Polypharmacy, functional outcome and treatment effect of intravenous alteplase for acute ischaemic stroke
Abstract
Background and purpose
Polypharmacy is an important challenge in clinical practice. Our aim was to determine the effect of polypharmacy on functional outcome and treatment effect of alteplase in acute ischaemic stroke.
Methods
This was a post hoc analysis of the randomized, placebo-controlled WAKE-UP trial of magnetic resonance imaging guided intravenous alteplase in unknown onset stroke. Polypharmacy was defined as an intake of five or more medications at baseline. Comorbidities were assessed by the Charlson Comorbidity Index (CCI). The primary efficacy variable was favourable outcome defined by a score of 0–1 on the modified Rankin Scale at 90 days. Logistic regression analysis was used to test for an association of polypharmacy with functional outcome, and for interaction of polypharmacy and the effect of thrombolysis.
Results
Polypharmacy was present in 133/503 (26%) patients. Patients with polypharmacy were older (mean age 70 vs. 64 years; p < 0.0001) and had a higher score on the National Institutes of Health Stroke Scale at baseline (median 7 vs. 5; p = 0.0007). A comorbidity load defined by a CCI score ≥ 2 was more frequent in patients with polypharmacy (48% vs. 8%; p < 0.001). Polypharmacy was associated with lower odds of favourable outcome (adjusted odds ratio 0.50, 95% confidence interval 0.30–0.85; p = 0.0099), whilst the CCI score was not. Treatment with alteplase was associated with higher odds of favourable outcome in both groups, with no heterogeneity of treatment effect (test for interaction of treatment and polypharmacy, p = 0.29).
Conclusion
In stroke patients, polypharmacy is associated with worse functional outcome after intravenous thrombolysis independent of comorbidities. However, polypharmacy does not interact with the beneficial effect of alteplase.
Introduction
Polypharmacy, commonly defined as an intake of five or more drugs [1], is considered an important and growing challenge in clinical practice. Whilst there is no strong evidence to support the use of any particular threshold, the risk of drug-related problems seems to increase with each additional medication prescribed. The most important factors driving polypharmacy are age and multimorbidity [2, 3]. Polypharmacy has been associated with a significant burden in frequency of hospitalization, length of hospital stay, in-hospital deaths, disability and adverse drug reactions [3]. In addition, previous studies revealed that polypharmacy is associated with higher rates of complications and mortality in elderly patients [4-7].
Stroke remains one of the leading causes of death and disability worldwide [8], and the risk of stroke is increased in the elderly and in patients with multiple comorbidities. It is well known that ischaemic stroke is associated with cardiovascular risk factors, such as arterial hypertension, diabetes, hypercholesterolaemia and atrial fibrillation (AF), which, if treated according to current guidelines, commonly lead to polypharmacy [9-11]. However, data on the association between polypharmacy and stroke outcome as well as acute stroke treatment are limited.
In the present study, our first objective was to study whether polypharmacy is associated with functional outcome in patients with acute ischaemic stroke. Secondly, the aim was to study a possible effect of polypharmacy on the benefit of treatment with intravenous alteplase. These objectives were investigated in a post hoc analysis of the WAKE-UP study.
Methods and materials
Study design
In this exploratory secondary post hoc analysis, the medical history of all patients randomized in WAKE-UP was reviewed to evaluate polypharmacy and pre-existing comorbidities. WAKE-UP was a multicentre, randomized, double-blind, placebo-controlled clinical trial to study the efficacy and safety of intravenous thrombolysis with alteplase in patients with an acute stroke of unknown onset time, guided by magnetic resonance imaging. Inclusion criteria comprised the mismatch between an acute ischaemic lesion visible on diffusion-weighted imaging but with no corresponding marked parenchymal hyperintensity on fluid-attenuated inversion recovery as a surrogate marker of lesion age, indicating that the stroke onset most probably lies within 4.5 h [12].
In this analysis, demographic characteristics, medical history, clinical and imaging data at baseline and follow-up, including final follow-up at 90 days after stroke, were examined.
