Experimental evidence for a time-integrated effect of productivity on diversity
Abstract
The time–area–productivity hypothesis is a proposed explanation for global biodiversity gradients. It predicts that a bioregion's modern diversity is the product of its area and productivity, integrated over evolutionary time. I performed the first experimental test of the time–area–productivity hypothesis using a model system for adaptive radiation – the bacterium Pseudomonas fluorescens SBW25. I initiated hundreds of independent radiations under culture conditions spanning a variety of productivities, spatial extents and temporal extents. Time-integrated productivity was the single best predictor of extant phenotypic diversity and richness. In contrast, ‘snapshots’ of modern environmental variables at the time of sampling were less useful predictors of diversity patterns. These results were best explained by marked variation in population growth parameters under different productivity treatments and the long periods over which standing diversity could persist in unproductive habitats. These findings provide the first experimental support for time-integrated productivity as a putative driver of regional biodiversity patterns.
Introduction
The differential distribution of biodiversity across biogeographic regions is simultaneously striking in its apparency and perplexing in its origins (Dobzhansky 1950; Hutchinson 1959; Pianka 1966). A remarkable number of hypotheses have been proposed to explain why such regional differences in diversity exist – many of which place primacy on the strength and fluctuations of physical and biotic processes over evolutionary time (e.g. Fischer 1960). In particular, the time–area–productivity hypothesis presents a compelling mechanism for explaining global gradients in biodiversity (Mittelbach et al. 2007; Jetz & Fine 2012; Belmaker & Jetz 2015). This hypothesis stresses the simultaneous interacting roles of spatial extent and energy availability integrated over evolutionary time in driving regional diversification. Here, I follow Jetz & Fine's (2012) definition of bioregions as ‘evolutionary arenas’ – geographical regions sharing few species and generally encompassing a single or multiple similar climatic biomes. Time integration of a bioregion's area and/or productivity allows their historical signatures on diversity dynamics to influence contemporary patterns of species diversity. In other words, time integration implies that communities equilibrate to a changing environment relatively slowly compared to the timescale over which diversification occurs.
The number of species a bioregion can support is predicted to positively correlate with its spatial extent (Chown & Gaston 2000). This prediction fits with the observation that the larger tropical bioregions generally contain many more species than smaller, globally discontinuous temperate regions (Terborgh 1973; Rosenzweig 1995). Originally, the effects of area were attributed to larger bioregions supporting greater population sizes than smaller regions. All else being equal, these larger populations have a greater probability of peripatric speciation (Nuismer et al. 2012) and a decreased probability of extinction due to buffering from catastrophic disturbances (Rosenzweig 1995; Kisel et al. 2011). Larger bioregions are also anticipated to contain more boundaries to gene flow, which should result in the evolution of reproductive isolation (Kisel & Barraclough 2010). These hypotheses have been met with mixed results when observational biodiversity data are fit to models incorporating their bioregional extents (Willig et al. 2003), suggesting that these contemporary ‘snapshot’ measures of area may not be the dominant factors driving global diversity patterns, especially among assemblages containing high levels of endemism.
As with geographic extent, a bioregion's energy supply can set the upper limit on population sizes (Wright 1983; Hurlbert & Jetz 2010). Regions receiving more energy per unit area, such as those in the tropics, can support larger populations of individual species than their temperate counterparts, making them less vulnerable to extinctions and more prone to speciation (Preston 1962; Srivastava & Lawton 1998; Hurlbert 2004). Through its effects on organismal metabolic rates, productivity is predicted to set limits on the total number of diverging populations that an ecosystem can support (Allen et al. 2002). If the assumption of diversity-dependent speciation rates holds true (see Rabosky 2013), then this theory provides an avenue for a positive feedback loop between standing and incipient diversity mediated by energy input. Productive environments are also predicted to be more heterogeneous with regard to resource availability and thus may also permit diversification and coexistence via niche partitioning and resource specialisation (Abrams 1995). It is widely believed that the fitness advantages of many life history specialists should manifest only above a minimum resource base (Wilbur et al. 1974).
