Spinal dural leaks in patients with infratentorial superficial siderosis of the central nervous system—Refinement of a diagnostic algorithm
Abstract
Background and purpose
Superficial siderosis of the central nervous system is a sporadic finding in magnetic resonance imaging, resulting from recurrent bleedings into the subarachnoid space. This study aimed to determine the frequency of spinal dural cerebrospinal fluid (CSF) leaks amongst patients with a symmetric infratentorial siderosis pattern.
Methods
In all, 97,733 magnetic resonance images performed between 2007 and 2018 in our neurocenter were screened by a keyword search for “hemosiderosis” and “superficial siderosis.” Siderosis patterns on brain imaging were classified according to a previously published algorithm. Potential causative intracranial bleeding events were also assessed. Patients with a symmetric infratentorial siderosis pattern but without causative intracranial bleeding events in history were prospectively evaluated for spinal pathologies.
Results
Forty-two patients with isolated supratentorial siderosis, 30 with symmetric infratentorial siderosis and 21 with limited (non-symmetric) infratentorial siderosis were identified. Amyloid angiopathy and subarachnoid hemorrhage were causes for isolated supratentorial siderosis. In all four patients with a symmetric infratentorial siderosis pattern but without a causative intracranial bleeding event in history, spinal dural abnormalities were detected. Dural leaks were searched for in patients with symmetric infratentorial siderosis and a history of intracranial bleeding event without known bleeding etiology, considering that spinal dural CSF leaks themselves may also cause intracranial hemorrhage, for example by inducing venous thrombosis due to low CSF pressure. Thereby, one additional spinal dural leak was detected.
Conclusions
Persisting spinal dural CSF leaks can frequently be identified in patients with a symmetric infratentorial siderosis pattern. Diagnostic workup in these cases should include magnetic resonance imaging of the whole spine.
INTRODUCTION
Superficial siderosis of the central nervous system (CNS) is characterized by hemosiderin deposits on the surface of the brain and spinal cord resulting from recurrent subarachnoid hemorrhage [1, 2]. After bleeding events, hemolysis causes free iron, which is bound by ferritin and finally converted to hemosiderin. Recurrent hemorrhage can deplete ferritin synthesis, leaving free iron deposited on the subpial layer. The accumulation of this neurotoxic iron leads to progressive cell damage and neurological deficits [3, 4].
In their recent landmark publication, Wilson et al evaluated an unselected cohort of 142 patients with superficial siderosis of the CNS derived from in-hospital radiological reports and external referrals [5]. Siderosis primarily affecting supratentorial regions of the brain was associated with cerebral amyloid angiopathy or a history of subarachnoid hemorrhage [5, 6]. Remarkably, a relevant proportion of patients (33%) had symmetric infratentorial superficial siderosis (iSS) (i.e., hemosiderin deposits along both sides of the cerebellum, the brain stem and the spinal cord) without a causative bleeding event in history. Here, 80% of cases were reported to have dural abnormalities (mostly spinal; e.g., resulting from surgery or spontaneous or traumatic dural tears) as the likely cause of siderosis. Consistently, case reports suggest that spinal dural cerebrospinal fluid (CSF) leaks may persist for years leading to recurrent bleedings into the subarachnoid space [7, 8]. Considering that progressive superficial siderosis is associated with chronic worsening of neurological symptoms, treatment of the underlying dural pathology is usually indicated in such cases [7, 9].
The aim of this study was to determine the frequency of spinal dural CSF leaks amongst patients with a symmetric iSS pattern on magnetic resonance imaging (MRI) scans. For this purpose the large MRI database of our tertiary care neurocenter (including cases between 2007 and 2018) was screened for patients with symmetric iSS without a causative intracranial bleeding event in history (i.e., patients suspicious for dural leaks according to Wilson et al). After that, patients were prospectively evaluated for the presence of spinal epidural fluid collections by means of MRI.
