Protein tyrosine phosphatase receptor type Q in cerebrospinal fluid reflects ependymal cell dysfunction and is a potential biomarker for adult chronic hydrocephalus
Abstract
Background and purpose
Protein tyrosine phosphatase receptor type Q (PTPRQ) was extracted from the cerebrospinal fluid (CSF) of patients with probable idiopathic normal-pressure hydrocephalus (iNPH) by proteome analysis. We aimed to assess the feasibility of using CSF PTPRQ concentrations for the additional diagnostic criterion of iNPH in Japanese and Finnish populations.
Methods
We compared PTPRQ concentrations among patients with probable iNPH and neurologically healthy individuals (normal control [NC] group), patients with normal-pressure hydrocephalus (NPH) of acquired and congenital/developmental aetiologies, patients with Alzheimer’s disease and patients with Parkinson’s disease in a Japanese analysis cohort. A corresponding iNPH group and NC group in a Finnish cohort was used for validation. Patients in the Finnish cohort who underwent biopsy were classified into two groups based on amyloid and/or tau deposition. We measured PTPRQ expression levels in autopsied brain specimens of iNPH patients and the NC group.
Results
Cerebrospinal fluid PTPRQ concentrations in the patients with NPH of idiopathic, acquired and congenital/developmental aetiologies were significantly higher than those in the NC group and those with Parkinson’s disease, but iNPH showed no significant differences when compared with those in the Alzheimer’s disease group. For the patients with iNPH, the area under the receiver-operating characteristic curve was 0.860 in the Japanese iNPH and 0.849 in the Finnish iNPH cohorts. Immunostaining and in situ hybridization revealed PTPRQ expression in the ependymal cells and choroid plexus. It is highly possible that the elevated PTPRQ levels in the CSF are related to ependymal dysfunction from ventricular expansion.
Conclusions
Cerebrospinal fluid PTPRQ levels indicated the validity of this assay for auxiliary diagnosis of adult chronic hydrocephalus.
Introduction
Idiopathic normal-pressure hydrocephalus (iNPH) is prevalent among older adults and is characterized by Hakim’s triad, which consists of gait disturbances, cognitive impairment and urinary incontinence [1]. Although these symptoms are improved by cerebrospinal fluid (CSF) shunts, the aetiology and physiopathology of iNPH remain enigmatic. However, iNPH is thought to stem from difficulties in the clearance of waste metabolites from the brain [2-4].
Diagnosis of iNPH is primarily based on symptoms and diagnostic imaging [5, 6]; however, CSF-based assays are used as auxiliary diagnostic tools. For example, studies have measured the levels of CSF biomarkers for iNPH, such as amyloid β 1-42 (Aβ42), tau phosphorylated at threonine 181 (p-tau), and total tau (t-tau), which are also associated with concomitant Alzheimer’s disease (AD) [7, 8]. In one study, these biomarkers predicted that CSF shunt placement would provide limited improvement of symptoms in patients with iNPH with concomitant AD [9]. Further, a variety of CSF protein biomarkers have been described [10-13], most of them being reduced in patients with iNPH compared with healthy individuals. Likely reasons for this reduction are delayed CSF clearance, an increase in the overall CSF volume due to iNPH [14], altered metabolism, and astrocyte activation [11, 15]. Although previous studies have focused on AD-related proteins for the diagnosis of iNPH, most protein biomarkers were not directly associated with the underlying mechanisms of iNPH pathology. The lack of knowledge regarding the specific pathology of iNPH presents a challenge for identifying potential biomarkers for this disorder, therefore, it is necessary to discover new diagnostic biomarkers to help understand the pathophysiology of iNPH.
