The APOE ε4 allele in relation to pre- and postsurgical cognitive functioning of patients with primary brain tumors
Funding information
Data collection for this study was funded by ZonMw, The Netherlands Organization for Health Research and Development (project nr. 824003007) and CZ fonds, a Dutch non-profit health insurer's foundation (project nr. 201700031 and 201300447).
Abstract
Background
Recent studies suggest a relationship between the APOE ε4 allele and cognitive outcome in patients treated for malignant brain tumors. Still, longitudinal investigations that include a pretreatment cognitive assessment are lacking and APOE’s effects in patients with benign tumors are understudied. This study investigated presurgical cognitive performance and postsurgical change in ε4-carrying and non-carrying patients with glioma and meningioma.
Methods
Neuropsychological test scores (CNS Vital Signs battery [seven measures], Digit Span Forward/Backward, Letter Fluency test) were obtained as part of a prospective study in which patients with meningioma and glioma underwent cognitive assessment 1 day before (T0, n = 505) and 3 (T3, n = 418) and 12 months after (T12, n = 167) surgery. APOE isoforms were identified retrospectively. ε4 carriers and non-carriers were compared with regard to pretreatment cognitive performance on the group and individual level. Changes in performances over time were compared with longitudinal mixed model analysis in the total sample and the subgroup receiving adjuvant treatment.
Results
Carriers and non-carriers did not differ with regard to pretreatment performance. No significant main effect of ε4 carrier status or interaction between time (T0–T12) and carrier status was found on any of the tests in the whole sample nor in the sample receiving adjuvant treatment.
Conclusions
This study found no evidence of increased vulnerability for pretreatment cognitive dysfunction or cognitive decline within 1 year after surgery in APOE ε4-carrying meningioma and glioma patients. Investigations that include larger samples at longer-term follow-up are recommended to investigate potential late treatment effects.
INTRODUCTION
Patients with primary brain tumors are at risk for cognitive dysfunction before and after treatment [1-4]. Sociodemographic, clinical and tumor-specific factors have been related to the variation in the affected domains and the severity of cognitive dysfunction [5-11]. Research into possible germ line genetic determinants, such as APOE, in this patient population is relatively limited.
The major alleles of the APOE gene – ε2, ε3, ε4 – code for three variants of the glycoprotein apolipoprotein E (ApoE2/E3/E4), which is a key player in lipid metabolism regulation in the central nervous system (CNS) [12], and facilitator of neuronal repair and plasticity processes [13, 14]. However, the three ApoE isoforms possess different structural and functional properties that determine their effects in case of injury through numerous cellular pathways [14-16].
ApoE4 specifically shows negative effects compared to the other isoforms [14] as it facilitates maladaptive responses to CNS damage and less effectively promotes repair [17, 18]. Cognitive outcome in clinical populations including Alzheimer's dementia [19], ischemic stroke [20, 21], Parkinson's disease [22] and breast cancer [23, 24] appear related to ApoE4. The detrimental effects of the isoform might also influence consequences of brain tumor growth and damage. Similarly to after acute injury [25, 26], ApoE4 may facilitate an enhanced inflammatory response that results in aggravated disruption of blood−brain barrier integrity and increased edema. In addition, less efficient myelin formation [27] may result in lower white matter integrity [28]. Adverse effects more specific to antitumor treatment, such as oxidative stress and alterations in neurogenesis, may also be isoform-dependent [17, 29-34].
Correa and colleagues were the first to study the role of the APOE ε4 allele in cognitive functioning in patients treated for CNS tumors [5]. They found that ε4 carriers showed poorer verbal learning and recall [35] and were more susceptible to decline of attention and working memory [36] as compared to non-carriers years after treatment. Currently, the absence of prospective longitudinal assessment of cognitive function in the literature and a lack of investigations into other common primary brain tumors, such as meningioma, limit our understanding of the role of APOE ε4 in the course of cognitive functioning in this population.
Prospective investigation of APOE ε4’s effects on cognition may improve our ability to (preoperatively) identify patients with a higher risk for tumor- and treatment-related dysfunction in clinical practice and inform them accordingly. Moreover, it could allow for more tailored planning of treatment to optimize the balance between maximal antitumor effect while limiting disruption of cognition, and thereby other relevant outcomes, such as quality of life [37]. In this study, we analyzed APOE genotypes in patients with glioma and meningioma who underwent neuropsychological assessment before and after surgical (and adjuvant) treatment in order to investigate differences between ε4 carriers and non-carriers with regard to (1) pretreatment cognitive performance (status) and (2) cognitive functioning over time (change) up to 12 months after surgery.
METHODS
Design and procedure
Patients with meningioma or glioma underwent surgical debulking between November 2010 and September 2017 at the Neurosurgery Department of Elisabeth-TweeSteden hospital, Tilburg, The Netherlands. Neuropsychological assessment (NPA) was performed as standard clinical care 1 day before (T0) and 3 months after (T3) surgery. All patients gave informed consent for the use of the T0 and T3 NPA data in research. For research purposes only, and with separate informed consent, patients underwent NPA 12 months after surgery (T12, from January 2014 onwards). NPA was administered by a neuropsychologist or neuropsychologist in training (MSc/graduate level).