METHODS
A single center study was conducted consisting of a retrospective and a prospective part. Both parts were approved by the ethics committee of the University Hospital (no. 19-321). Informed consent was obtained from each patient for prospective evaluation.
In the first part patients with superficial siderosis of the CNS were retrospectively identified by a keyword search (terms “hemosiderosis” and “superficial siderosis”) in our MRI database comprising the radiological reports of all MRI scans performed between 2007 and 2018 in our tertiary care neurocenter (Institute of Neuroradiology, Center of Neurology and Neurosurgery, University Hospital/Goethe University, Frankfurt am Main, Germany).
Brain MR images were evaluated by two experienced neuroradiologists (B.R., R.dM.dR.) blinded to patients’ clinical details. Superficial siderosis was diagnosed according to pre-specified radiological criteria (homogeneous low signal on T2- or blood-sensitive sequences—T2* gradient echo or susceptibility-weighted imaging) [5, 10]. According to Wilson et al, patients were classified into four different types: (i) isolated siderosis involving supratentorial brain regions only; (ii) infratentorial siderosis with symmetric involvement of at least two of three predefined regions (brainstem [including midbrain, pons, medulla], cerebellum [including the cerebellar folia, vermis and cerebellar peduncles], spinal cord or craniocervical junction) without a causative intracranial bleeding event in history (iSS type 1); (iii) infratentorial siderosis with symmetric involvement of regions as described above with a causative intracranial bleeding event in history (e.g., intracerebral or subarachnoid hemorrhage in the posterior fossa; iSS type 2); and (iv) limited (non-symmetric) infratentorial siderosis [5]. Additional radiological findings (e.g., vascular abnormalities, intracranial hemorrhage) were registered.
Two neurologists (C.F., L.F.) reviewed the medical records of the patients with primary respect to the clinical course and the presence of typical symptoms of infratentorial siderosis (i.e., cerebellar gait ataxia, bilateral hearing loss and spinal symptoms) [1, 2] but also with respect to other neurological deficits. The two neurologists also documented the reported intracranial bleeding events that probably caused superficial siderosis. Moreover, it was evaluated whether the etiology of the reported intracranial bleeding event was known and plausible (e.g., vascular malformations, cerebral amyloid angiopathy, arterial hypertension) or whether the hemorrhage itself could have been a consequence of cerebral venous thrombosis resulting from low intracranial pressure due to a persisting dural CSF leak. According to the standard of practice in our hospital and depending on individual decision making by the treating physician, diagnostic procedures in patients with intracranial hemorrhage include cranial computed tomography (CT) and MR imaging, cerebral and spinal digital subtraction angiography, CSF analysis and blood pressure evaluation.
In the second part all patients with symmetric iSS without a causative intracranial bleeding event in history (iSS type 1) were evaluated for the presence of spinal epidural fluid collections on MR imaging of the spinal cord as an indicator of a potential dural leak. This included previously performed scans (if available) and prospective whole spine MR imaging including sagittal fat-suppressed T2-weighted sequences. Areas with suspected dural leaks were supplemented by axial sequences focusing on extra-arachnoid spinal fluid collection. Patients with iSS type 2 (i.e., with a causative intracranial bleeding event in history) were also screened for dural CSF leaks if the etiology of the bleeding event could not be determined from the treating physician despite routine diagnostic workup (i.e., the diagnosis at that time was “intracerebral or subarachnoid hemorrhage of unknown etiology”). As described above, this condition may point to hemorrhage resulting from venous pathologies associated with low intracranial pressure.
Statistics
SPSS version 25 was used for the statistical evaluation of our dataset. Baseline variables and distribution of bleeding etiologies between groups were compared using parametric and non-parametric statistics, respectively. Relative frequencies for siderosis groups for both the dataset from Wilson et al and our dataset were calculated and compared by chi-squared test statistics for significant differences.
Data availability statement
Source data are available on reasonable request via the corresponding author. The principal author has access to all the data and takes responsibility for the data, accuracy of the data analysis and the conduct of the research.