Recently, protein tyrosine phosphatase receptor type Q (PTPRQ) was extracted from the CSF of patients with iNPH by proteome analysis. PTPRQ has been reported to be involved in iNPH only once before [16]. The estimated molecular weight of PTPRQ in the CSF was approximately 170 kDa when the antibody for its extracellular domain was used [16]. The PTPRQ gene is involved in hearing loss (i.e., deafness autosomal recessive 84A [DFNB84A]) [17]. Nayak et al. [18] reported that the extracellular region of PTPRQ in the vestibular end organ is modified with dermatan sulphate, and, depending on the location of the dermatan sulphate on PTPRQ, neighbouring stereocilia adhere to or repel each other in order to maintain a proper distance between the cilia. However, the function of PTPRQ in the central nervous system is largely unknown. In the present study, we investigated the role of PTPRQ in probable iNPH and carried out the first intracerebral analysis of PTPRQ expression in autopsied brains of patients with iNPH.
Material and methods
Ethics statement
This study was reviewed and approved by the ethics committees of Juntendo University Hospital and Kuopio University Hospital (KUH) and conforms to the World Medical Association Declaration of Helsinki. All patients included in the study or their relatives gave informed consent for participation in the study. If the clinician suspected that dementia would significantly affect the capacity of the patient to consent, the next of kin or guardian consented on behalf of the patient. When consent was obtained from a participant’s proxy, the patient’s opinion was asked and considered, and no patients were recruited against their will.
Study design
In the first part of the study, we evaluated two cohorts of patients with probable iNPH: a Japanese cohort for analysis and a Finnish cohort for validation. We measured and compared the differences in the concentrations of CSF protein biomarkers for differential diagnosis among four groups: Japanese patients with probable iNPH (Japanese iNPH group), cognitively normal healthy individuals, hereafter referred to as the normal control (NC) group, patients with Alzheimer’s disease (AD) and patients with Parkinson’s disease (PD; Fig. 1). We validated the results for the NC group and patients with iNPH (Finnish iNPH group) in the Finnish cohort. Next, we intended to clarify the role and significance of PTPRQ in acquired (secondary) normal-pressure hydrocephalus (NPH; in which symptoms appeared subsequent to a preceding neurological injury, e.g., subarachnoid haemorrhage, meningitis) and congenital/developmental aetiologies, which present with panventriculomegaly, wide foramen of Magendie and large cisterna magna [19].
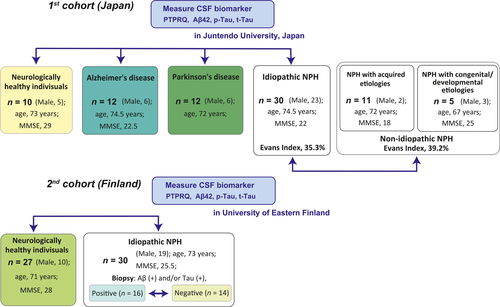
In the second part of the study, immunostaining and mRNA expression analyses were performed in autopsied brain specimens of NCs and patients with iNPH.
Study population
This study included two cohorts: one from Japan and the other from Finland. Patients who had at least one component of the NPH triad (gait disturbance, dementia, and urinary incontinence), and those with ventriculomegaly on computed tomography (CT)/magnetic resonance imaging (MRI) were enrolled as possible iNPH patients. In accordance with the Japanese guidelines for the management of iNPH, probable iNPH was defined as the presence of at least one component of the NPH triad with tight high convexity on CT/MRI or improvement of symptoms after CSF tap test or drainage [6]. CSF shunt surgeries were performed in patients with probable iNPH. A retrospective analysis of the detailed course of clinical symptoms was performed. Evaluation items included Mini Mental State Examination (MMSE) scores [19, 20] and iNPH Grading Scale (iNPHGS) scores [21].