Clinically obtained blood samples were analyzed retrospectively if patients had not formally objected to the use of samples for purposes other than clinical monitoring. Consent was recorded by the Clinical Pathology Laboratory. The study was conducted according to the principles of the Declaration of Helsinki (Fortaleza, Brazil revision 2013), and in accordance with the Medical Research Involving Human Subjects Act (WMO). The study protocol was approved by the Medical Ethics Trial Committee Brabant (file NL41351.008.12).
Sample
Data were used from adult patients with a newly diagnosed diffuse glioma (World Health Organization [WHO] grade II-IV) or meningioma (grade I-II) who had completed at least T0 NPA. Further exclusion criteria were: previous intracranial surgery, a recent history (≤2 years) of severe psychiatric or neurologic disorder, other major medical illnesses in the last year (eg, cancer), no basic proficiency in Dutch, and inability to undergo NPA (eg, due to severe visual or motor problems). Patient data described in the current study are partly described in previous studies [10, 38, 39].
Measures
Sociodemographic data
Age, sex, level of education (low, middle, high) were obtained through standardized interview at T0.
Clinical data
Histopathological diagnosis, tumor location, use of corticosteroids, use of anti-epileptic drugs (AED) and adjuvant treatment were obtained from electronic medical records. Adjuvant treatment was dichotomized for the analyses (chemotherapy and/or radiotherapy vs no adjuvant modality). Preoperative tumor volume was obtained through semi-automatic segmentation with either Brainlab Elements software or ITK-snap software, and expressed in cubic centimeters.
Cognitive data
NPA comprised the formal Dutch translation of the CNS Vital Signs (CNS VS) computerized test battery (see Table S1 for a description of the seven tests that were used): Verbal Memory test (VEM), Visual Memory test (VIM), Symbol Digit Coding test (SDC), Shifting Attention test (SAT), Continuous Performance test (CPT), Stroop test I and Stroop test III. The local software application of CNS VS was used on a notebook computer. Three paper-and-pencil tests were administered: a Letter fluency task [40] and, from 2015 onwards, a Digit Span task (Forward and Backward) [41].
Standardization of test scores
Patients’ raw scores on CNS VS were converted into Z-scores using data from 158 previously recruited Dutch healthy controls. Z-scores were adjusted for demonstrated effects of age, sex and educational level. Follow-up measurements were also corrected for practice effects [42]. Digit Span scores were standardized in a comparable manner using data from a healthy Dutch control group obtained as part of an ongoing clinical trial (CAR Study A, ClinicalTrials.gov reference NCT02953756). Fluency scores were standardized into Z-scores, using published norms [40]. These scores were standardized for educational level, but not sex or age, since these were not demonstrated to influence performance. Z-scores of each patient on each test were also dichotomized into impaired (Z-score ≤ −1.5) or unimpaired.
APOE genotype
APOE isoforms were determined by the Department of Laboratory Medicine using assay kits (ViennaLab, Diagnostics GmbH) involving a procedure of DNA isolation, polymerase chain reaction (PCR) amplification using biotinylated primers, and reverse-hybridization. Obtained genotypes were dichotomized into ε4 carrier (heterozygous or homozygous) versus non-carrier (ie, ε2 or ε3 carrier).
Psychological data
The Dutch translation of the Hospital Anxiety and Depression Scale (HADS) [43] was administered at each time-point (T0, T3, T12) to screen for symptoms of anxiety and depression.
Statistical analyses
Characteristics of APOE ε4 carriers and non-carriers
Potential baseline differences regarding sociodemographic (age, education level, sex), clinical (histopathology, frontal lobe involvement, tumor hemisphere, tumor volume, use of AED, use of corticosteroids and adjuvant treatment) and psychological (HADS Anxiety and Depression) scores between ε4 carriers and non-carriers were investigated in the total sample and stratified by diagnosis. Chi-square tests of independence were used for categorical variables, independent samples t-tests for continuous variables with normal distributions and Mann–Whitney U tests for continuous variables with skewed distributions (α = 0.05).
Preoperative cognitive performance
Mean performance of the entire sample was compared to healthy controls using Z-tests. Subsequently, mean performances of carriers versus non-carriers in the patient sample were compared for each test with independent samples t-tests and Mann–Whitney U tests. The proportions of impairment for carriers versus non-carriers on each test were compared using Chi-square tests. In case of baseline differences on any of the sample characteristics previously described, that variable was adopted as a covariate in analysis of variance (ANCOVA) or as a layer in Chi-square tests. To inspect potential bias in the long-term follow-up sample, we compared preoperative performances (mean performances and impairment proportions) of patients who completed T12 assessment and those who dropped out before T12.
Cognitive functioning over time
We conducted linear mixed model (LMM) analyses to investigate the course of cognitive performances over time (one model per cognitive test), initially in the total patient sample. In the longitudinal LMM, time (T0, T3, T12) was level 1 and its measurements were nested in the patients at level 2. Because only three time points were involved, we adopted a linear effect of time for all models. Intercepts were specified as random effects, allowing for individual estimations of the data of each patient. Random slopes were added to those models if they significantly improved model fit (likelihood ratio test, α = 0.05). Among the tested correlation structures (autoregressive, continuous autoregressive, compound symmetry, general correlation matrix, scaled identity), the one providing the best fit based on the Akaike information criterion (AIC) for the majority of the models was adopted uniformly.