RESULTS
The radiological reports of all 97,733 MRI scans (including 79,168 brain MRI scans) acquired within the 12-year study period (2007–2018) in our neurocenter were screened for the presence of the search terms and 127 patients were identified with “superficial siderosis” or “hemosiderosis,” respectively. Eighteen patients aged below 18 years were excluded as were another 16 patients where the search terms described alternative constellations (e.g., “no hemosiderosis”) (Figure 1).
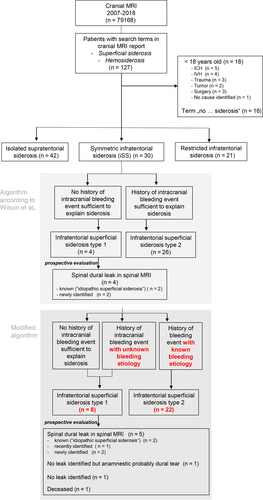
The algorithm published by Wilson et al was then applied to the remaining 93 patients [5]. The baseline variables of this cohort are described in Table 1. Of the 93 included cases of siderosis, 42 had isolated supratentorial siderosis, four had iSS type 1 (i.e., iSS without history of a causative intracranial bleeding event), 26 had iSS type 2 (i.e., iSS with history of a causative intracranial bleeding event) and another 21 patients had limited infratentorial siderosis (Figure 1).
| sSS | iSS type 1 | iSS type 2 | Restricted iSS | |
|---|---|---|---|---|
| N | 42 | 4 | 26 | 21 |
| Age in years (mean ± SD) | 64.7 ± 16.7 | 62.8 ± 6.3 | 57.2 ± 17.1 | 58.1 ± 14.6 |
| Sex (f) | 23 | 2 | 13 | 10 |
| Intracranial bleeding event due to | ||||
| Aneurysmal SAH | 15 | 7 | 7 | |
| CAA | 12 | 2 | ||
| Brain tumor | 3 | 7 | 1 | |
| Primary ICH | 3 | 4 | 1 | |
| Vasculitis | 2 | 1 | ||
| Trauma | 2 | 3 | 5 | |
| Surgery | 1 | 2 | ||
| SDH | 1 | |||
| Ischemic stroke | 1 | |||
| Unidentified | 2 | 4a | ||
| No intracranial bleeding event | 4a | |||
- Abbreviations: CAA, cerebral amyloid angiopathy; ICH, intracerebral hemorrhage; iSS, infratentorial superficial siderosis; SAH, subarachnoid hemorrhage; SDH, subdural hematoma; sSS, supratentorial superficial siderosis.
- a Due to siderosis localization and symmetric configuration suspicious for dural abnormality.
This corresponds to relative frequencies of 0.4 per 1,000 brain MRI scans for symmetric iSS (i.e., iSS types 1 and 2) (95% CI 0.256–0.541) and of 0.05 per 1,000 scans for iSS type 1 (95% CI 0.014–0.129). In our dataset, the relative frequency for iSS (types 1 and 2) differed significantly from that described in the Wilson cohort (0.323, 95% CI 0.229–0.427 vs. 0.452, 95% CI 0.348–0.558; p = 0.039) [5].
Table 1 displays the distribution of the presumed etiologies of the siderosis. Cerebral amyloid angiopathy and aneurysmatic subarachnoid hemorrhage were the predominant causes for isolated supratentorial siderosis. The predominant causes in iSS type 2 patients were aneurysmatic subarachnoid hemorrhage and brain tumors.