The first cohort in Japan comprised the Japanese NC group, which consisted of 10 subjects (median age 73 years; five men); these patients were aged ≥60 years and had no known brain diseases or subjective declines in cognition and MMSE scores ≥26; the Japanese iNPH group consisted of 30 consecutive patients with iNPH (median age 74.5 years; 23 men) who received a lumboperitoneal shunt intervention between February 2013 and April 2015; the AD group consisted of 12 patients with AD (median age 74.5 years; six men), diagnosed by a neurologist according to the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association criteria for probable AD [22]; finally, the PD group consisted of 12 patients with PD (median age 72 years; six men), diagnosed by a neurologist according to the Movement Disorder Society Clinical Diagnostic Criteria for PD [23] (Table 1). An additional survey was conducted to compare iNPH with non-idiopathic NPH: 11 NPH patients with acquired aetiologies (median age 72 years; two men) and five NPH patients with congenital/developmental aetiologies (median age 67 years; three men; Table 2). The whole Japanese cohort was recruited from the Department of Neurology and Neurosurgery, Juntendo University, Tokyo, Japan.
| NC (n = 10) | AD (n = 12) | PD (n = 12) | iNPH (n = 30) | P | |
|---|---|---|---|---|---|
| Age, years | 73 (66.5–80.5) | 74.5 (72–80.5) | 72 (66.25–79) | 74.5 (72–80.5) | 0.809 |
| Men, n (%) | 5 (50) | 6 (50) | 6 (50) | 23 (77) | 0.308 |
| MMSE scores at entry | 28 (28–30) | 22.5 (20–27) | 26 (25–29.25) | 22 (20–26) |
P1: 0.002**, P2: 1.000, P3: <0.001***, P4: 0.488, P5: 0.702 |
| CSF biomarker, mean (SD) | |||||
| PTPRQ, pg/ml | 296 (94) | 365 (225) | 338 (230) | 619 (398) |
P1: 0.921, P2: 0.994, P3: 0.001**, P4: 0.077, P5: 0.042* |
| Aβ42, pg/ml | 633 (217) | 420 (145) | 657 (241) | 500 (236) | P1: 0.103, P2: 1.000, P3: 0.533, P4: 0.725, P5: 0.395 |
| p-tau, pg/ml | 25.1 (7.5) | 103 (38.5) | 22.8 (8.2) | 25.6 (13.0) |
P1: <0.001***, P2: 0.986, P3: 1.000, P4: <0.001***, P5: 0.960 |
| t-tau, pg/ml | 170 (86) | 595 (276) | 116 (120) | 117 (90.7) |
P1: 0.001**, P2: 0.797, P3: 0.513, P4: <0.001***, P5: 1.000 |
- Aβ42, amyloid β 1-42; AD, Alzheimer’s disease; CSF, cerebrospinal fluid; iNPH, idiopathic normal-pressure hydrocephalus; MMSE, Mini-Mental State Examination; NC, normal control (neurologically healthy; PD, Parkinson’s disease; p-tau, tau phosphorylated at threonine 181; PTPRQ, protein tyrosine phosphatase receptor type Q; t-tau, total tau. Data are expressed as median (25%−75%), unless otherwise indicated; *P < 0.05, **P < 0.01, ***P < 0.001. P1, comparison between NC and AD groups; P2, comparison between NC and PD groups; P3, comparison between NC and iNPH groups; P4, comparison between iNPH and AD groups; P5, comparison between iNPH and PD groups.
