dementia diagnosis | |
| 1998–2009 | 15,340 (50.4) |
| 2010–2017 | 15,123 (49.6) |
| Geographical region | |
| Northeast | 718 (2.4) |
| Northwest | 5167 (17.0) |
| Yorkshire & the Humber | 1191 (3.9) |
| East Midlands | 751 (2.5) |
| West Midlands | 3958 (13.0) |
| East of England | 3287 (10.8) |
| Southwest | 4133 (13.6) |
| South central | 4079 (13.4) |
| London | 2954 (9.7) |
| Southeast coast | 4225 (13.9) |
| Deprivation | |
| 1, least deprived | 6875 (22.6) |
| 2 | 6817 (22.4) |
| 3 | 6568 (21.6) |
| 4 | 5601 (18.4) |
| 5, most deprived | 4585 (15.1) |
- a Unless otherwise stated.
Prevalence and incidence of markers and domains
In the 24 months prior to index (diagnosis) date, 68% of patients had at least one recorded marker from the Comorbidity domain and 62% from the Cognitive Function domain (Table 3). Home Pressures (4%) and Care (5%) were the least prevalent domains.
| Domain | Prevalence in 24 months before index date | Incidencea per 1,000 person-years in 36 months after index date | |
|---|---|---|---|
| n (%) | n (%)b | Incidence (95% CI) | |
| Care | 1432 (4.7) | 5567 (19.1) | 88.17 (85.89–90.52) |
| Home Pressures | 1178 (3.9) | 775 (2.7) | 11.24 (10.48–12.06) |
| Severe Neuropsychiatric | 3145 (10.3) | 5068 (18.6) | 85.64 (83.31–88.03) |
| Neuropsychiatric | 13,834 (45.4) | 5922 (35.6) | 186.36 (181.68–191.17) |
| Cognitive Function | 18,979 (62.3) | 3099 (27.0) | 136.78 (132.05–141.68) |
| Daily Functioning | 2558 (8.4) | 4684 (16.8) | 76.45 (74.30–78.68) |
| Safety | 7177 (23.6) | 6617 (28.4) | 137.93 (134.65–141.29) |
| Comorbidity | 20,555 (67.5) | 4919 (49.7) | 299.93 (291.67–308.43) |
| Diet/Nutrition | 5388 (17.7) | 5971 (23.8) | 112.41 (109.59–115.29) |
| Symptoms | 13,921 (45.7) | 6933 (41.9) | 230.47 (225.11–235.96) |
| Imaging | 14,659 (48.1) | 5481 (34.7) | 185.56 (180.72–190.54) |
| Increased Multimorbidity | 12,501 (41.0) | 13,696 (76.3) | 468.54 (460.76–476.45) |
| Dementia-Related Drug | 1623 (5.3) | 10,335 (35.8) | 206.65 (202.71–210.67) |
- a Incidence defined as first record of any marker within domain, in those with no marker from that domain recorded in 24 months prior to index date.
- b In those with no record of domain in 24 months before index date.
Increased Multimorbidity (based on increase in polypharmacy) was the most common domain newly recorded after the index date. The highest incidence for the other domains was observed for Comorbidity (300/1000 py) and Symptoms (230/1000 py). Home Pressures had the lowest incidence (11/1000 py). Prevalence and incidence of markers are reported in Table S1.
Associations of domains with outcomes
Seventy-four percent of patients were hospitalised at least once after the index date (430/1000 py; 95% confidence interval [CI] = 424–436), 8% of patients received palliative care (26/1000 py; 95% CI = 25–27), and 37% of patients died (115/1000 py; 95% CI = 112–116) during follow-up.
The majority of domains were associated with increased risk of all three outcomes (Table 4). The Safety, Comorbidity, and Symptoms domains had the strongest associations with hospital admission (hazard ratios [HRs] = 1.36–1.40), whereas the Severe Neuropsychiatric, Diet/Nutrition, Increased Multimorbidity, and Daily Functioning domains had the strongest associations with palliative care (HRs = 1.97–2.23) and mortality (HRs = 1.92–2.84). The Home Pressures domain was only associated with palliative care. The Cognitive Function domain was negatively associated with hospital admission (HR = 0.94; 95% CI = 0.91–0.97) and mortality (HR = 0.95; 95% CI = 0.92–0.99). A new or change in dementia-related drug was protective of all three outcomes.