With respect to clinical data, all four patients with iSS type 1 showed at least two of the three typical progressive symptoms of “classical” infratentorial siderosis (gait ataxia, hypacusis, spinal symptoms). Most patients with iSS type 2 presented with an acute to sub-acute development of neurological symptoms, each consistent with the underlying cause of bleeding (in the case of patients with subarachnoid hemorrhage or intracerebral hemorrhage, typically sudden headache, vigilance reduction and focal neurological deficits; in the case of a tumor, recurrent headache with nausea and/or loss of vision). However, in three of the iSS type 2 patients, a single severe headache event in history was followed by a progressive worsening of neurological symptoms (gait ataxia, hypacusis, oculomotor abnormalities; Table 2, patients 5, 6, 7). In all three patients the presence of spinal dural CSF leaks was evaluated (see below).
| Patient | Imaging characteristics | Clinical symptoms | Latency between onset of symptoms and diagnosis of dural leak | ||
|---|---|---|---|---|---|
| Superficial siderosis | Epidural fluid collection | Additional findings | |||
| 1 | Hemosiderin deposits centred on brainstem, cerebellum and spinal cord | Ventral epidural fluid collection (C2–T12) | Myelopathy |
Progressive hearing loss Progressive gait ataxia Hypesthesia of the legs Vertigo Urinary symptoms |
13 years |
| 2 | Hemosiderin deposits centred on brainstem, cerebellum and spinal cord | Ventral epidural fluid collection (C6–T6) | Extended myelopathy in the cervical and thoracic section |
Low CSF pressure headache Progressive gait ataxia Hypesthesia of the legs |
13 years |
| 3 | Hemosiderin deposits centred on brainstem, cerebellum and spinal cord | Epidural fluid collection (T2/3 downwards) |
Progressive hearing loss Progressive gait ataxia |
8 years | |
| 4 | Hemosiderin deposits centred on brainstem, cerebellum and spinal cord | Ventral epidural fluid collection (T5–T7) | Pronounced intradural venous vessels in the upper thoracic section (without angiographic evidence of a dural fistula) |
Progressive hearing loss Progressive gait ataxia |
17 years |
| 5 | Hemosiderin deposits centred on brainstem, cerebellum and spinal cord | Epidural fluid collection (C5–T5) |
Myelopathy Status after atypical ICH in the right cerebellum Status after vein of Labbé thrombosis in the right cerebellum |
Low CSF pressure headache Progressive hearing loss Progressive gait ataxia |
34 years |
| 6 | Hemosiderin deposits centred on brainstem, cerebellum and spinal cord | Atypical intracerebral hemorrhage parieto-occipital |
Headache Hearing loss Homonymous hemianopia Progressive gait ataxia |
Deceased | |
| 7 | Hemosiderin deposits centred on brainstem and cerebellum |
Status after SAH without causative aneurysm Unclear glial scar in the left midbrain Hypertrophic olivary degeneration |
Vertical nystagmus Double vision Dysarthria Progressive gait ataxia |
No leak detected | |
| 8 | Hemosiderin deposits centred on brainstem, cerebellum and spinal cord | Partially thrombosed giant fusiform aneurysm of the basilar artery | No focal neurological deficit | No leak detected | |
Note
- Patients 1–4, iSS type 1 patients according to Wilson et al; patients 5–8, additional iSS type 1 patients after reclassification according to the new algorithm.
- Abbreviations: CSF, cerebrospinal fluid; ICH, intracerebral hemorrhage; iSS, infratentorial superficial siderosis; SAH, subarachnoid hemorrhage.
In the next step, the four patients with iSS type 1 were prospectively evaluated for the presence of spinal dural CSF leaks. All four patients had a diagnosis of “idiopathic superficial siderosis” (Table 2, patients 1–4).
In two patients, a persisting spinal dural CSF leak had already been described in previous spinal MR images. However, the “idiopathic” siderosis had not been linked to the dural leak so far, and therapeutic measures had not been undertaken (Table 2, patients 1 and 2). In the third patient (patient 3) a spinal dural CSF leak was newly identified on a previous spinal MRI scan that had not been recognized before according to the available medical and radiological documentation. In the fourth patient (patient 4) full spinal MR imaging had never been performed. Follow-up MRI revealed an epidural fluid collection ventral to the thoracic cord, indicating a persisting spinal dural CSF leak. In summary, in all patients (100%) with iSS type 1 a dural leak has prospectively been detected in our study.