Until now, I have ignored the importance of time as both as a driver of diversity gradients and as a mediator of areal and productivity effects on diversification rates. All else being equal, older bioregions are anticipated to have accumulated more diversity than younger ones (Fischer 1960; Ricklefs & Schluter 1994). This hypothesis is supported by the observation that many extant clades originated in older, tropical bioregions (Mittelbach et al. 2007). Furthermore, because the tempo of diversification occurs on timescales encompassing major climatic and tectonic events, a bioregion's area and productivity are expected to fluctuate to varying degrees which should in turn influence the region's diversification dynamics (Fischer 1960; Terborgh 1973; Ricklefs & Schluter 1994). Opponents of contemporary area–productivity explanations often cite as a counterargument small and/or resource-poor habitat patches containing high biodiversity (McGlone 1996; Fine & Ree 2006). If these patches are relics of once-large and productive habitats, then their contemporary diversities may simply be due to past events promoting diversification and the subsequent long-term maintenance of that diversity in the face of environmental change. By scaling a bioregion's time-integrated area by its average productivity, Fine & Ree (2006) and Jetz & Fine (2012) were able to predict with remarkable accuracy the species richness of their ‘evolutionary arenas’ – a strong argument for the joint roles of historical area, productivity and temporal stability in promoting and maintaining diversity. Crucially, the authors were unable to make similarly accurate predictions using contemporary ‘snapshot’ measures of area and productivity for trees, endemic vertebrates and ectotherms – the majority of species included in their analyses.
The environmental stability of a bioregion can theoretically both promote and hinder the generation and maintenance of diversity over evolutionary time. First, greater stability implies more time is available for niche specialisation to evolve and selects for continuous, rather than discrete, generations, accelerating rates of recombination (Klopfer 1959; Fischer 1960; Connell & Orias 1964). Likewise, organisms adapted to fluctuating environments are often generalist phenotypes adapted to tolerate ephemeral resources and fluctuating abiotic conditions. Alternatively, environmental fluctuations can lead to negative frequency-dependent population dynamics and create temporal niche opportunities, leading to a greater number of competitors able to coexist (Levins 1979; Petraitis et al. 1989; Chesson & Huntly 1997). These contrasting effects of stability in both promoting and inhibiting coexistence and diversification are not mutually exclusive, and likely depend on spatial and temporal scales.
The bacterium Pseudomonas fluorescens SBW25 is a model system for the experimental study of adaptive diversification and coexistence (Rainey & Travisano 1998). Under homogeneous (i.e. shaken) culture conditions, colonies of the strain remain uniform in morphology (called smooth spreaders). If cultured under static conditions, however, this ancestral type quickly diversifies into a variety of morphologically distinct niche specialists. These mutants can be categorised into two distinct groups based on colony morphology: the wrinkly spreaders and fuzzy spreaders, each of which encompasses several unique subtypes including wheel-like, lobate, filamentous and undulate forms (Fukami et al. 2007). Wrinkly spreaders predictably evolve to exploit the air–water interface by excreting acetylated cellulose and forming a thick biofilm (Spiers et al. 2003). This niche construction results in a sharp oxygen gradient, paving the way for additional diversification. The genetic basis for these changes is well understood, with the paradox of random beneficial mutations leading to predictable independent radiations explained by large population sizes and rapid growth rates (Spiers et al. 2002). Smaller culture volumes are said to inhibit predictable radiations due to reduced population sizes (Rainey & Travisano 1998). Similarly, the productivity of the growth medium (carbon substrate concentration) also affects the population sizes, growth rates and relative fitness of P. fluorescens morphotypes, and therefore can also constrain adaptive radiation (Kassen et al. 2000). Finally, the stability of the culture habitat also influences its diversity dynamics. Intermediate rates of disturbance via imposed population bottlenecks drives diversification rates and resulting community composition in a unimodal pattern, indicating that negative frequency-dependent selection at intermediate productivity and disturbance was equalizing the population sizes (and relative fitness) of competing phenotypes (Buckling et al. 2000; Kassen et al. 2004).