First, we created models with only time as predictor to investigate the overall course of performances without any other predictors. In the final models, we included a time*carrier status interaction (non-carrier as reference group). We also included a time*diagnosis interaction to account for possible differences in performance over time between meningioma and glioma patients (glioma as reference group). Similar models were constructed to investigate the effect of carrier status for the T0−T3 interval and T3−T12 interval separately, using time as factor instead of a continuous variable (no random slopes). Within the group of patients who received adjuvant treatment, regardless of diagnosis, we performed ancillary analyses, again of time*carrier status.
We used the restricted maximum likelihood (REML) algorithm to estimate model parameters. Global fits of the models (models with only time as predictor vs the final models) were compared using AIC, and tested with likelihood ratio tests in case of a significant effect of carrier status. Analyses of the data [44] were performed using SPSS software (version 24) and Rstudio software (lme4 and nlme packages [45, 46]). We adopted a correction for multiple testing (taking into account the 10 tests we performed to investigate all cognitive measures) per main analysis (pretreatment performance, posttreatment change with time only, and posttreatment change with APOE carrier status) using the false discovery rate (FDR) correction procedure of Benjamini and Hochberg (BH) [47] (original α = 0.05).
RESULTS
Characteristics of APOE ε4 carriers and non-carriers
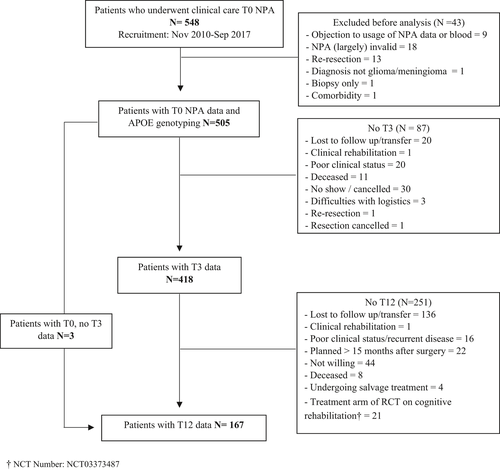
Figure 1 shows a flow chart of patient inclusion. Baseline characteristics of the sample are displayed in Table 1. There were no significant differences for any of the inspected sociodemographic, psychological or clinical variables between APOE ε4 carriers and non-carriers in the total sample (p > 0.05). In the meningioma group, there was a significantly larger proportion of frontal lobe tumors among non-carriers as compared to carriers (p = 0.03).
| Characteristic | Glioma (n = 263) | Meningioma (n = 242) | Total (n = 505) | |||
|---|---|---|---|---|---|---|
| ε4 carrier (n = 64) | Non-carrier (n = 199) | ε4 carrier (n = 64) | Non-carrier (n = 178) | ε4 carrier (n = 128) | Non-carrier (n = 377) | |
| Sociodemographic | ||||||
| Age, M ± SD | 53.2 ± 14.8 | 53.2 ± 13.8 | 55.9 ± 12.7 | 57.0 ± 11.6 | 54.9 ± 13.9 | 55.3 ± 13.1 |
| Female, n (%) | 28 (44) | 71 (36) | 41 (64) | 135 (76) | 69 (54) | 206 (55) |
| Education, n (%) | ||||||
| Low | 20 (31) | 57 (29) | 25 (39) | 63 (35) | 45 (35) | 120 (32) |
| Middle | 19 (30) | 69 (34) | 14 (22) | 57 (32) | 40 (31) | 126 (33) |
| High | 25 (39) | 73 (37) | 25 (39) | 58 (33) | 43 (34) | 131 (35) |
| Clinical | ||||||
| Diagnosis, n (%) | ||||||
| LGG WHO II | 21 (30) | 59 (30) | n/a | n/a | 19 (15) | 59 (16) |
| HGG WHO III/IV | 45 (70) | 140 (70) | n/a | n/a | 45 (35) | 140 (37) |
| MEN WHO I | n/a | n/a | 61 (95) | 166 (93) | 64 (50) | 178 (47) |
| MEN WHO II | n/a | n/a | 3 (5) | 12 (7) | ||
| Frontal involvement | 34 (53) | 101 (51) | 31 (48)a | 114 (64)a | 65 (51) | 215 (57) |
| Lesion hemisphere | ||||||
| Left | 30 (47) | 81 (40) | 24 (38) | 68 (38) | 54 (42) | 149 (40) |
| Right | 33 (52) | 117 (59) | 36 (56) | 87 (49) | 69 (54) | 204 (54) |
| Bilateral | 1 (1) | 1 (1) | 4 (6) | 23 (13) | 5 (4) | 24 (6) |
| Tumor volume (n = 235), median (range) | 24.0 (15.3–80.1) | 42.8 (2.0–139.1) | 35.1 (30.4–128.2) | 29.5 (4.5–150.2) | 30.9 (3.4–128) | 31.0 (4.5–150) |
| AED use, n (%) | 32 (53) | 83 (43) | 58 (33) | 42 (23) | 47 (38) | 125 (34) |
| Corticosteroid use, n (%) | 29 (48) | 107 (56) | 21 (33) | 15 (23) | 50 (41) | 165 (45) |
| Adjuvant treatment, n (%) | 46 (72) | 146 (73) | 4 (6) | 13 (7) | 50 (39) | 159 (42) |
| Rtx | 46 (72) | 146 (73) | 4 (6) | 13 (7) | 50 (39) | 159 (42) |
| Chtxb | 36 (56) | 117 (59) | 0 (0) | 0 (0) | 36 (29) | 117 (31) |
| Concurrent Rtx Chtx | 36 (56) | 117 (59) | 0 (0) | 0 (0) | 36 (29) | 117 (31) |
| Psychological | ||||||
| HADS anxiety M ± SD | 7.0 ± 4.5 | 6.9 ± 4.3 | 7.1 ± 4.3 | 7.1 ± 4.3 | 7.0 ± 4.4 | 7.0 ± 4.2 |
| HADS depression M ± SD | 5.3 ± 3.7 | 4.8 ± 3.4 | 5.8 ± 4.7 | 6.1 ± 4.9 | 5.6 ± 4.3 | 5.4 ± 4.2 |
- Information was available for AED use at T0 n = 124 (ε4 carriers) versus n = 367 (non-carriers), corticosteroid use at T0 n = 128 versus n = 367 and adjuvant treatment administration n = 126 versus n = 372.