A slightly modified diagnostic algorithm was then applied to the dataset, taking into account that spinal dural CSF leaks may themselves cause intracranial hemorrhage, for example by inducing venous thrombosis in the case of low CSF pressure. Therefore, all patients classified as iSS type 2 (i.e., iSS with history of a causative intracranial bleeding event) who had an unknown etiology of the bleeding event despite routine diagnostic workup (n = 4) were reclassified as iSS type 1 (Figure 1; Table 2, patients 5–8). Interestingly, three of those four patients also showed progressive clinical symptoms of “classical” infratentorial siderosis (as already mentioned above). This reclassification enriched the group by a severely affected patient with years of past history of cerebellar hemorrhage caused by venous thrombosis in whom a spinal dural CSF leak (Table 2, patient 5, initially triggering the refinement of the diagnostic algorithm) was only recently identified, by one patient with incomplete spinal imaging and two patients without spinal imaging at all. One patient died before prospective evaluation could be performed (patient 6). Prospective spinal MR imaging of the remaining two patients (patients 7 and 8) excluded dural leaks. However, anamnestic exploration in one of the cases (patient 7) revealed sudden-onset severe neck pain during dumbbell workout with occurrence of posterior fossa hemorrhage in immediately performed CT scan of the brain, suggesting a transient cervical dural tear.
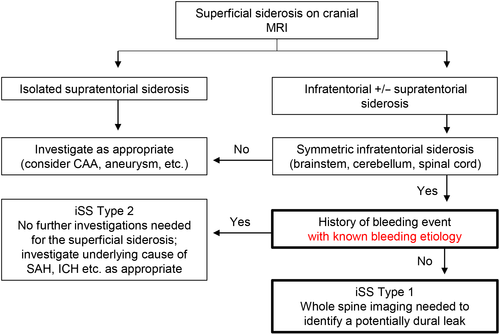
The diagnostic algorithm suggested by Wilson et al was modified by addition of the stratification regarding the etiology of the bleeding event in iSS type 2 (known vs. unknown) (Figure 2). This modification increased the number of patients with iSS type 1 by four (100%) in whom in one a dural leak could be detected. Thus, excluding the patient lost to follow-up, in five of seven (71%) patients with modified iSS type 1 a spinal dural CSF leak could be identified.
DISCUSSION
Our investigation resulted in three major findings. First, estimates were provided of the frequency of different types of superficial siderosis in a large sample of MR brain scans performed in a tertiary care neurocenter. Secondly, it was confirmed that persisting spinal dural CSF leaks are frequent findings in patients with iSS type 1 (i.e., iSS without history of a causative intracranial bleeding event) and, if previously unidentified, are likely to be discovered upon reevaluation. Thirdly, the diagnostic algorithm suggested by Wilson et al [5] was refined by adding a stratification regarding the etiology of some cases of iSS type 2, which may also be caused by dural leaks leading to intracerebral or subarachnoid hemorrhages via cerebral venous thrombosis [8, 11].
The prevalence of superficial siderosis has not been assessed in detail before. In our large dataset of MRI examinations collected over a 12-year period at a tertiary care neurocenter, symmetric iSS was found in 0.4 per 1,000 brain MRI scans. In about 13% of these cases a causative intracranial bleeding event could not be identified in history (i.e., iSS type 1 according to Wilson et al [5]). The Wilson cohort showed a significantly higher relative frequency of iSS amongst the included patients with superficial siderosis, primarily due to the higher frequency of patients with iSS having post-surgical dural abnormalities (mainly suboccipital pseudomeningoceles) compared to our center (n = 22 vs. n = 0) [5]. Although screening of MRI reports in our neurocenter also included neurosurgery patients, it was not possible to identify a relevant number of post-surgery siderosis patients (and none with an iSS pattern). Differences in the frequency of neurosurgical procedures in the posterior fossa and in follow-up imaging between centers may account for this finding.