| iNPH (n = 30) | Non-idiopathic NPH (① + ②, n = 16) | P value comparison between idiopathic and non-idiopathic | ① NPH with acquired aetiologies (n = 11) | ② NPH with congenital/developmental aetiologies (n = 5) | P value for multiple comparisons | |
|---|---|---|---|---|---|---|
| Age, years | 74.5 (72–80.5) | 71.5 (66.25–75.5) | 0.182 | 72 (67–76) | 67 (62–76) | 0.125 |
| Men, n (%) | 23 (77) | 5 (31) | 0.267 | 2 (18) | 3 (60) | 0.007** |
| Mean (SD) Evans Index, % | 35.3 (4.3) | 39.2 (4.7) | 0.011* | 38.5 (4.6) | 40.0 (5.2) | 0.035* |
| MMSE scores at entry | 22 (20–26) | 20 (15–24) | 0.716 | 20 (14.25–20.75) | 26 (21–28) | 0.015* |
| iNPHGS total score | 5 (5–7) | 5 (4.5–8.5) | 0.870 | 7 (5–9.75) | 5 (3–5) | 0.048* |
| Gait disturbance score | 2 (2–2.25) | 2 (1–3) | 0.076 | 3 (1.25–3.75) | 2 (1–2) | 0.079 |
| Cognitive impairment score | 2 (1–3) | 2 (1.5–3) | 0.256 | 3 (2–3) | 1 (0.5–2.5) | 0.024* |
| Urinary impairment score | 2 (1–2) | 1 (1–2.5) | 0.857 | 1.5 (1–3) | 1 (1–1.5) | 0.424 |
| CSF biomarker, mean (SD) | ||||||
| PTPRQ, pg/ml | 619 (398) | 1704 (861) | <0.001*** | 1697 (969) | 1720 (656) |
P1: 0.013*, P2: 0.053 |
| Aβ42, pg/ml | 500 (236) | 444 (204) | 0.518 | 383 (146) | 576 (266) |
P1: 0.192, P2: 0.464 |
| p-tau, pg/ml | 25.6 (13.0) | 22.4 (11.5) | 0.229 | 19.4 (6.6) | 29 (17.6) |
P1: 0.150, P2: 0.972 |
| t-tau, pg/ml | 117 (90.7) | 237 (184) | 0.004** | 216 (148) | 280 (260) |
P1: 0.162, P2: 0.551 |
- CSF, cerebrospinal fluid; iNPH, idiopathic normal-pressure hydrocephalus; iNPHGS, idiopathic normal-pressure hydrocephalus grading scale; MMSE, Mini Mental State Examination; NPH, normal-pressure hydrocephalus; p-tau, tau phosphorylated at threonine 181; PTPRQ, protein tyrosine phosphatase receptor type Q; t-tau, total tau.
- Data are expressed as median (25%−75%), unless otherwise indicated; *P < 0.05, **P < 0.01, ***P < 0.001. P1, comparison between iNPH and NPH with acquired aetiologies; P2, comparison between iNPH and NPH with congenital/developmental aetiologies.
The KUH Neurosurgery Department provides full-time acute and elective neurosurgical services for the KUH patient population from a recruitment area of Eastern Finland. The Kuopio NPH registry comprises all presumed iNPH patients from the KUH patient population and contains clinical baseline and follow-up data, diagnoses from other hospitals, medications and causes of death from national registries, and neuropathological findings of these patients [24, 25]. The Finnish cohort included the Finnish NC group and patients with probable iNPH. The Finnish NC group consisted of 30 patients (median age 71 years; 13 men) who were scheduled for knee or hip replacement surgeries at the KUH. The Finnish iNPH group consisted of 30 consecutive patients (median age 73 years;19 men) who consented to a lumbar puncture with CSF removal. The Finnish iNPH group had undergone ventriculoperitoneal shunt with a right frontal cortical biopsy [24, 26] at KUH between December 2013 and February 2015. Patients in the Finnish cohort who underwent biopsy at the time of CSF shunting were classified into two groups based on the presence or absence of amyloid and/or tau deposition in the frontal lobe cortex following histological examination (Table S1) [27-30].
Expression analysis using autopsied brains was performed during the second part of the study. Immunohistochemical staining was performed to identify the expression levels of PTPRQ in the frontal lobe cortex, cingulate gyrus at the level of mamillary bodies, hippocampus and thalamus, including the paraventricular system of the autopsied brains of patients with iNPH (10 patients: Japanese-iNPH [n = 3], Finnish iNPH [n = 7]) and NC subjects (n = 6) without significant AD pathology [28-30] (Table S2). All patients were clinically followed up until death, and the development of symptoms was documented by neurologists, neurosurgeons, and general practitioners.
Patients were diagnosed with dementia according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, criteria [31]. Cognitive status was evaluated at the time of surgery and retrospectively at the time of death. Cognition was classified as normal, mild cognitive impairment, mild dementia, moderate dementia, or severe dementia (bedridden).