Here, I use the P. fluorescens SBW25 model system to experimentally test the time–area–productivity hypothesis as it relates to the tempo and extent of adaptive diversification. Because the air–water interface habitat is crucial for diversification, I used air–water interface area as a proxy for bioregion extent, though it scales exactly with culture volume in this study. I tested the hypothesis that time-integrated, productivity-scaled area (TimeAreaProductivity) is the best predictor of P. fluorescens phenotypic diversity owing to the positive effects of area on population size and the positive effects of productivity on both population size and growth rate. Furthermore, I investigated whether the temporal stability of the culture conditions experienced by each cell line either increased or decreased the extent of P. fluorescens diversification. My goal was to compare the utility of these time-integrated variables as predictors of diversity to ‘snapshots’ of the ecosystem taken at the time of sampling. I anticipated time-integrated measures would outperform ‘snapshot’ measures if the time it took diversity to equilibrate to changing abiotic conditions was long relative to the frequency of disturbance. I performed this test in order to experimentally verify the mechanism and relative importance of time-integrated productivity and area as drivers of P. fluorescens diversity dynamics.
Material and Methods
Time–area–productivity experiment
Ancestral Pseudomonas fluorescens SBW25 cells were grown in 20 mL KB broth under shaken conditions at 26° C for 2 days (attaining a population density of 3.4 × 1010 cells mL−1). Approximately 1 mL of this culture was spun at 10 000×g for 5 min. The supernatant was replaced with sterile phosphate buffer and the pelleted cells re-suspended. The centrifugation and resupply of fresh buffer was repeated three times to wash cells of any residual medium. Cells were then diluted to 105 mL−1 and starved for 2 h. I prepared three growth media encompassing a 100-fold difference in nutrient availability – a proxy for productivity. This order of magnitude approximates the variance of primary productivity experienced across all terrestrial biomes (Yuan et al. 2010). Media were prepared by diluting 1× M9-KB broth (NH4Cl 1 g L−1, Na2HPO4 6 g L−1, KH2PO4 3 g L−1, NaCl 0.5 g L−1, glycerol 6 g L−1, proteose peptone #3 20 g L−1) 10-fold and 100-fold in M9 salts solution. These three media were aliquoted into flat-bottomed culture vessels spanning two orders of magnitude in volume: 10 mL (6-well plates), 1 mL (48-well plates) and 0.1 mL (96-well plates). This experimental design allowed for growth of bacterial populations in nine different combinations of habitat volume (0.1, 1 and 10 mL) and resource availability (0.01×, 0.1×, 1×).
Into each habitat, I inoculated 50 μL of starved ancestral P. fluorescens cells (approximately 1000 isogenic cells). Each of the nine culture conditions were replicated in triplicate and stored at 26° C under static conditions for 24 h. Aliquots of these cultures were then sampled, briefly vortexed and stored 20% glycerol at −20° C. From two of the three replicate sets of cultures, I removed, washed and starved 50 μL, and inoculated them into three different randomly assigned volume × productivity treatments, for a total of 54 randomised habitats (Fig. S1). These cultures were grown for 2 days and sampled as previously described. Aliquots were once again taken from 27 of these cultures, washed and starved before using each one to seed three new randomised habitats for a total of 81 cultures. These were incubated for 3 days and then sampled and preserved. This schedule forced bacterial populations to remain in or near the exponential phase of growth for the duration of the experiment and did not allow thick biofilms to form and collapse inside of the culture medium, nor allow cultures to deplete the medium and starve. Transfers contained approximately 106 cells, which did not represent a significant genetic bottleneck. Dilutions of each 1-, 3- and 6-day population were made in phosphate buffer and spread on KB agar plates for enumeration. I identified bacterial morphotypes by scoring 100 random colonies per plate. All questionable colonies were re-plated to ensure that they were genetically distinct.
Time-to-equilibrium experiment
I conducted a second experiment to estimate the time scale over which diversity equilibrates to a particular productivity. Washed and starved ancestral bacteria were inoculated into wells containing 1 mL of high, medium, or low-resource broth as previously described. Every 3 days, a 50 μL aliquot of each culture was used to seed a new well of the same productivity and the rest of the culture was preserved and frozen. After 24 days, 50 μL aliquots from the eight cultures containing the greatest number of unique morphotypes (all of the high and two of the medium productivity cultures) were then washed of media, starved, added into 1 mL low-productivity wells and serially transferred and preserved every 3 days for an additional 21 days. Samples from the entire 45-day time series were then plated and scored for morphotype richness and diversity.