Infratentorial siderosis subsequent to persisting dural leaks is known for causing progressive and disabling clinical symptoms, in particular gait ataxia and hearing loss [2]. If identified leaks can be treated by epidural patching or neurosurgical repair [7, 9] this can prevent further neurological deterioration, even if leaks have persisted for many years. The diagnostic algorithm suggested by Wilson et al was applied to our dataset and identified four patients with iSS type 1. Two of these patients had a known spinal dural CSF leak that, according to clinical documentation, had not been linked to the siderosis before. Rather, after excluding cranial and spinal bleeding sources (such as arteriovenous malformations and dural fistula) by intra-arterial angiography, the diagnosis of idiopathic superficial siderosis was established. Even recently, a case report on a patient with idiopathic superficial siderosis was published although spinal MR imaging had not been performed within the diagnostic workup [12]. Both of our patients had disabling neurological deficits. However, the so far unknown association between dural leaks and superficial siderosis precluded the consideration of a possible role of the dural defect. In the two other patients persisting spinal dural CSF leaks were newly diagnosed. Remarkably, in our study, spinal dural CSF leaks could be detected in all (100%) patients with iSS type 1. Thus, reevaluation for dural leaks in patients with iSS type 1 will probably result in treatment-relevant findings.
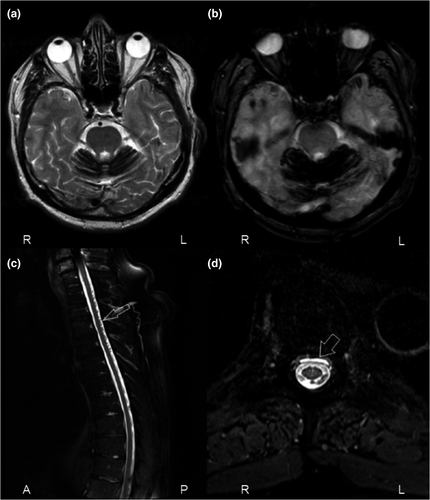
Dural leaks causing recurrent subarachnoid bleedings can represent the single underlying cause for the superficial siderosis [8]. Recurrent capillary or venous low-pressure hemorrhage originating from perilesional tissue around the dural tear has been described as a bleeding source [5, 13]. In addition, the persistent loss of CSF and the resulting intracranial hypotension are associated with an increased risk for brain sagging causing traction to bridging veins as well as cerebral venous thrombosis, both leading to subsequent intracranial hemorrhage [11]. Based on the Monro–Kellie hypothesis implying that intracranial volume is always constant, any decrease in CSF volume leads to a compensatory increase in intracranial blood volume, recognizable by subsequent dilatation of venous structures, venous stasis and venous engorgement [11, 14]. Our prospective screening for dural leaks revealed meandering and diluted spinal veins along the myelon in one patient (Figure 3). Another patient had cerebellar hemorrhage associated with venous thrombosis of cerebellar veins. Consequently, any intracranial bleeding event in patients with iSS that is not otherwise explained should trigger further diagnostic steps to identify spinal dural CSF leaks. Until now, the Wilson et al algorithm suggested not to screen for dural abnormalities in iSS patients with a “clear single spontaneous or traumatic intracranial bleeding event” (iSS type 2), regardless of bleeding etiology [5]. Their algorithm was therefore modified by reclassifying patients with a history of an intracranial bleeding event but an unidentified bleeding etiology (originally iSS type 2) into iSS type 1. This reclassification of four patients (one died before follow-up) led to the identification of one additional spinal dural CSF leak, resulting in an overall relative frequency of spinal dural CSF leaks in patients with modified iSS type 1 of 71%. Therefore, full MRI of the spine for dural leak detection is indicated in these patients.