All clinical data from the hospitals in Juntendo University Hospital or the KUH catchment areas were collected.
Collection of cerebrospinal fluid
Cerebrospinal fluid was collected via lumbar puncture with the patient in the recumbent position (sitting position for the Finnish iNPH group). All CSF samples were centrifuged to remove cells and debris, aliquoted, and immediately stored in polypropylene tubes at −80°C until biochemical analysis.
Enzyme-linked immunosorbent assay
We measured the concentration of PTPRQ in the CSF of patients with probable iNPH and in NCs using an enzyme-linked immunosorbent assay (ELISA; Cloud-Clone Corp., Houston, TX, USA) according to the manufacturer’s protocol.
Additionally, we used Innotest assays for the quantitative determination of other CSF biomarkers, including p-tau (NIPRO, Osaka, Japan or Fujirebio, Ghent, Belgium), t-tau (NIPRO or Fujirebio), and Aβ42 (Fujirebio), which are markers of AD pathology. We also analysed the sensitivity and specificity of PTPRQ for the auxiliary diagnosis of probable iNPH.
Immunohistochemistry and histological evaluation
Post-mortem brain tissues were fixed in 10% neutral formalin for at least 1 week and then cut into 1-cm-thick coronal slices. The brain specimens were taken from 16 standard regions [25], embedded in paraffin, and cut into 6-µm sections. The sections were dewaxed using xylene, rehydrated in a graded series of alcohol to water, and subjected to antigen retrieval using Dako Target Retrieval Solution, Citrate pH 6 (×10; Agilent Technologies, Inc., Santa Clara, CA, USA) in an autoclave at 121°C for 10 min. Endogenous peroxidase was blocked by incubation of the brain sections with 0.3% hydrogen peroxide for 30 min. The sections were blocked with 5 × SEA BLOCK Blocking Buffer (Thermo Fisher Scientific Inc., Waltham, MA, USA) and 1% donkey serum phosphate-buffered saline (PBS) at 25°C for 30 min to prevent non-specific antibody binding.
The sections were incubated with rabbit polyclonal IgG anti-PTPRQ (immunized with amino acids 2208–2299; 1:50 dilution; Cloud-Clone Corp.) overnight at 4°C. The following day, the sections were incubated with EnVision System Labelled Polymer (DAKO, Glostrup, Denmark), which was used as the secondary antibody, for 30 min at room temperature. Following each treatment, the slides were washed with PBS (three times for 5 min). Finally, the sections were stained with 3,3′-diaminobenzidine (DAB) and counterstained with Mayer’s haematoxylin, dehydrated, cleared and mounted.
Protein tyrosine phosphatase receptor type Q staining was viewed under an E800 microscope (Nikon, Tokyo, Japan), and images were captured with an AxioCam HRc CCD camera using AxioVison Rel 4.7.2.0 imaging processing software (Carl Zeiss, Microimaging GmbH, Jena, Germany). Whole-slide images of Aβ42, tau, and haematoxylin and eosin stains were captured with a NanoZoomer Digital slide scanner (Hamamatsu Photonics K.K., Hamamatsu City, Japan) [32, 33]. An experienced pathologist evaluated all the slides without knowledge of the patient’s outcome.
Immunofluorescence staining
The sections were blocked using a method similar to that used for single immunohistochemical staining, and stained with rabbit polyclonal IgG anti-PTPRQ (1:50) overnight at 4°C. The following day, the sections were incubated with secondary antibodies, including goat anti-rabbit IgG (H + L) Alexa Fluor™Plus488 (A32731; diluted 1:100, Thermo Fisher Scientific Inc.), for 60 min at room temperature. Next, the nuclei in the sections were stained with Hoechst 33342, trihydrochloride, trihydrate (H3570; Thermo Fisher Scientific Inc.) for 2 min at room temperature, and a glass coverslip was mounted onto each section using VECTASHIELD Mounting Medium (H-1000; Vector Laboratories Inc., Burlingame, CA, USA). Finally, the sections were examined under a confocal scanning microscope (Leica TCS-SP5) using a Leica Application SuiteX 3.4.2.18368 (Leica Microsystems, GmbH, Wetzlar, Germany).