Growth curve measurement
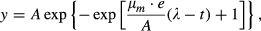
where t is the culture age (hours), e is base of the natural logarithm and y is the change in OD600 values from t0. Once each culture reached stationary phase, I estimated its population density by plating dilutions of the culture onto solid medium and counting the colonies.
Data collection and statistical analyses
I estimated diversity as previously described (Kassen et al. 2004). Alongside morphotype richness, I estimated the complement (1 − λ) of Simpson's index, λ, where λ = Σ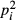 and pi is the frequency of each morphotype in the 100 censused colonies. This metric takes a value between 0 and 1 and represents the likelihood that any two randomly selected colonies belong to different morphotypes. This index was logit-transformed to fulfil the assumptions of linear regression. Time-integrated area (TimeArea) and productivity (TimeProductivity) were measured by integrating the total areas and productivities experienced by each cell line over their 1-, 3- or 6-day evolutionary histories. Similarly, TimeAreaProductivity is the time-integrated product of culture area and productivity (Jetz & Fine 2012) (Fig. S1). I estimated the stability of TimeArea, TimeProductivity and TimeAreaProductivity using the coefficient of variation (CV = standard deviation/mean). I fit a series of generalised linear (GLM) and generalised additive models (GAM) to the hypothesised drivers of diversity. I used the slope parameters of GLM fits to interpret the magnitude and direction of covariance, while GAM fits were used to estimate the shape of the response surface and estimate the proportion of variance explained. Additive models were constrained to a maximum of five basis dimensions to avoid overfitting while permitting quadratic and logistic-like response surfaces. The response variables were richness (modelled as Poisson distributed) and logit-transformed diversity (modelled as Gaussian distributed). Best-fit models were selected using AICc and coefficients of determination (R2). To avoid pseudoreplication, I only analysed endpoint communities – those that were not used to seed new microcosms at 1- and 3-day transfers.
and pi is the frequency of each morphotype in the 100 censused colonies. This metric takes a value between 0 and 1 and represents the likelihood that any two randomly selected colonies belong to different morphotypes. This index was logit-transformed to fulfil the assumptions of linear regression. Time-integrated area (TimeArea) and productivity (TimeProductivity) were measured by integrating the total areas and productivities experienced by each cell line over their 1-, 3- or 6-day evolutionary histories. Similarly, TimeAreaProductivity is the time-integrated product of culture area and productivity (Jetz & Fine 2012) (Fig. S1). I estimated the stability of TimeArea, TimeProductivity and TimeAreaProductivity using the coefficient of variation (CV = standard deviation/mean). I fit a series of generalised linear (GLM) and generalised additive models (GAM) to the hypothesised drivers of diversity. I used the slope parameters of GLM fits to interpret the magnitude and direction of covariance, while GAM fits were used to estimate the shape of the response surface and estimate the proportion of variance explained. Additive models were constrained to a maximum of five basis dimensions to avoid overfitting while permitting quadratic and logistic-like response surfaces. The response variables were richness (modelled as Poisson distributed) and logit-transformed diversity (modelled as Gaussian distributed). Best-fit models were selected using AICc and coefficients of determination (R2). To avoid pseudoreplication, I only analysed endpoint communities – those that were not used to seed new microcosms at 1- and 3-day transfers.
To estimate whether equilibrium diversities were reached in the productivity treatments and whether they differed, I fit a series of generalised additive models to the 24-day time series begun with the isogenic ancestral cells. I visually compared the shapes of each productivity treatment's curves and assessed whether the 95% confidence intervals surrounding each estimated mean differed between productivities. I compared these curves to intercept-only null models using likelihood ratio tests and R2 values. I then performed the same set of analyses on the high-diversity, low-productivity treatments started at day 24. I anticipated that these cultures' diversities would decrease over time, as less fit morphotypes are driven to rarity or extinction and that their diversities would eventually equal those of low-productivity cultures started with ancestral cells. All statistical operations were performed using r (R Development Core Team 2014) and code is available from the author on request.