- AED, anti-epileptic drugs; Chtx, chemotherapy; HADS, Hospital Anxiety and Depression Scale; HGG, high-grade glioma; LGG, low-grade glioma; MEN, meningioma; n/a, not available; Rtx, radiotherapy; WHO, World Health Organization.
- a Significant difference between carriers and non-carriers within the diagnostic group (p < 0.05).
- b Temozolomide, lomustine or PCV (procarbazine-lomustine-vincristine).
Baseline cognitive performances in the total sample
Z-tests showed that our sample performed worse than healthy controls on all NPA measures (p < 0.001, data not shown). As a group, patients who returned for T12 follow-up showed better presurgical performances than those who did not return for T12 on all tests (p < 0.05, data not shown) except Finger Tapping, Continuous Performance, Fluency and Digit Span Forward and Backward. The baseline proportion of impaired performances was also lower among patients who returned for T12 follow-up for Symbol Digit Coding, Shifting Attention, Stroop III and Fluency tests (p < 0.05, data not shown).
Baseline cognitive performances of ε4 carriers and non-carriers
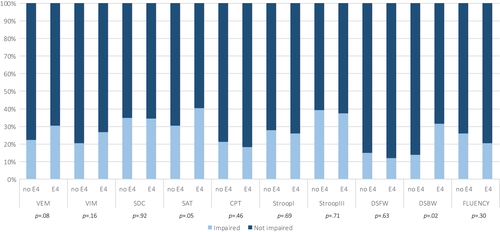
No significant differences were found between carriers and non-carriers in mean performance on any of the tests under the adjusted α (BH-corrected α = 0.005) (Table 2). No significant differences were found between carriers and non-carriers with regard to the proportions of impaired performances (BH-corrected α = 0.005) (Figure 2.
| T | Carrier status | N | VEMa | VIMa | SDCa | SATa | CPTa | Stroop test Ia | Stroop test IIIa | Fluency | DSFW | DSBW | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GLIO | T0 | ε4 carrier | 64 | −0.70 ± 1.37 | −0.59 ± 1.39 | −0.97 ± 1.27 | −1.21 ± 1.66 | −0.79 ± 1.58 | −0.71 ± 1.71 | −1.40 ± 2.51 | −0.36 ± 1.12 | −0.40 ± 0.91 | −0.71 ± 0.51 |
| Non-carrier | 199 | −0.66 ± 1.27 | −0.59 ± 1.37 | −1.06 ± 1.46 | −0.87 ± 1.62 | −0.62 ± 1.45 | −1.05 ± 1.97 | −1.40 ± 2.32 | −0.86 ± 1.12 | −0.25 ± 1.30 | −0.45 ± 1.14 | ||
| p | 0.80 | 0.99 | 0.68 | 0.16 | 0.42 | 0.32 | 0.73 | 0.01 | 0.91 | 0.41 | |||
| T3 | ε4 carrier | 50 | −0.91 ± 1.46 | −0.52 ± 1.41 | −0.99 ± 1.08 | −1.10 ± 1.49 | −1.14 ± 1.93 | −0.69 ± 1.46 | −1.25 ± 1.86 | −0.56 ± 1.15 | −0.73 ± 0.97 | −1.00 ± 1.35 | |
| Non-carrier | 149 | −0.72 ± 1.29 | −0.58 ± 1.23 | −0.90 ± 1.25 | −0.94 ± 1.60 | −0.97 ± 1.58 | −1.02 ± 1.85 | −1.37 ± 1.83 | −0.71 ± 1.17 | −0.59 ± 1.11 | −0.71 ± 1.45 | ||
| T12 | ε4 carrier | 12 | −0.26 ± 0.85 | −0.77 ± 0.78 | −0.92 ± 1.11 | −0.36 ± 1.06 | −0.61 ± 1.10 | −0.82 ± 1.27 | −0.27 ± 1.72 | 0.01 ± 1.33 | −0.83 ± 0.98 | −0.59 ± 0.22 | |
| Non-carrier | 51 | −0.18 ± 0.94 | −0.41 ± 1.17 | −0.46 ± 1.31 | −0.42 ± 1.20 | −0.27 ± 1.01 | −0.65 ± 1.38 | −0.47 ± 1.70 | −0.44 ± 1.16 | −0.79 ± 1.20 | −0.63 ± 1.27 | ||