Polypharmacy was defined as the intake of five or more medications at baseline according to the most common definition [1]. Pre-existing comorbid conditions were assessed and summarized in the Charlson Comorbidity Index (CCI) [13]. The CCI is an extensively validated index and includes 19 diseases, which are weighted according to their association with mortality. A modified version of the CCI score, which has shown its validity for use in stroke outcome studies, was used [14, 15]. In this adjusted version, diagnosis and symptoms of the acute ischaemic stroke are disregarded, and age also does not count for the score.
Outcome measures and end-points
Clinical outcome was assessed at 90 days after stroke. Evaluation of efficacy outcomes followed the clinical end-points as defined in the WAKE-UP trial. The primary end-point was favourable outcome defined as a score of 0–1 on the modified Rankin Scale (mRS). The secondary end-point in this analysis comprised the ordinal analysis of the mRS ('shift analysis'). Safety outcomes were mortality and the incidence of symptomatic intracranial haemorrhage according to the protocol of SITS-MOST on follow-up imaging 22–36 h after treatment.
Statistical analysis
Baseline characteristics were compared between patients with and without polypharmacy. Statistical analyses of treatment effects were performed in the intention-to-treat population for all patients with available information for clinical end-points. Primary and secondary efficacy outcome were assessed using an unconditional logistic regression analysis including polypharmacy and the CCI score as a continuous parameter, fitted to estimate the odds ratio (OR) and its 95% confidence interval (CI). The categorical shift in the distribution of mRS scores was analysed by fitting a proportional-odds logistic regression model.
The CCI score comprises a wide range of comorbidities but does not include important cardiovascular diseases such as arterial hypertension, AF and hypercholesterolaemia, which are frequent in stroke patients and may also be associated with stroke outcome. To account for this, and to test for the association of individual comorbidities with favourable outcome, another model was calculated including polypharmacy, the National Institutes of Health Stroke Scale (NIHSS) at baseline, age, treatment and the individual comorbidities summarized in the CCI amended by arterial hypertension, AF and hypercholesterolaemia. Comorbidities which were present in fewer than 10 patients were excluded. To identify significant predictors of favourable outcome amongst this large list of variables, a stepwise backwards multivariable logistic regression analysis based on the Akaike information criterion (AIC) was performed.
To investigate the interaction between polypharmacy and treatment effect on the primary end-point, an unconditional logistic regression model relating the log-odds of the primary outcome with the covariate of interest, the treatment group and their interaction was used. The interaction term was tested with the Wald chi-squared test, and the treatment effect (OR) and its 95% CI was estimated for each category of the categorical variable. All analyses were adjusted for the stratification parameters age and NIHSS. All tests were carried out with a two-sided alpha level of 5% without correction for multiple comparisons.
Standard protocol approvals, registrations and patient consents
Patients or their legal representatives provided written informed consent according to national and local regulations. There was an exception from explicit informed consent in emergency circumstances in some countries. For each study site, the competent authorities and the corresponding ethics committee approved the trial. The detailed trial protocol has been published together with its main results [12]. The trial was registered at ClinicalTrials.gov (NCT01525290) and EudraCT (2011-005906-32).
Data availability statement
The full trial protocol and the statistical analysis plan of WAKE-UP have already been published along with the main trial publication [12]. Individual patients’ data, after de-identification, will be shared with the Virtual International Stroke Trials Archive (VISTA) and be accessible for researchers who provide a methodologically sound proposal according to the VISTA rules (http://www.virtualtrialsarchives.org/vista/).
Results
Patient characteristics
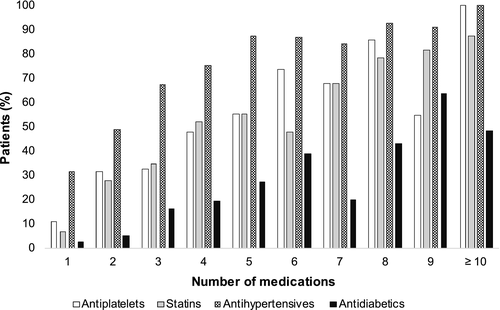
Of 503 patients randomized in WAKE-UP, polypharmacy was present in 133 patients (26%, Figure 1). A continuous increase of patients taking typical drugs for cardiovascular prevention (i.e., antiplatelets, statins, antihypertensives and antidiabetics) was observed with increasing number of drugs (Figure 2). In the polypharmacy group, more than 50% of patients were on a combination of antiplatelets, statins and antihypertensives.