Results
Time–productivity drives diversity dynamics
A total of 116 independent microcosms were scored for diversity. In total, I identified eight distinct, heritable colony morphotypes, though no single culture contained more than four and all but three were variants of the wrinkly spreader phenotype (see Fukami et al. 2007 for descriptions) (Fig. S2). In only one case was a morphotype observed to go extinct. I confirmed a relatively weak relationship between TimeAreaProductivity and diversity, but not for morphotype richness (Table 1). However, the covariate TimeProductivity was the strongest single predictor for both diversity and richness (Fig. 1). Furthermore, linear models incorporating time-integrated productivity explained approximately 33% (diversity) and 26% (richness) more variance than did models using only ‘snapshot’ productivity measured at the time of sampling (Table 1). Neither contemporary ‘snapshots’ of area nor TimeArea were found to be associated with diversity or richness. As anticipated, culture age per se (Time) was also a good predictor of culture diversity. The stability of area over time (CVarea) was positively associated with diversity and richness. Inclusion of CVarea into the TimeProductivity linear and nonlinear models delivered the best predictive accuracies (Fig. 2). For logit-transformed diversity and richness, this increased the percentage variance explained by the best-fit GAM models to 72 and 60% respectively.
| Predictor variables | Richness | Diversity | ||||||
|---|---|---|---|---|---|---|---|---|
| GLM models | GAM models | GLM models | GAM models | |||||
| ΔAICc | R 2 | ΔAICc | R 2 | ΔAICc | R 2 | ΔAICc | R 2 | |
| Time | 17 | 0.21 | 20 | 0.18 | 93 | 0.21 | 114 | 0.21 |
| Area | 28 | 0.01 | 31 | 0.00 | 120 | 0.00 | 141 | 0.00 |
| Productivity | 13 | 0.28 | 15 | 0.30 | 69 | 0.36 | 89 | 0.36 |
| AreaProductivity | 26 | 0.05 | 28 | 0.08 | 105 | 0.12 | 121 | 0.17 |
| TimeArea | 29 | 0.00 | 32 | 0.00 | 120 | 0.00 | 141 | 0.00 |
| TimeProductivity | 0 | 0.49 | 0 | 0.56 | 5 | 0.63 | 6 | 0.69 |
| TimeAreaProductivity | 25 | 0.06 | 28 | 0.06 | 107 | 0.11 | 126 | 0.06 |
| CVarea | 22 | 0.12 | 25 | 0.10 | 108 | 0.10 | 129 | 0.10 |
| CVproductivity | 28 | 0.02 | 28 | 0.10 | 116 | 0.04 | 123 | 0.10 |
| CVarea + CVproductivity | 24 | 0.12 | 26 | 0.16 | 109 | 0.11 | 118 | 0.16 |
| TimeProductivity + CVarea | 0 | 0.54 | 0 | 0.59 | 0 | 0.65 | 0 | 0.72 |
| TimeAreaProductivity + CVarea | 21 | 0.18 | 24 | 0.16 | 95 | 0.22 | 115 | 0.16 |
| Intercept-only null | 27 | 0.00 | 30 | 0.00 | 118 | 0.00 | 139 | 0.00 |
- GAM, generalised additive models; GLM, generalised linear.