| MEN | T0 | ε4 carrier | 178 | −0.93 ± 1.47 | −0.65 ± 1.26 | −0.92 ± 1.36 | −0.88 ± 1.82 | −0.16 ± 1.28 | −0.98 ± 2.35 | −0.40 ± 2.41 | −0.62 ± 1.05 | −0.41 ± 1.19 | −1.21 ± 0.99 |
| Non-carrier | 64 | −0.55 ± 1.24 | −0.46 ± 1.18 | −1.08 ± 1.37 | −0.75 ± 1.50 | −0.47 ± 1.52 | −0.76 ± 1.96 | −0.11 ± 1.92 | −0.81 ± 1.05 | −0.53 ± 1.12 | −0.71 ± 0.92 | ||
| p | 0.05 | 0.28 | 0.45 | 0.60 | 0.15 | 0.41 | 0.36 | 0.37 | 0.71 | 0.06 | |||
| T3 | ε4 carrier | 59 | −1.07 ± 1.32 | −0.32 ± 1.33 | −0.70 ± 1.18 | −0.77 ± 1.67 | −0.56 ± 1.24 | −0.89 ± 1.95 | −1.00 ± 1.78 | −0.05 ± 1.37 | −0.66 ± 1.32 | −0.95 ± 1.45 | |
| Non-carrier | 160 | −0.80 ± 1.29 | −0.34 ± 1.24 | −0.83 ± 1.18 | −0.69 ± 1.39 | −0.70 ± 1.29 | −0.62 ± 1.69 | −0.90 ± 1.67 | −0.42 ± 1.10 | −0.53 ± 0.82 | −0.41 ± 1.15 | ||
| T12 | ε4 carrier | 29 | −0.63 ± 1.17 | −20 ± 1.17 | −0.49 ± 1.03 | −0.23 ± 1.17 | −0.35 ± 1.08 | −0.52 ± 1.76 | −0.51 ± 1.77 | −0.27 ± 0.95 | −0.85 ± 1.02 | −0.83 ± 1.37 | |
| Non-carrier | 75 | −0.35 ± 1.06 | −0.35 ± 1.13 | −0.48 ± 0.86 | −0.22 ± 1.12 | −0.35 ± 1.10 | 0.00 ± 1.07 | −0.50 ± 1.47 | −0.07 ± 1.08 | −0.27 ± 0.89 | −0.38 ± 0.92 | ||
| Total | T0 | ε4 carrier | 128 | −0.82 ± 1.42 | −0.62 ± 1.28 | −0.95 ± 1.31 | −1.04 ± 1.74 | −0.48 ± 1.47 | −0.85 ± 2.06 | −1.40 ± 2.46 | −0.52 ± 1.09 | −0.41 ± 1.02 | −0.92 ± 1.32 |
| Non-carrier | 377 | −0.61 ± 1.26 | −0.53 ± 1.28 | −1.07 ± 1.42 | −0.81 ± 1.56 | −0.55 ± 1.48 | −0.91 ± 1.96 | −1.26 ± 2.14 | −0.83 ± 1.08 | −0.38 ± 1.22 | −0.57 ± 1.04 | ||
| p | 0.12 | 0.48 | 0.46 | 0.31 | 0.63 | 0.56 | 0.97 | 0.02 | 0.91 | 0.10 | |||
| T3 | ε4 carrier | 109 | −1.00 ± 1.38 | −0.42 ± 1.37 | −0.83 ± 1.14 | −0.91 ± 1.59 | −0.81 ± 1.59 | −0.81 ± 1.75 | −1.11 ± 1.80 | −0.27 ± 1.30 | −0.69 ± 1.15 | −0.97 ± 1.38 | |
| Non-carrier | 309 | −0.76 ± 1.29 | −0.45 ± 1.24 | −0.86 ± 1.21 | −0.81 ± 1.49 | −0.83 ± 1.43 | −0.81 ± 1.78 | −1.10 ± 1.76 | −0.56 ± 1.14 | −0.56 ± 0.96 | −0.61 ± 1.29 | ||
| T12 | ε4 carrier | 41 | −0.39 ± 1.16 | −0.36 ± 1.10 | −0.61 ± 1.05 | −0.26 ± 1.13 | −0.43 ± 1.08 | −0.60 ± 1.63 | −0.44 ± 1.73 | −0.18 ± 1.06 | −0.85 ± 0.96 | −0.75 ± 1.10 | |
| Non-carrier | 126 | −0.28 ± 1.01 | −0.37 ± 1.15 | −0.47 ± 1.07 | −0.30 ± 1.15 | −0.31 ± 1.06 | −0.27 ± 1.25 | −0.49 ± 1.56 | −0.22 ± 1.12 | −0.48 ± 1.04 | −0.48 ± 1.06 |
Note
- Values displayed are M ± SD. p values refer to group comparison at T0 (FDR adjusted α = 0.005).
- Sample sizes (n) for the Digit Span test: T0 = 42 vs 94, T3 36 vs 81, T12 12 vs 24; for the Verbal Fluency test: T0 97 vs 281, T3 87 vs 255, T12 30 vs 91.
- CPT, Continuous Performance test; DSBW, Digit Span Backward; DSFW, Digit Span Forward; FDR, false discovery rate; Fluency, Verbal Fluency test; GLIO, glioma; MEN, meningioma; SAT, Shifting Attention test; SDC, Symbol Digit Coding test; T, time; VEM, Verbal Memory test; VIM, Visual Memory test.