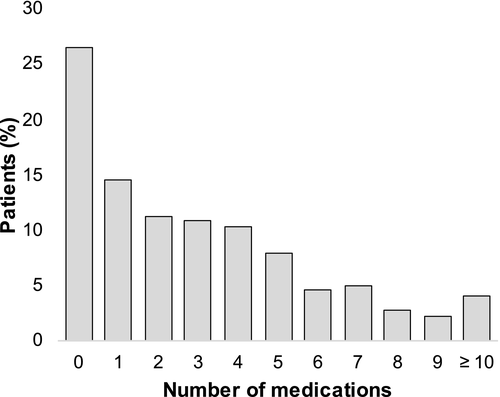
Clinical characteristics in patients with and without polypharmacy are shown in Table 1. Patients with polypharmacy were older, with a mean age (SD) of 70 (8) years compared with 64 (12) years in patients without polypharmacy (p < 0.0001). Those with polypharmacy were more severely affected, with a higher median NIHSS score on admission of 7 points compared to 5 points in those without polypharmacy (p = 0.0007). Overall, patients with polypharmacy had more comorbidities, with 64 patients (48%) with a CCI score ≥ 2 in the polypharmacy group, compared to 30 patients (8%) in the no polypharmacy group (p < 0.0001). Regarding cardiovascular risk factors, arterial hypertension (115 [86%] vs. 151 [41%] patients; p < 0.0001), diabetes mellitus (55 [41%] vs. 27 [7%] patients; p < 0.0001), hypercholesterolaemia (82 [62%] vs. 96 [26%] patients; p < 0.0001) and AF (29 [22%] vs. 30 [8%] patients; p = 0.0002) were more prevalent in patients with polypharmacy. Diffusion-weighted imaging lesion volume at baseline, frequency of vessel occlusion, as well as time from recognition of symptoms to treatment initiation did not differ between the groups.
| Variable | Patients, no. (%) | p value | |
|---|---|---|---|
| Polypharmacy (n = 133) | No polypharmacy (n = 370) | ||
| Age, mean (SD), years | 70 (8) | 64 (12) | <0.0001 |
| Women | 57 (43) | 121 (33) | 0.04 |
| Medical history or risk factors | |||
| Charlson Comorbidity Index score ≥ 2 | 64 (48) | 30 (8) | <0.0001 |
| Arterial hypertension | 115 (86) | 151 (41) | <0.0001 |
| Diabetes mellitus | 55 (41) | 27 (7) | <0.0001 |
| Hypercholesterolaemia | 82 (62) | 96 (26) | <0.0001 |
| Atrial fibrillation | 29 (22) | 30 (8) | 0.0002 |
| History of ischaemic stroke | 35 (26) | 33 (9) | <0.0001 |
| Medication classes | |||
| Antiplatelets | 94 (71) | 69 (19) | <0.0001 |
| Statins | 86 (65) | 67 (18) | <0.0001 |
| Antihypertensives | 119 (89) | 127 (34) | <0.0001 |
| Antidiabetics | 47 (35) | 24 (6) | <0.0001 |
| National Institute of Health Stroke Scale score, median (IQR) | 7 (4–12) | 5 (3–8) | 0.0007 |
| Diffusion-weighted imaging lesion volume at baseline, median (IQR), ml | 2.6 (0.9–9) | 2.2 (0.7–8.2) | 0.51 |
| Vessel occlusion on time-of-flight magnetic resonance angiography | |||
| Large vessel occlusion | 28 (21) | 70 (19) | 0.61 |
| Any vessel occlusion | 52 (40) | 135 (37) | 0.6 |
| Time from symptom recognition to treatment initiation, median (IQR), min | 200 (159–237) | 185 (148–230) | 0.13 |
| Treatment with alteplase | 63 (47) | 191 (52) | 0.42 |
- Abbreviation: IQR, interquartile range.