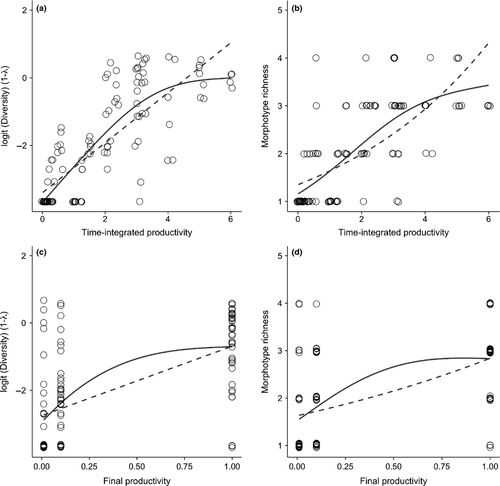
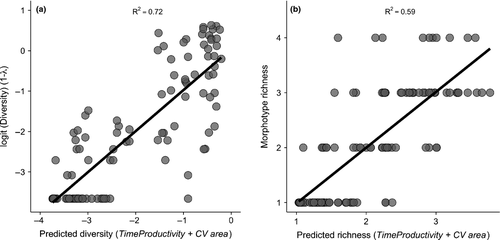
Growth rates vary by productivity
By analyzing the strain's growth kinetics under varying nutrient concentrations, I determined that differences exist in the strain's growth rates (μ) and carrying capacities (A) among productivity treatments (Table 2). Specifically, diluting the standard KB+M9 medium by 100 has the effect of decreasing the bacterium's growth rate and carrying capacity approximately 10-fold (Fig. 3). At steady state, the cultures had estimated population densities of 2.3 × 109, 1.8 × 109 and 2.8 × 108 cells mL−1 for the high, medium and low-productivity treatments respectively.
| Productivity | Parameter estimate (95% CI) | ||
|---|---|---|---|
| μ | A | λ | |
| 1× | 0.037–0.055 | 1.054–1.225 | 16.5–19.8 |
| 0.1× | 0.025–0.030 | 0.830–0.927 | 13.8–16.0 |
| 0.01× | 0.004–0.006 | 0.112–0.132 | 4.5–10.3 |
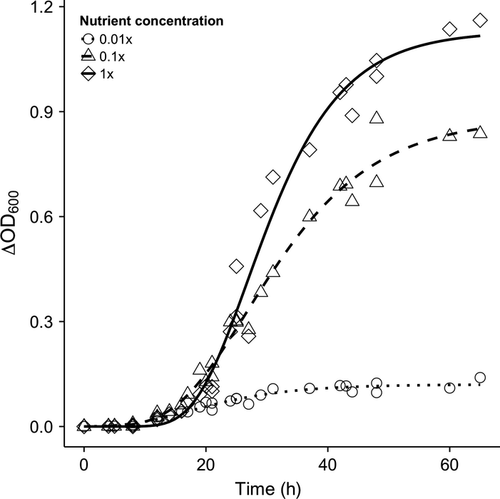
Historical signatures of productivity are slow to disappear
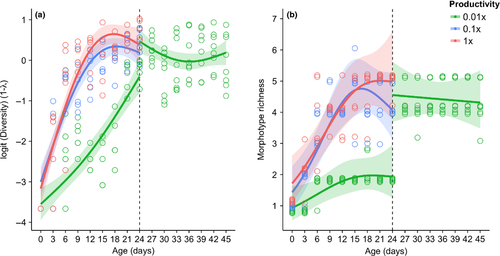
Diversity and richness in all cultures initially increased over a period of approximately 6–9 days (Fig. 4 and Fig. S3). In high and medium cultures, these values initially increased and then appeared to remain unchanged after 9 days. Diversity in low-productivity cultures steadily increased throughout the 24-day period and ended lower than those in high and medium productivities. Average morphotype richnesses for high (five morphotypes) and medium (four morphotypes) productivities were both greater than morphotype richness in low-productivity cultures (two morphotypes) (Fig. 4). For cultures started with ancestral smooth cells, AICc and R2 metrics favoured time-variant models of diversity over their intercept-only counterparts (Table S1). Morphotype-rich cultures moved into low-productivity media did not decrease in diversity nor richness. For these cultures, time-dependent models were not improvements over intercept-only null models (Table S1). The expected values from these intercept-only models were within bounds of the endpoint mean diversity and richness values from the high and medium productivity cultures started with the ancestral strain but greater than the mean endpoints of the low-productivity cultures (Fig. 4).