- a CNS Vital Signs computerized test battery.
Cognitive functioning over time of ε4 carriers and non-carriers
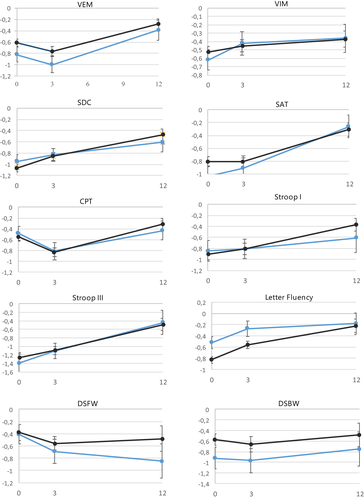
Table 2 and Figure 3 show group performances on each test for carriers and non-carriers over time. Table 3 shows results of the LMM. We found a positive effect of time for scores on the Verbal Memory test, Symbol Digit Coding test, Shifting Attention test, Stroop test I and II and Fluency test (BH-corrected adjusted α = 0.03, range β = 0.02 to β = 0.05, p < 0.01). In the final models, we found no significant main effects of ε4 carrier status nor time*ε4 carrier status interactions (BH-corrected α = 0.005). No significant effects were found for time*diagnosis, except for Fluency performance in the T0−T3 interval. Meningioma patients showed more improvement than glioma patients on this test (p = .001) (Table 3). Analyses in the group of patients who received adjuvant treatment (chemotherapy and/or radiotherapy) revealed no significant main effect of carrier status or time*carrier status interaction (data not shown) (p > 0.10).
| Parameter | VEMa | VIM | SDCa | SATa | CPT | Stroop test Ia | Stroop test IIIa | Fluency | DSFW | DSBW | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time model | Model AIC | 3290 | 3352 | 3156 | 3451 | 3470 | 4026 | 4012 | 2411 | 813 | 893 |
| Time | b (SE) | 0.02 (0.01) | 0.00 (0.01) | 0.03 (0.01) | 0.03 (0.01) | 0.01 (0.01) | 0.04 (0.01) | 0.05 (0.01) | 0.03 (0.01) | −0.02 (0.01) | 0.01 (0.01) |
| p | 0.003 | 0.563 | <0.001 | <0.01 | 0.328 | <0.001 | <0.001 | <0.001 | 0.132 | 0.615 | |
| Final model | Model AIC | 3308 | 3372 | 3173 | 3467 | 3484 | 4035 | 4026 | 2424 | 832 | 906 |
| Time*diagnosisb | b (SE) | −0.02 (0.01) | 0.01 (0.02) | 0.01 (0.01) | 0.02 (0.02) | −0.02 (0.02) | 0.04 (0.02) | −0.00 (0.02) | 0.02 (0.01) | 0.04 (0.02) | 0.07 (0.03) |
| p | 0.198 | 0.678 | 0.398 | 0.241 | 0.313 | 0.108 | 0.979 | 0.207 | 0.104 | 0.022 | |
| Time*carrierc | b (SE) | 0.00 (0.02) | .01 (0.02) | −0.02 (0.01) | 0.00 (0.02) | −0.03 (0.02) | −0.05 (0.03) | −0.01 (0.02) | −0.02 (0.02) | −0.03 (0.02) | −0.00 (0.03) |
| p | 0.903 | 0.779 | 0.137 | 0.904 | 0.088 | 0.073 | 0.899 | 0.123 | 0.252 | 0.962 | |
| Diagnosis | b (SE) | 0.00 (0.11) | 0.16 (0.10) | 0.05 (0.12) | 0.15 (0.14) | 0.29 (0.12) | 0.13 (0.17) | 0.30 (0.18) | 0.02 (0.11) | −0.13 (0.17) | −0.15 (0.19) |
| p | 0.968 | 0.119 | 0.698 | 0.275 | 0.020 | 0.443 | 0.110 | 0.346 | 0.457 | 0.355 | |
| Carrier | b (SE) | −0.22 (0.13) | −0.07 (0.12) | 0.13 (0.13) | −0.21 (0.16) | 0.02 (0.14) | 0.08 (0.19) | −0.16 (0.21) | 0.32 (0.12) | −0.04 (0.19) | −0.37 (0.20) |
| p | 0.092 | 0.572 | 0.335 | 0.181 | 0.881 | 0.673 | 0.452 | 0.010 | 0.810 | 0.069 | |
| Final model: interval T0 T3 | Model AIC | 3293 | 3364 | 3179 | 3476 | 3451 | 4042 | 4035 | 2404 | 824 | 879 |
| T0 T3*diagnosis | b (SE) | −0.25 (0.18) | 0.22 (0.13) | 0.15 (0.10) | 0.11 (0.13) | 0.03 (0.12) | 0.07 (0.19) | 0.34 (0.16) | 0.35 (0.10) | 0.16 (0.18) | 0.53 (0.22) |
| p | 0.167 | 0.093 | 0.126 | 0.156 | 0.826 | 0.699 | 0.038 | 0.001 | 0.383 | 0.016 | |
| T0 T3*carrier | b (SE) | −0.08 (0.12) | 0.13 (0.15) | −0.06 (0.11) | −0.00 (0.15) | −0.12 (0.14) | −0.09 (0.22) | 0.01 (0.19) | 0.03 (0.12) | −0.07 (0.19) | −0.01 (0.23) |
| p | 0.523 | 0.400 | 0.608 | 0.983 | 0.379 | 0.669 | 0.966 | 0.818 | 0.731 | 0.976 | |
| Diagnosis | b (SE) | 0.03 (0.12) | 0.08 (0.11) | −0.00 (0.12) | 0.14 (0.14) | 0.29 (0.13) | 0.14 (0.17) | 0.17 (0.18) | −0.03 (0.11) | −0.13 (0.18) | −0.30 (0.20) |
| p | 0.802 | 0.458 | 0.996 | 0.324 | 0.028 | 0.395 | 0.345 | 0.810 | 0.479 | 0.144 | |
| Carrier | b (SE) | −0.23 (0.13) | −0.11 (0.13) | 0.13 (0.13) | −0.31 (0.16) | 0.03 (0.15) | 0.07 (0.20) | −0.16 (0.21) | 0.28 (0.13) | −0.05 (0.20) | −0.37 (0.22) |
| p | 0.075 | 0.407 | 0.378 | 0.183 | 0.842 | 0.736 | 0.446 | 0.029 | 0.794 | 0.091 | |
| Final model: interval T3 T12 | Model AIC | 3293 | 3364 | 3179 | 3476 | 3451 | 4042 | 4035 | 2404 | 824 | 897 |