Influence of polypharmacy on stroke outcome
Information on the primary end-point was available for 490 patients (130 with polypharmacy and 360 without polypharmacy). Amongst all randomized patients, polypharmacy was independently associated with lower odds of favourable outcome, whilst pre-existing comorbidities, indicated by the sum score of the CCI, were not (Table 2). Favourable outcome was observed in 43 of 130 patients (33%) in the polypharmacy group and in 190 of 360 patients (53%) in the no polypharmacy group (absolute difference 20%; adjusted OR 0.50, 95% CI 0.30–0.85; p = 0.0099).
| Assessment variable at 90 days | Adjusted odds ratioa (95% CI) | p value |
|---|---|---|
| Primary end-point (modified Rankin Scale score 0–1) | ||
| Polypharmacy | 0.50 (0.30–0.85) | 0.0099 |
| Charlson Comorbidity Index score | 1.04 (0.85–1.26) | 0.74 |
| Treatment with alteplase | 1.60 (1.09–2.36) | 0.018 |
| Secondary end-point ('shift analysis') | ||
| Polypharmacy | 0.68 (0.45–1.03) | 0.070 |
| Charlson Comorbidity Index score | 0.94 (0.81–1.10) | 0.47 |
| Treatment with alteplase | 1.60 (1.16–2.21) | 0.004 |
- Abbreviation: CI, confidence interval.
- a The odds ratio was adjusted for the stratification factors age and symptom severity assessed by the National Institutes of Health Stroke Scale (NIHSS).
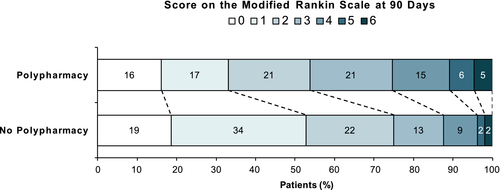
In the ordinal analysis of the mRS at 90 days, polypharmacy was associated with a shift towards worse functional outcome (adjusted common OR 0.68, 95% CI 0.45–1.03; p = 0.070; Figure 3). The CCI score again was not significantly associated with functional outcome (p = 0.47).
In backwards multivariable logistics regression analysis including polypharmacy, CCI sub-items and additional cardiovascular comorbidities, the most parsimonious model showed that polypharmacy (adjusted OR 0.56, 95% CI 0.35–0.88; p = 0.0013) and NIHSS score (adjusted OR 0.12, 95% CI 0.06–0.21; p < 0.0001) were associated with lower odds of favourable outcome, whilst treatment with alteplase showed a positive association with favourable outcome (adjusted OR 1.60, 95% CI 1.08–2.36; p = 0.023). Sub-items of the CCI, arterial hypertension, AF and hypercholesterolaemia were not associated with functional outcome.
Interaction of polypharmacy and treatment effect
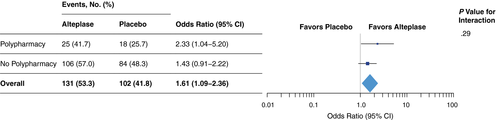
Amongst all randomized patients, treatment with alteplase was associated with higher odds of favourable outcome with no heterogeneity of treatment effect for subgroups defined by polypharmacy (test for interaction, p = 0.29; Figure 4). The adjusted OR for favourable outcome with alteplase was 2.33 (95% CI 1.04–5.20) in patients with polypharmacy and 1.43 (95% CI 0.91–2.22) in patients without polypharmacy.
Mortality and safety outcomes in patients with polypharmacy
At 90 days, six patients in the polypharmacy group had died (4.5%), whilst seven patients (1.9%) had died in the no polypharmacy group. There were two (1.5%) cases of symptomatic intracranial haemorrhage meeting the criteria of SITS-MOST in the polypharmacy group and four (1.1%) cases in the no polypharmacy group.
Discussion
In this secondary post hoc analysis of the WAKE-UP trial, the effect of polypharmacy on outcome and efficacy of reperfusion treatment in acute ischaemic stroke was studied, motivated by the growing prevalence of polypharmacy and missing recommendations for action in clinical practice. Our study yielded two major findings. First, it was observed that polypharmacy, independently of pre-existing comorbidities, was associated with worse functional outcome after ischaemic stroke. Secondly, there was no heterogeneity of treatment effect with regard to the presence of polypharmacy. Thus, our data suggest that treatment with alteplase is of similar benefit in patients with polypharmacy as in those without polypharmacy.