| Hospital Admission | Palliative Care | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Domain | n a | n (%) with outcome | HR (95% CI)b | n a | n (%) with outcome | HR (95% CI)b | n a | n (%) with outcome | HR (95% CI)b |
| Care | 4605 | 2684 (58) | 1.06 (1.02–1.11) | 6539 | 684 (10) | 1.64 (1.50–1.80) | 6999 | 2496 (36) | 1.63 (1.56–1.70) |
| Home Pressures | 1677 | 1196 (71) | 1.04 (0.97–1.10) | 1929 | 171 (9) | 1.23 (1.05–1.43) | 1953 | 621 (32) | 0.98 (0.91–1.05) |
| Severe Neuropsychiatric | 6006 | 4415 (74) | 1.26 (1.21–1.31) | 7890 | 729 (9) | 2.23 (2.05–2.44) | 8213 | 4292 (52) | 2.50 (2.41–2.59) |
| Neuropsychiatric | 17,511 | 12,995 (74) | 1.25 (1.22–1.29) | 19,500 | 1789 (9) | 1.78 (1.63–1.93) | 19,756 | 7984 (40) | 1.73 (1.67–1.79) |
| Cognitive Function | 20,895 | 15,359 (74) | 0.94 (0.91–0.97) | 21,891 | 1916 (9) | 1.04 (0.95–1.13) | 22,078 | 7829 (35) | 0.95 (0.92–0.99) |
| Daily Functioning | 5018 | 3632 (72) | 1.26 (1.21–1.31) | 7043 | 759 (11) | 1.97 (1.81–2.14) | 7242 | 3441 (48) | 1.92 (1.85–1.98) |
| Safety | 10,322 | 7780 (75) | 1.40 (1.35–1.44) | 13,596 | 1275 (9) | 1.67 (1.55–1.81) | 13,794 | 5894 (43) | 1.73 (1.68–1.79) |
| Comorbidity | 23,823 | 17,976 (75) | 1.37 (1.33–1.42) | 25,255 | 2249 (9) | 1.60 (1.44–1.79) | 25,474 | 9724 (38) | 1.41 (1.35–1.47) |
| Diet/Nutrition | 8440 | 5913 (70) | 1.15 (1.12–1.19) | 11,048 | 1228 (11) | 2.05 (1.90–2.21) | 11,359 | 5090 (45) | 2.02 (1.95–2.09) |
| Symptoms | 18,301 | 13,824 (76) | 1.36 (1.32–1.40) | 20,627 | 1940 (9) | 1.82 (1.67–1.99) | 20,854 | 8343 (40) | 1.63 (1.57–1.69) |
| Imaging | 17,920 | 13,369 (75) | 1.26 (1.23–1.30) | 19,934 | 1833 (9) | 1.30 (1.20–1.42) | 20,140 | 7316 (36) | 1.19 (1.15–1.23) |
| Increased Multimorbidity | 19,165 | 13,419 (70) | 1.01 (0.99–1.04) | 24,675 | 2111 (9) | 2.01 (1.82–2.21) | 25,048 | 9887 (39) | 2.84 (2.70–2.98) |
| Dementia-Related Drug | 9821 | 6329 (64) | 0.85 (0.83–0.88) | 11,849 | 927 (8) | 0.91 (0.84–0.99) | 11,958 | 3471 (29) | 0.93 (0.89–0.96) |
- Abbreviations: CI, confidence interval; HR, hazard ratio.
- a Number with recorded marker from domain in 24 months before index to earliest of outcome or 36 months after index date.
- b Reference is those with no marker from domain; HR is adjusted for age and gender.
Accumulation of markers and association with outcomes
In the 12 months after the index date, 26,055 (86%) patients had at least one recorded marker not previously recorded, and 5594 (18%) had at least four domains with a new recorded marker. Increasing number of domains with new recorded markers in the 1 year after the index date was linearly associated with increasing risk of hospital admission, palliative care, and mortality (Table 5). Adjusted HRs when comparing patients with new markers in four or more domains to patients with no new recorded markers were 1.24 (95% CI = 1.15–1.33) for hospital admission, 1.87 (95% CI = 1.62–2.15) for palliative care, and 1.57 (95% CI = 1.47–1.67) for mortality. Restricting analysis to the 15,123 patients diagnosed with dementia from 2010 onward gave similar strengths of association (Table S2).