| T3 T12*diagnosis | b (SE) | −0.17 (0.18) | −0.13 (0.20) | 0.03 (0.15) | 0.17 (0.20) | −0.29 (0.18) | 0.33 (0.28) | −0.26 (0.240 | −0.14 (0.17) | 0.26 (0.28) | 0.20 (0.35) |
| p | 0.349 | 0.494 | 0.817 | 0.391 | 0.109 | 0.244 | 0.286 | 0.447 | 0.363 | 0.573 | |
| T3 T12*carrier | b (SE) | −0.06 (0.21) | −0.07 (0.22) | −0.18 (0.17) | −0.05 (0.23) | −0.21 (0.21) | −0.49 (0.32) | −0.12 (0.28) | −0.32 (0.19) | −0.18 (0.30) | 0.00 (0.36) |
| p | 0.770 | 0.760 | 0.300 | 0.838 | 0.323 | 0.130 | 0.664 | 0.086 | 0.338 | 0.993 | |
| Diagnosis | b (SE) | −0.05 (0.13) | 0.31 (0.12) | 0.15 (0.12) | 0.25 (0.15) | 0.31 (0.14) | 0.22 (0.18) | 0.51 (0.19) | 0.33 (0.12) | 0.03 (0.19) | 0.23 (0.21) |
| p | 0.692 | 0.014 | 0.219 | 0.099 | 0.024 | 0.237 | 0.341 | 0.005 | 0.897 | 0.278 | |
| Carrier | b (SE) | −0.19 (0.14) | 0.02 (0.14) | 0.07 (0.14) | −0.22 (0.17) | −0.10 (0.16) | −0.03 (0.21) | −0.17 (0.22) | 0.31 (0.12) | −0.11 (0.21) | −0.38 (0.23) |
| p | 0.190 | 0.888 | 0.619 | 0.209 | 0.546 | 0.892 | 0.449 | 0.020 | 0.572 | 0.104 |
- Autoregressive correlation matrix was adopted for the final models with time as continuous predictor. In the interval analyses (time as factor, T0−T3, T3−T12), scaled identity was specified (covariances set to 0).
- Corrected α of the final models: α = 0.005, corrected α of the models with time only: α = 0.03.
- AIC, Akaike information criterion; CPT, Continuous Performance test; DSBW, Digit Span Backward; DSFW, Digit Span Forward; Fluency, Verbal Fluency test; SAT, Shifting Attention test; SDC, Symbol Digit Coding test; T, time; VEM, Verbal Memory test; VIM, Visual Memory test.
- a Random slopes of time.
- b Glioma (as reference category) versus meningioma.
- c ε4 non-carrier (as reference category) versus ε4 carrier.
DISCUSSION
The current prospective longitudinal study investigated whether patients with glioma or meningioma carrying the APOE ε4 allele showed greater vulnerability for cognitive dysfunction before treatment (1 day before surgery) and worse cognitive functioning over the course of treatment (3 and 12 months after surgery) as compared to non-carriers. We found no evidence for significantly worse pretreatment cognitive performance, that is, a lower group performance or higher prevalence of impairment, in ε4 carriers. Overall (without distinction based on APOE genotype), patients showed significant improvement from the presurgical to the 12-month postsurgical measurement on tests tapping into verbal memory (Verbal Memory test), psychomotor speed (Symbol Digit Coding test) and executive functioning (Shifting Attention test, Stroop test and Verbal fluency). We found no significant differences in performance over time between carriers and non-carriers on any of the tests.
As previous investigation of APOE’s effects in brain tumor patients did not include a pretreatment measurement, it remained unknown to what extent worse cognition in carriers after treatment was actually related to preexisting dysfunction [48]. We expected a small negative effect in the ε4 allele carriers before the start of treatment, based on ApoE4’s modulation of cerebrovascular function [16, 26] and white matter integrity [49] in response to injury. The lack of differences in pretreatment performances between carriers and non-carriers may be related to the temporal pattern of brain tumor injury. Brain tumor growth involves diffuse infiltration and/or compression over a period of years, as opposed to acute damage. Especially in the case of tumors with lower lesion momentum, APOE ε4 carriers may exert greater compensatory neural recruitment or “cognitive effort” that may be reflected in altered functional connectivity [28], but not a poorer test performance. We also note that large standard deviations were present for most of the (computerized) test scores at baseline. Substantial within-group variation is not uncommon in brain tumor patients, but it could have complicated detection of potential small effect sizes from an allelic variation.