Polypharmacy is a growing challenge in clinical practice, with 10% of the population and 30% of older adults in the USA taking five or more drugs simultaneously [16]. A similarly high prevalence is reported in other countries (e.g., the UK [17], Germany [18] and China [19]). Due to ageing of the population, the importance of polypharmacy on a societal level is likely to increase over the next years. With 26%, the prevalence of polypharmacy amongst the patients randomized in WAKE-UP was high, although trial design criteria led to exclusion of very elderly patients (>80 years of age) and those with pre-stroke disability (mRS > 1). Given that both polypharmacy and stroke are associated with higher age and comorbidities, it is assumed that polypharmacy is even more common in the general stroke population.
Previous studies have identified polypharmacy as an indicator of mortality in elderly patients [5, 6]. In our study, patients with polypharmacy were older and had more comorbidities, including cardiovascular risk factors and diseases, but our analysis did not show an influence of polypharmacy on mortality. This may be explained by the fact that the cohort size was small, the patients’ age was relatively low and premorbid disability was excluded, compared to unselected stroke populations, and finally overall mortality in our trial was low (<3%). Additionally, it has to be considered that in recent studies mortality was assessed over a longer period of time and not restricted to 3-months follow-up of a certain event.
In the context of polypharmacy and stroke, there are few studies investigating the influence of polypharmacy on stroke occurrence. In patients with AF, which in turn represent a population at risk for stroke, polypharmacy was associated with a higher risk for cardiovascular death independently of age and comorbidities. However, a significant association between polypharmacy and stroke was not observed [20]. A retrospective study showed that an increased number of drugs during hospitalization was negatively associated with both functional recovery and the possibility of home discharge amongst geriatric stroke patients [21]. Based on these findings, it is reasonable to assume that polypharmacy adversely affects stroke outcome. Our study enabled the first systematic investigation of the effect of polypharmacy on outcome and intravenous thrombolysis amongst patients with ischaemic stroke in a randomized clinical trial. Indeed, our analysis revealed that polypharmacy, independently of pre-existing comorbidities, was associated with worse functional outcome after ischaemic stroke compared to no polypharmacy, with an absolute 20% fewer patients achieving a favourable outcome at 3 months after stroke.
Of note, this effect was not driven by the higher burden of comorbidities in patients with polypharmacy, as might have been conceivable. Moreover, in multivariable analysis, neither individual CCI items nor other important cardiovascular comorbidities such as arterial hypertension, AF and hypercholesterolaemia showed a significant association with functional outcome, whilst polypharmacy did consistently across all models. It has to be considered that this finding may be attributable to a selection effect in our study based on data from a randomized controlled clinical trial with strict inclusion and exclusion criteria. These comprise amongst others the exclusion of patients with malignant diseases and other severe comorbidities, as well as an upper age limit. As a result, the proportion of patients with severe comorbidities indicated by a CCI score ≥ 2 in our population was rather small (19%). Previous studies of unselected cohorts of stroke patients reported higher rates of severe comorbidities ranging from 32% to 43% [14, 15]. It cannot be ruled out that in other stroke cohorts comorbidities may show a greater influence on outcome. Nevertheless, our findings suggest that specific effects of polypharmacy might drive worse outcome in stroke patients taking five or more medications. A possible mechanism that might add to the deleterious effect of polypharmacy is drug–drug interactions which were reported to be frequent in acute stroke patients [22]. Especially serious drug–drug interactions are likely to occur more frequently with polypharmacy.
Although the WAKE-UP trial was not powered to show a significant treatment effect in subgroups of patients, it was found that alteplase was associated with favourable outcome in both the polypharmacy as well as the no polypharmacy group. There was no significant heterogeneity of treatment effect between the subgroups. This finding may be surprising, as it could have been assumed that drug–drug interactions also adversely influence the effect of intravenous thrombolysis. Obviously, this is not the case, and the result is reassuring as it demonstrates that intravenous thrombolysis is both effective and safe even in patients on multiple drugs. Thus, polypharmacy should not affect the decision for thrombolysis.