In situ hybridization
In situ hybridization was performed on formalin-fixed, paraffin-embedded sections of periventricular samples in the iNPH and NC groups using the GeneticLab QuantiGene ViewRNA in situ hybridization tissue evaluation kit (Thermo Fisher Scientific Inc.). After deparaffinization, the sections were boiled in pretreatment solution for 10 min, digested with protease for 20 min, and then hybridized with designed probes against PTPRQ (VA1-3017466). Fast Red substrates were used to produce signals. The sections were examined under an E800 microscope (Nikon), and images were captured with an AxioCam HRc CCD camera using AxioVison Rel 4.7.2.0 imaging processing software (Carl Zeiss, Microimaging GmbH).
Data analyses and statistics
Descriptive statistics were used to summarize the data. The Shapiro–Wilk test and QQ plots were used to assess the normality of distributions. In each comparison, we used Tamhane’s T2 multiple comparison test after a one-way analysis of variance for continuous variables and Kruskal–Wallis’s H test for categorical variables. The Mann–Whitney U-test with Bonferroni correction was used to compare among the different groups. Wilcoxon signed-rank test was used for within-group comparisons of MMSE and iNPHGS scores before and 3 months after CSF shunting. Pearson correlation coefficient was estimated for finding linear association between two continuous variables. A chi-squared test was conducted to compare proportions.
The receiver-operating characteristic (ROC) curve was calculated to evaluate the goodness-of-fit for predictors of probable iNPH versus healthy neurological state. Statistical analyses were performed using IBM Statistical Package for the Social Sciences Version 25.0 (SPSS, Cary, NC, USA) for Windows. For all analyses, P < 0.05 was considered statistically significant.
Results
Differentiation of iNPH from other neurodegenerative diseases
We confirmed that the preoperative CSF concentrations of p-tau, t-tau, and Aβ42 did not differ significantly between the Japanese iNPH and the NC groups (Table 1). Conversely, CSF PTPRQ concentrations were significantly higher in the Japanese and Finnish iNPH groups (mean [SD] 619 [398] pg/ml and 762 [570] pg/ml, respectively) compared to the respective NC groups (296 [94] pg/ml, **P = 0.001; and 371 [94] pg/ml, ***P < 0.001, respectively) and in the PD group (338 [230] pg/ml, *P = 0.041). However, the Japanese iNPH and AD groups showed no significant difference in CSF PTPRQ concentrations (619 [398] pg/ml vs. 365 [225] pg/ml; P = 0.075 [Table 1, Fig. 2]).
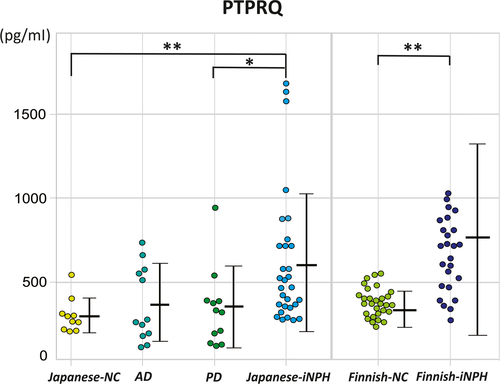
Using a cut-off PTPRQ concentration of 313 pg/ml, iNPH was detected with a sensitivity of 83.3%, specificity of 80%, and an area under the ROC curve (AUC) of 0.860 in the Japanese iNPH group. In the validation analysis, using a cut-off PTPRQ concentration of 555 pg/ml, iNPH was detected with a sensitivity of 63.3%, specificity of 100%, and an AUC of 0.849 in the Finnish iNPH group (Fig. S1).