Based on longitudinal research in treated (non-)CNS cancer patients, we expected ε4 carriers to show worse performances over time (ie, less recovery) compared to non-carriers on tests of executive functioning, (working) memory and processing speed [24, 35, 36, 50, 51]. A myriad of pathways [47], including vascular abnormalities, subefficient myelin regulation, increased oxidative stress and treatment-related toxicity [17, 30, 31, 34], could contribute to this difference in cognitive outcome. Our results did not, however, illustrate poorer trajectories of cognitive functioning in the total sample nor in the subgroup that received adjuvant treatment.
We note some methodological differences between studies that might account for the different findings. The longitudinal study by Correa and colleagues [36] that reported a ε4-related risk for decline in Digit Span performance obtained cognitive measurements at later time points (first assessment 4 ± 3.4 years after completion of treatment and second assessment 5.2 ± 0.8 years after that). Similarly, a study by Ahles and colleagues included long-term survivors of breast cancer 8.8 ± 4.3 years posttreatment [23]. Our measurements were obtained up to 12 months post-surgery (about 9 months after completion of radiotherapy, and about 3 months after completion of chemotherapy, depending on clinical and tumor characteristics). A longitudinal study by Ahles et al. [50] investigating changes from pre- up to 18 months post-chemotherapy in breast cancer patients also found no main effect of APOE. Late cognitive effects of treatment-induced processes that continue >6 months after radiation, such as capillary loss [52] and apoptosis [53], may be captured better at later follow-ups than those in our study.
We applied correction for multiple testing, thereby holding a more stringent cutoff for significant effects than other studies. Still, differences for baseline proportions of impairment Digit Span Backward and Shifting Attention tests were relatively large (>10% more impairment in carriers as compared to non-carriers) and could have been considered significant under an unadjusted significance level. In addition, mean performances for Letter Fluency appeared higher in carriers than non-carriers at baseline, but similar at 12-month follow-up, which indicates more improvement in non-carriers. These tests measure different facets of executive function, and a significant difference for Digit Span Backward was also found in previous research [36]. Future investigations may therefore focus primarily on executive measures.
While our sample sizes at pre- and first postsurgical measurement were large, 41 ε4 carriers and 126 non-carriers remained for the relevant time point 12 months post-surgery. This left us unable to include additional variables that might moderate the relationship between APOE and long-term cognition. For example, preclinical research has shown that adverse cognitive effects of radiation in ε4 carriers may manifest particularly in females [34]. Our adjuvant treatment sample naturally comprised a large proportion of high-grade glioma that occur more commonly in males [54]. In addition, mixed results regarding the effect of APOE ε4 on cognition have been found for different age groups, namely a positive effect in middle-aged or younger adults versus a negative effect in older adults [27, 55]. Our sample reflected the prevalence of brain tumors across age groups.
The degree to which APOE ε4 moderates cognition in patients with brain tumors remains somewhat inconclusive. While APOE ε4 might be related to a cognitive phenotype [27] conflicting results have also been reported in other neurological samples, such as traumatic brain injury [56]. Still, elucidating the effect of APOE allelic variation on cognition is important, especially for patients with low-grade or benign tumors who are expected to return to daily activities, such as work, that are associated with cognitive fitness [57]. We identify multiple potential areas of interest for future research. First, although APOE has received most attention in studies on cancer-related cognitive function [58] other genetic polymorphisms should also be recognized and investigated further as potential (interacting) markers for risk of cognitive dysfunction. For example, catechol-O-methyltransferase (COMT) and brain-derived neurotrophic factor (BDNF) have been associated with cognition independently [46, 59, 60] as well as in interaction with APOE genotype [59, 61]. Several genes associated with DNA repair, oxidative stress and inflammation have also been described [58]. APOE may also be investigated as a moderating factor for the effect of behavior on cognitive outcomes. For example, longitudinal findings by Ahles et al. [50] suggested that the association between APOE and cognition over the course of oncological treatment may be moderated by smoking behavior. In addition, individuals who carry ε2/ε3 alleles have been reported to benefit more from engaging in complex cognitive activities – as opposed to cognitively less challenging activities – than those who carry ε4 [62]. Finally, ε4-carrying men with low levels of physical activity appear to be more at risk for cognitive decline as compared to their non-carrying counterparts [63]. Individual brain tumor patients receiving cognitive or physical rehabilitation might benefit to different extents based on APOE genotype.
CONCLUSIONS
The current prospective longitudinal study was the first to investigate the association between APOE ε4 carrier status and both pre- and posttreatment cognition in patients with primary brain tumors. We found no statistical evidence for a negative effect of ε4 on pretreatment cognitive performance nor cognitive functioning over time up to 12 months after surgery. Research with larger samples at longer-term follow-up and investigations of the potential for APOE to interact with other (genetic) patient characteristics to influence cognitive outcome are warranted.
CONFLICT OF INTEREST
The authors report no financial, personal or professional conflict of interest for this study and the findings specified in this article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.