There are limitations to our study. Due to the retrospective observational design of the analysis of polypharmacy effects, a causal relationship of polypharmacy and functional outcome cannot be established. In addition, as study inclusion and exclusion criteria entailed a selected and relatively young sample of stroke patients, the generalizability of our results is limited. Finally, our analysis was not powered to study specific effects of individual drugs or medication classes in detail.
The results of our study provide novel information on the impact of polypharmacy on stroke outcome. There are still only scarce data on polypharmacy in stroke, and further research is needed. Polypharmacy is a complex phenomenon, which, besides potential negative effects such as the increased risk of side effects and drug–drug interactions, may also reflect a high quality of medical care and adherence to guideline recommendations.
Conclusions
Polypharmacy is highly prevalent in stroke patients and associated with worse functional outcome after acute ischaemic stroke. Polypharmacy does not influence the beneficial effect of alteplase, and therefore polypharmacy should not affect the decision for intravenous thrombolysis. Further research is required to determine whether strategies for reducing polypharmacy may improve the outcome of stroke patients.
Acknowledgements
Funding: WAKE-UP received funding from the European Union Seventh Framework Program (FP7/2007-2013) under grant agreement no. 278276 (WAKE-UP). The European Union Seventh Framework Program (FP7/2007-2013) had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication. Open access funding enabled and organized by Projekt DEAL.
Disclosure of conflicts of interest
FB reports grants from University Medical Center Hamburg-Eppendorf during the conduct of the study. BC reports grants from University Medical Center Hamburg-Eppendorf during the conduct of the study. MEb reports grants from University Medical Center Hamburg-Eppendorf during the conduct of the study. MEn reports grant support from Bayer, the German Research Foundation (DFG), the German Federal Ministry of Education and Research (BMBF), the German Center for Neurodegenerative Diseases (DZNE), the German Center for Cardiovascular Research (DZHK), the European Union, Corona Foundation and Fondation Leducq; and fees paid to the Charité from Boehringer Ingelheim, Bristol-Myers Squibb, BMS/Pfizer, Daiichi Sankyo, Amgen, GlaxoSmithKline (GSK), Sanofi, Covidien, Novartis, all outside the submitted work. JBF reports grants from European Union 7th Framework Program during the conduct of the study and personal fees from Bioclinica, Artemida, Cerevast, Brainomix, BMS, Merck, Eisai, Biogen, Guerbet and Nicolab outside the submitted work. JF reports grants and personal fees from Acandis, personal fees from Cerenovus, grants and personal fees from Medtronic, grants and personal fees from Microvention, personal fees from Penumbra, personal fees from Route92, outside the submitted work. IG reports grants from European Union 7th Framework Program during the conduct of the study. AK reports grants from European Union 7th Framework Program during the conduct of the study. VT has received personal fees as consultant or lecturer from Boehringer-Ingelheim, outside the submitted work. RL reports institutional fees from Bayer, Boehringer Ingelheim, Genentech, Ischemaview, Medtronic and Occlutech outside the submitted work. KWM has participated in advisory boards for Boehringer Ingelheim; Boehringer Ingelheim supplies medication for the ATTEST-2 clinical trial, funded by the Stroke Association and British Heart Foundation, for which he is Chief Investigator. SP reports grants from European Union 7th Framework Program during the conduct of the study and personal fees from Lundbeck, outside the submitted work. CZS is supported by a research grant from Novo Nordisk, outside the submitted work. CG reports, outside the submitted work, funding from German Research Council (DFG), European Union, Federal Ministry of Education and Research (BMBF), German Statutory Pension Insurance Scheme (RV Nord), National Innovation Fond, Wegener Foundation, Hertie Foundation and Schilling Foundation; CG received, outside the submitted work, personal fees from Abbott, Amgen, Bayer Vital, Bristol-Myers-Squibb, Boehringer Ingelheim, Daiichi-Sankyo, Sanofi Aventis and Prediction Biosciences. GT reports personal fees as consultant or lecturer from Acandis, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, Portola, Stryker and research grants from Bayer, Federal Ministry for Economic Affairs and Energy (BMWi), Corona-Foundation, German Research Foundation (DFG), Else Kröner-Fresenius Foundation, European Union (Horizon 2020), German Innovation Fund, all outside the submitted work. All remaining authors declare no competing interests.