Difference between iNPH with and without Alzheimer’s disease comorbidity
Analysis of the pathological tissues obtained by cortical biopsy at the time of CSF shunting showed that there were significant differences in age (*P = 0.01), MMSE scores at biopsy (*P = 0.012), MMSE scores after shunt (**P = 0.003), iNPHGS scores at biopsy (*P = 0.04), iNPHGS scores after shunt (*P = 0.018), concentrations of Aβ42 (**P = 0.004) and p-tau (*P = 0.048) between the group with amyloid deposits and/or tau deposits (n = 16) and the group without deposits (n = 14). However, the concentration of PTPRQ and t-tau did not differ significantly between the two groups (P = 0.257 and P = 0.525, respectively; Table S1).
Difference of PTPRQ between shunt responders and non-responders
We confirmed that there were no statistically significant differences between the Japanese and Finnish iNPH groups. The Japanese and Finnish iNPH groups were thus combined to create a single probable iNPH group, and no significant differences were found in PTPRQ concentrations between the shunt responder group (n = 46) and shunt non-responder group (n = 14; P = 0.432 [Table S3]).
Differences between iNPH and non-idiopathic NPH
The patients with non-idiopathic NPH (acquired + congenital/developmental) had significantly dilated ventricles compared to those with iNPH (*P = 0.011; Table 2). CSF PTPRQ concentrations were significantly higher in non-idiopathic NPH (mean [SD] 1704 [861] pg/ml) compared to the iNPH group (***P < 0.001; Fig. 3). There was a positive correlation between the Evans Index and CSF PTPRQ concentrations in NPH (correlation coefficient = 0.384, *P = 0.013).

Validation of PTPRQ expression in the brain tissues
Immunohistochemistry was performed to examine the localization of PTPRQ in the brain. We performed an absorption test to identify the specificity of PTPRQ immunostaining, and the results demonstrated that the addition of the antigen peptide to the primary antibody solution completely abolished PTPRQ immunostaining. The choroid plexus (Fig. 4a,b) and ependymal cells in patients with iNPH (Fig. 4c–e) were positively stained with the anti-PTPRQ antibody, as was the surface area of the ventricle that was directly in contact with the CSF in both iNPH and NC subjects.
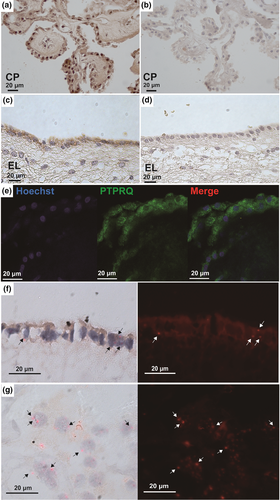
Immunostaining and in situ hybridization also revealed that PTPRQ was expressed in the ependymal cells (Fig. 4f) and choroid plexus (Fig. 4g) of iNPH subjects.
Discussion
The phosphorylation/dephosphorylation of phosphotyrosine in proteins, and of the inositol phosphate moiety of phosphatidylinositol phospholipids, are important mechanisms through which cells regulate proliferation, survival, migration and secretion. In particular, PTPRQ is upregulated in a model of renal injury [34]. In this study, we confirmed that CSF PTPRQ levels in patients with probable iNPH were significantly increased compared to NCs and other neurodegenerative diseases. Further, the PTPRQ concentration in non-idiopathic NPH, which generally showed more extensive ventricular enlargement than iNPH, was higher than in iNPH. In addition, the non-iNPH patients had significantly dilated ventricles compared to those with iNPH.
This was the first study to demonstrate the expression of PTPRQ in the human brains of patients with iNPH. We showed that the expression of PTPRQ in pathological tissues was confined to the ependymal cells and choroid plexus, which are directly in contact with the CSF. PTPRQ includes an extracellular domain that contains 18 fibronectin III repeats, a membrane-spanning domain, and a cytoplasmic domain with phosphatidylinositol phosphatase activity [34, 35]. PTPRQ is a membrane protein localized to the apical surface and lower region of the hair bundle of inner ear hair cells, and the extracellular domain of PTPRQ has been reported to be involved in regulating the function of the hair cell bundle [36]. Therefore, we hypothesize that ependymal cells and choroidal epidermal cells are involved in actin polymerization and ciliary movement, and these ciliary structures have an important role in CSF dynamics [37, 38].