| Number of domains | Number of patients | n (%) with outcome | Unadjusted HR (95% CI) | Adjusted HR (95% CI)a |
|---|---|---|---|---|
| Hospital admissionb | ||||
| 0 | 3540 | 2216 (62.6) | 1.00 | 1.00 |
| 1 | 5702 | 3350 (58.8) | 0.99 (0.94–1.05) | 1.03 (0.97–1.08) |
| 2 | 4672 | 2797 (59.9) | 1.06 (1.00–1.13) | 1.09 (1.03–1.16) |
| 3 | 2838 | 1711 (60.3) | 1.13 (1.05–1.22) | 1.16 (1.07–1.25) |
| 4+ | 2353 | 1375 (58.4) | 1.20 (1.12–1.29) | 1.24 (1.15–1.33) |
| Palliative carec | ||||
| 0 | 4353 | 285 (6.6) | 1.00 | 1.00 |
| 1 | 7725 | 528 (6.8) | 1.14 (0.99–1.31) | 1.11 (0.96–1.28) |
| 2 | 7243 | 554 (7.6) | 1.31 (1.14–1.51) | 1.28 (1.11–1.47) |
| 3 | 5152 | 410 (7.0) | 1.48 (1.25–1.74) | 1.44 (1.22–1.69) |
| 4+ | 5411 | 502 (9.3) | 1.96 (1.70–2.26) | 1.87 (1.62–2.15) |
| Mortality | ||||
| 0 | 4408 | 1632 (37.0) | 1.00 | 1.00 |
| 1 | 7839 | 2731 (34.8) | 1.01 (0.96–1.07) | 1.05 (0.99–1.11) |
| 2 | 7369 | 2731 (37.1) | 1.11 (1.05–1.18) | 1.16 (1.09–1.23) |
| 3 | 5253 | 2040 (38.8) | 1.24 (1.16–1.33) | 1.26 (1.18–1.36) |
| 4+ | 5594 | 2394 (42.8) | 1.56 (1.46–1.67) | 1.57 (1.47–1.67) |
- Abbreviations: CI, confidence interval; HR, hazard ratio.
- a HR adjusted for age, gender, body mass index, smoking status, alcohol consumption, deprivation, year of index date, and geographical region.
- b Excludes those with record of hospitalisation in the 12 months after index date.
- c Excludes those with record of palliative care in 24 months before index date or in the 12 months after index date.
Sensitivity analysis exploring the associations between domains recorded in the 3 years after the index date with outcomes after 3 years provided similar results. HRs for the comparison of patients with four or more domains with newly recorded markers compared to those with no domains were 1.28 (95% CI = 1.05–1.57) for hospital admission, 2.31 (95% CI = 1.27–4.19) for palliative care, and 1.64 (95% CI = 1.33–2.03) for mortality (Table S3). Repeating the main analyses using flexible parametric models yielded similar HRs to the Cox proportional hazards models (Table S4).
DISCUSSION
This study of more than 30,000 patients with dementia in England aimed to determine the feasibility and validity of using markers of dementia-related health as indicators of dementia progression in primary care. The study showed that these markers, nested in domains, can be identified in primary care EHR, and their recording is associated with recognised endpoint outcomes (hospital admission, palliative care, mortality). There was a dose–response effect after diagnosis, with an increase in risk of poor outcomes as the number of domains for which new markers were recorded soon after diagnosis increased. These results demonstrate the potential to identify indicators of progression for those with dementia using primary care EHR.
Although there was variation in their prevalence and incidence, all domains had evidence of regular recording in primary care. However, some individual markers were infrequently recorded and use of these markers as indicators of progression may be more feasible at the domain level. Construct validity of the domains was shown through testing of an a priori hypothesis of their association with recognised outcomes. A record of a marker such as psychosis within the Severe Neuropsychiatric domain increased risk of all outcomes, concordant with a previously identified link of behavioural and psychological symptoms and/or antipsychotic use with risk of hospitalisation, nursing home admission, and mortality [28-31]. The Increased Multimorbidity, Comorbidity, and Symptoms domains were commonly recorded and strongly associated with the outcomes, supporting reviews showing increases in comorbidity and health-related burden related to poor dementia outcomes [30, 32, 33]. The Care domain showed associations across all outcomes; research shows that caregiver coping and stress is associated with poor outcomes in the person with dementia [30, 34], but also will be reflective of increased provision of care as demonstrated by the strong association with palliative care.
Having a record of a marker (e.g., cognitive decline or memory loss) from the Cognitive Function domain did not increase risk of the outcomes. One explanation for this might be the level of information available. A recent EHR study interrogated narrative text (i.e., clinical notes) to give a gradient of cognitive function severity and demonstrated that greater severity is associated with mortality [35]. A finer grade indicator inclusive of severity would be beneficial in the prediction of outcomes. It is also possible cognitive function is less commonly assessed or completed when the patient is nearing end of life [36], or that noncognitive measures are more strongly associated with the outcomes used here.
A prescription for a new dementia-related drug was associated with a reduced risk of the outcomes. Previous research has shown that use of cholinesterase inhibitors is associated with lower mortality in people living with Alzheimer's disease [37, 38]. This domain does not take into account reason for change or whether change was to a more or less potent drug (related to stage of dementia) and therefore may be a less valid indicator of progression.
The domains of Daily Functioning, Diet/Nutrition, and Safety showed consistent associations with our outcomes, which concurs with research that has shown associations between functional ability and mortality [39], and nutrition and outcomes for those with dementia [40], and is reflective of broader function, safety issues (e.g., falls), and frailty in elderly populations [41, 42].