Discussion
Using a model microbial adaptive radiation, I have shown that time-integrated productivity is a primary driver of diversification dynamics in the P. fluorescens model system. These data represent the first experimental test of the time–area–productivity hypothesis and are consistent with results obtained observational studies (Jetz & Fine 2012; Belmaker & Jetz 2015). Contrary to their findings, however, I failed to identify spatial extent as an important predictor of diversification in this system. A reason for this discrepancy may be that the previous studies were unable to explicitly model TimeProductivity separately from TimeArea due to a lack of pre-Holocene productivity data. Instead, the authors scaled TimeArea by the bioregion's modern productivity. Therefore, this study represents the first to independently test for TimeProductivity and TimeArea effects. It remains to be determined whether time-integrated productivity is able to explain more variation than time-integrated area in global datasets.
The differences in diversification observed among experimental cultures stemmed from the maximisation of population carrying capacities and growth rates during periods of high productivity. Because cultures were maintained at or near exponential phase of growth during the experiment, the positive effects of productivity on growth rates allowed for beneficial mutants to accumulate more rapidly and persist in more productive environments. Contrary to anecdotal evidence (Rainey & Travisano 1998) neither culture volume (i.e. area) nor its time-integrated measure was positively associated with the extent of Pseudomonas diversification. This occurred despite a two order-of-magnitude difference in population sizes between 10 mL and 0.1 mL cultures. Thus, for P. fluorescens SBW25, time-integrated air–water interface area (or culture volume) cannot be considered an important driver of diversification in the presence of other environmental variables affecting population growth.
One explanation for why productivity, rather than area, drove diversification in this experiment is the competitive suppression of de novo niche specialists at low nutrient concentrations. Similar to Kassen et al. (2004), even small (0.1 mL), low-productivity cultures at 48 h contained populations of P. fluorescens large enough to produce wrinkly spreader mutants. However, the relative fitness of these morphotypes rely on a nutrient supply large enough to allow coordinated expression of cellulosic polymer (Spiers et al. 2003; Kassen et al. 2004). Lacking a surplus of growth substrate, wrinkly spreader populations cannot exploit the air–water interface niche and are either driven extinct or maintained at low-frequencies via competition with the ancestral smooth morphotype (Kassen et al. 2004). At intermediate and high productivities, all culture sizes contained nutrient concentrations necessary for wrinkly spreaders to invade, despite the pleiotropic fitness costs to biofilm production via decreased carbon metabolism (MacLean et al. 2004). In contrast, populations of macro-organisms are often constrained by geographic barriers to sizes much smaller than those observed in this experiment (Preston 1962). Such populations should not diversify, particularly if they are simultaneously being constrained by a low-productivity environment and/or competition (Wright 1983; Rosenzweig & Abramsky 1993). Additionally, larger bioregions are anticipated to contain more barriers to gene flow, which can promote non-adaptive diversification (Terborgh 1973; Gittenberger 1991; Rosenzweig 1995). Such allopatric diversification is not permitted in the relatively homogeneous environment of microbial culture vessels due to their lack of geographic boundaries. However, it is worth noting that no single culture contained all eight morphotypes, and a summation of morphotypes in 10 isolated 1 mL (or <100 isolated 0.1 mL) wells was unanimously greater than or equal to the diversity of any single 10 mL well, given similar productivities. This finding lends support to the role of geographic barriers in promoting regional diversity through the ecological saturation of convergent morphotypes (Terborgh & Faaborg 1980; Ricklefs 2004). The large effective population sizes (106 cells) and overall magnitude and replicability of diversification indicate that divergence via neutral drift is not the cause of this pattern, and it is instead due to selection. In a culture without geographically isolated populations and limited resources over which to compete, clonal interference could limit the number of coexisting niche specialists (Gerrish & Lenski 1998).
My results are similar to those of Kassen et al. (2000, 2004) in demonstrating inhibition of P. fluorescens diversification in low-productivity habitats. Unlike these previous studies, however, I did not encounter a negative-quadratic shape to the productivity–diversity relationship. Instead, diversity and richness appeared to asymptote at high time-integrated productivities. This difference may be because Kassen et al.'s ‘high-productivity’ cultures contained nutrient concentrations far greater than the standard 1× King's medium formulation. Furthermore, the extent of P. fluorescens diversification tends to follow a sigmoidal trend over the time periods used in this experiment – a pattern observed elsewhere (Fukami et al. 2007). Because two sources of environmental variation are incorporated into the TimeProductivity variable, it is probable that the saturating dynamics I observed represent a combination of constraints on diversification imposed by culture age (sigmoidal) and productivity (negative-quadratic). However, equivalent values of TimeProductivity can be achieved in a variety of ways. For instance, an intermediate productivity culture running for 3 days can equal a high-productivity culture at 1 day, or a low-productivity culture at 6 days. Productivity, however, is one constraint on the diversification dynamics of P. fluorescens SBW25. A number of other factors, most notably habitat heterogeneity, temperature, disturbance and community interactions (e.g. predation and competition) also mediate this model radiation (Rainey & Travisano 1998; Kassen et al. 2004; Fukami et al. 2007; Meyer & Kassen 2007).