The concentrations of other CSF biomarkers that have been used for the diagnosis of iNPH, such as Aβ42 and tau, were lower in patients with iNPH than in healthy subjects. The reduced levels of Aβ42 observed in iNPH cases may be attributable to a general reduction in the production of amyloid precursor protein-derived proteins, and this may be attributable to reduced brain metabolism in the periventricular zone as seen in positron-emission tomography and MRI studies [39]. Additionally, the observed reduction in Aβ peptide and tau protein levels may be explained by a reduced clearance from the extracellular fluid due to a reduced centripetal flow of extracellular fluid caused by the reversed CSF dynamics in iNPH. In addition, studies have reported that reduced CSF Aβ42 in iNPH is associated with Aβ pathology in the brain [40, 41]. In the present study, a high concentration of PTPRQ was observed in the CSF of patients with probable iNPH and non-idiopathic NPH. It is highly possible that the elevated PTPRQ levels in CSF are related to ependymal dysfunction from ventricular expansion. This finding may be useful for the diagnosis of NPH. However, the underlying cause of increased PTPRQ expression in NPH requires further research.
This study had several limitations. First, selection bias may have affected the results of this two-cohort retrospective study. In addition, the sample size was small, and this may have increased the rate of type II errors. Additionally, the evaluation period after the CSF shunt was only 3 months. In previous studies, the CSF concentrations of p-tau were higher and the CSF concentrations of Aβ42 were lower in the shunt non-responder than in the shunt responder group [42, 43]. Further, these differences were observed for more than 2 years. However, even if AD pathology coexisted with iNPH, we observed an improvement in symptoms at 3 months after performing the shunt [9]. Finally, the low specificity in the statistical analysis PTPRQ levels does not allow its use as a strong differential diagnostic criterion.
Nagata et al. [16] reported that PTPRQ concentrations were lower in AD patients than in iNPH patients; however, as revealed by the brain biopsy results of patients with iNPH in the present study, amyloid and tau were discovered in the frontal lobe of patients, and there were no statistically significant differences in PTPRQ concentrations between probable iNPH patients with and without AD pathology. Furthermore, there were no significant differences between the iNPH and AD groups in the first cohort. Further studies with larger samples are required to evaluate the relationship between PTPRQ concentrations and CSF shunting outcomes.
Overall, our findings demonstrated that PTPRQ levels were significantly higher in patients with probable iNPH than in NCs, regardless of the population of origin. Therefore, measurements of PTPRQ may be used for the auxiliary diagnosis of NPH.
Acknowledgements
The authors acknowledge: Marita Parviainen, RN; Ulla Mönkkönen, RN; Sisko Juutinen; and the Laboratory of Morphology and Image Analysis, the Research Support Centre. The Graduate School Research Programme of Juntendo University and the Juntendo University Research Institute for Diseases of Old Age (Tokyo, Japan) supported the present study. This work was supported in part by the Private University Research Branding Project by the Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health, Labour and Welfare of Japan [2017-Nanchi-General-037] and Grants-in-Aid for Scientific Research [grant numbers 16KK0187, 17K10908, 18H02916, 20K09398] from the Japan Society for the Promotion of Science. This work was also supported by the Academy of Finland [grant number 307866]; EVO/VTR [grant number 5053149] of Kuopio University Hospital, Sigrid Juselius Foundation; the Strategic Funding of the University of Eastern Finland (UEF-Brain), FP7, Grant Agreement [grant number 601055]; and VPH Dementia Research Enabled by IT VPH-DARE@IT and BIOMARKAPD project in the JPND Programme.
Disclosure of conflicts of interest
The authors declare that they have no financial or other conflicts of interest.
Open Research
Data availability statement
All data not published within the article are available, and will be shared in anonymized form upon request from any qualified investigator.