Strengths and limitations
This study tested a rigorously developed set of domains previously demonstrated to be associated with dementia. These domains had been produced via a review of EHR dementia research and expert consensus to ensure clinical relevance, mapped to an internationally agreed set of outcomes [43], and initial testing within a regional EHR database [10]. The CPRD database is broadly reflective of the UK population [44] with established validity for coding and accuracy of dementia diagnosis [45]. Although measures of frailty designed for use within EHR for the elderly population [41] may overlap with some of the domains, we have derived domains specifically relevant to dementia. Future studies could compare our indicators of early progression with general measures of frailty.
Lewy body and frontotemporal dementia are associated with greater behavioural disturbance and have a faster progression rate to mortality [46, 47]. Given these types of dementia are less common, larger studies should compare indicators of progression by dementia type. Whereas a person may consult with a number of problems, the clinician might decide to only code one problem. A coded record for a marker would suggest this was a key reason for a consultation and of importance to the clinician and patient/caregiver. Some markers may have been recorded prior to the window of time we examined, and although most chronic problems should be entered on a regular basis, our markers reflect recent issues. It is possible that some markers in a domain may be acute in nature and have less impact on long-term outcomes. Some markers could be sequentially related (e.g., a fall before a fracture), or have a gradient of severity (e.g., neuropsychiatric). This study aimed to determine the validity of markers and domains as indicators of progression, and not as independent predictors of poor outcome. As such, some of the associations identified with long-term outcomes may have arisen through chance given the multiple testing, and there may be other factors predictive of poor outcome, including socioeconomic status, ethnicity, social support, and social care provision [24, 30, 32, 33, 48, 49], and measures obtainable by invasive testing such as pathogen presence (e.g., cerebrospinal fluid t-tau) and cortical atrophy (volumetric magnetic resonance imaging). Future prognostic research should assess which domains improve prediction of long-term outcomes when added to other factors. Such research would need to address the potential for collinearity between domains. We could not use care home admission as an outcome, as this is not comprehensively recorded in primary care EHR. Future studies could compare recording of these markers and domains with changes on instruments designed to measure cognitive function such as the Mini-Mental State Examination, commonly used in secondary care dementia services.
Clinical relevance
This study is a key step in the development of an approach for managing people with dementia, where individuals who are at increased risk of a poor health outcome can be identified in a primary care setting. This has potential advantages. First, good information on prognosis can contribute to shared decisions on care between patients, carers, and clinicians. Second, “dementia” often overshadows other comorbidities within the consultation [50, 51], and systematic information about such additional concerns contributes to more holistic approaches to care [52, 53] and better health care coordination. Third, early identification and targeted care using a stratified-risk approach within the primary care population with dementia may offer the possibility of intervening to slow progression. This may be either by concentrating resources on patients with higher risk of poor outcomes or, if these markers and domains can be shown to be independent predictors of poor outcome, by targeting those that are modifiable such as prescribing.
The increase in risk as number of domains with new markers increases suggests that, although some domains had stronger associations with outcomes than others, it is the cumulative burden of indicators rather than particular combinations that increases risk of poor outcomes. This may advocate a simple approach to monitoring early dementia progression based on count of new indicators as measured by our domains.
These indicators may be useful in prognostic models to improve identification of patients with a poorer long-term outcome. It may be possible in the future to use these EHR-recorded markers and domains as short-term outcomes, reducing the time and costs of research studies, given that dementia observational and intervention studies often have to use long-term outcomes such as mortality and care home admission.
CONCLUSIONS
The validity of potential indicators of progression of dementia derived from primary care EHR is supported by their association with recognised outcomes. In particular, early accumulation of these indicators after diagnosis is associated with hospital admission, palliative care, and mortality. Combined with their prevalence and incidence in primary care EHR that illustrate their potential feasibility for use in practice, our study has established a case for future investigation of whether this set of markers can be applied to identify patients with a poorer long-term prognosis to improve their outcomes through stratified care and targeted support.
ACKNOWLEDGEMENTS
The study team would like to acknowledge the Patient and Public Involvement and Engagement Dementia Group within the School of Medicine for their input into the development and management of the overall MEDDIP project, of which this study is a part. The study team would also like to thank the project steering group for the MEDDIP study. This study is based in part on data from the CPRD obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the National Health Service as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. Use of HES and ONS data: © 2019, reused with the permission of the Health & Social Care Information Centre. All rights reserved.
CONFLICT OF INTEREST
None.
ETHICAL APPROVAL
This study was approved by the CPRD Independent Scientific Advisory Committee, ref 19_002.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the CPRD (www.cprd.com). Restrictions apply to the availability of these data. Access is subject to protocol approval by an independent scientific advisory committee.