The following refined algorithm is proposed for clinical practice. If superficial siderosis is diagnosed in cranial MRI, the distribution of siderosis should be assessed accurately. In the case of infratentorial siderosis with symmetric involvement of at least two of three predefined regions (brainstem, cerebellum, spinal cord) and lack of history of a bleeding event or history of a bleeding event without known bleeding etiology, patients should receive further spinal MR imaging to clarify a potential dural leak causative for the siderosis (e.g., via sagittal and transversal fat-suppressed T2 sequences of the entire spine) (Figure 2). The proposed algorithm may offer patients with dural leaks the opportunity to receive causal therapy after the exact localization of the leak by means of CT or MR myelography [7, 9]. As reported in case series, dural closure by graft application or surgical repair can stabilize or even improve clinical symptoms in siderosis patients [7]. However, in the case of a long history of a persisting spinal dural CSF leak, neurological symptoms may deteriorate even after successful dural closure, underlining the relevance of early diagnosis and treatment initiation [7, 15]. Here, alternative therapeutic strategies targeting the reduction of hemosiderin deposits come into play. A prospective observational study reported that therapy with the iron chelator deferiprone over 2 years resulted in clinical stabilization or improvement of neurological symptoms in 63% of the investigated cases [16]. In a subpopulation a comparison of pre-study and post-study MRI suggested a decrease of the total iron content of 50%. In this group of “MRI responders,” 88% of the subjects reported an improvement of neurological symptoms.
The strengths of our study include the large dataset of MR examinations used for screening purposes and both retrospective and prospective evaluation of cases. Limitations of our investigation are its single center design and the missing information on the precise location of the leaks (e.g., detected by CT myelography or intraspinal gadolinium-enhanced MR imaging) [15]. In a strict sense, epidural fluid collections in spinal MRI are indicative of and not confirmative of a persisting dural leak. Furthermore, the frequency estimates for iSS in MRI scans did not take into account that multiple scans might have been performed for individual patients. In addition, our study did not yet allow an estimation of the rate for occurrence of siderosis amongst patients with spinal dural CSF leaks. In accordance with the diagnostic algorithm proposed by Wilson et al, spinal dural CSF leaks were evaluated only in patients with symmetrical infratentorial siderosis. The assessment of occurrence of spinal dural CSF leaks in patients with other types of siderosis should be the subject of a future prospective study.
In summary, persisting spinal dural CSF leaks can frequently be identified in patients with a symmetric iSS pattern. Diagnostic workup in these cases should include MRI of the whole spine, in particular also in patients having a history of a causative intracranial bleeding event of unknown etiology. This may avoid delays in detection and treatment of spinal dural CSF leaks.
ACKNOWLEDGEMENTS
The authors have not declared any specific grant for this study from any funding agency in public, commercial or not-for-profit sectors. Open access funding enabled and organized by Projekt DEAL.
DISCLOSURE OF CONFLICTS OF INTEREST
The authors declare no financial or other conflicts of interest.
AUTHOR CONTRIBUTIONS
Lucie Friedauer, University Hospital Frankfurt: Guarantor for the overall article, contributed to the conception and design of the study, to the acquisition and analysis of data and to the drafting the manuscript and figures. Beata Rezny-Kasprzak, University Hospital Frankfurt: Involved in the acquisition and analysis of data. Helmuth Steinmetz, University Hospital Frankfurt: Contributed to the conception and design of the study and gave final approval of the article. Richard du Mesnil de Rochemont, University Hospital Frankfurt: Involved in the acquisition and analysis of data. Christian Foerch, University Hospital Frankfurt: Guarantor for the overall article, contributed to the conception and design of the study, to the acquisition and analysis of data and to the drafting the manuscript and figures. All authors have reviewed and approved the content of the manuscript. Requirements for authorship are met by all authors.
Open Research
DATA AVAILABILITY STATEMENT
Source data are available on reasonable request via the corresponding author. The principal author has access to all the data and takes responsibility for the data, accuracy of the data analysis and the conduct of the research.