I detected an effect of culture area stability on morphotype diversity and richness, but I did not anticipate the association to be positive in direction. Moreover, inclusion of this covariate in my models only resulted in an improvement of 5% variance explained. Models of species accumulation over time often cite the importance of climate-driven habitat stability in driving niche specialisation and speciation (Klopfer 1959; Fischer 1960; Connell & Orias 1964). My finding that morphotype richness and diversity decreased with environmental stability does not support the prediction that environmental stability per se promotes diversification. Rather, this observation suggests that disturbance in terms of periodically imposed population bottlenecks promotes the maintenance of incipient niche specialists. However, these bottlenecks were not especially strong, since approximately 106 cells were used to seed new cultures and transfers were made at time intervals over which both community interactions and adaptive diversification can influence diversity dynamics. Buckling et al. (2000) obtained a similar result and concluded that intermediate levels of disturbance acting on diversifying P. fluorescens cultures allowed rare genotypes the opportunity to invade an otherwise resistant community. Using the same system, Tan et al. (2013) found evidence that temporal variation in niche availability promoted coexistence among morphotypes. However, the positive covariance of CVarea with both Time and transfer frequency hinders the interpretation of this effect in my study. Nonetheless, whichever stability metric was used in my models, the qualitative result was consistent: a negative trend exists between diversity and environmental stability in the model P. fluorescens radiation.
Although I only detected a single extinction event during my experiment, it is probable that many undetected de novo morphotypes arose in all cultures and were rapidly driven extinct by established competitors. In both experiments, neither diversity nor richness decreased when diverse, high-productivity assemblages were transplanted into low-productivity media. In other words, niche specialist phenotypes unable to establish in low-productivity cultures could persist at their historical relative frequencies despite the reduced habitat quality. Extinction debt is a hypothesis used to explain the maintenance of hyper-diverse assemblages in deteriorating or shrinking habitats (Tilman et al. 1994). In both this experiment and many observational studies, present day area and productivity were poor predictors of regional variance in diversity (e.g. Fine & Ree 2006; Jetz & Fine 2012). Rather, time-integrated area and productivity were much better explanatory variables. It is noteworthy, however, that Jetz & Fine (2012) found evidence for the primacy of non-time-integrated ‘snapshot’ measures in explaining the richness of non-endemic bird and mammal assemblages. This suggests that time-integrated measures may be less useful when applied to assemblages capable of crossing bioregional boundaries (e.g. via migration). Further work is needed to determine whether biota better modelled with time-integrated variables share certain key traits predisposing them to endemicity.
The primacy of snapshot vs. time-integrated variables in explaining regional diversity patterns depends in part on the relative rates at which these systems equilibrate following a disturbance. Historical signatures of area and productivity should manifest if communities return to equilibrium slowly, but should be erased if communities either do not reach a steady state or return to it very quickly. My finding that high-richness cultures placed into low-productivity environments do not lose their derived morphotypes confirms the importance of the cultures' historical conditions in explaining contemporary diversity patterns. The time it took for high, medium and low-productivity cultures to reach initial equilibria was on the order of 1 week, whereas once the communities had developed, they were capable of remaining unchanged for 3 weeks, despite being moved into low-productivity media. While this timeframe is partly a product of the study system's large population sizes, rapid generation times and relative simplicity, it nonetheless verifies that given certain conditions, time-integrated effects on community structure can persist over evolutionary timescales. Fitness differences that precluded de novo morphotypes from establishing in unproductive medium did not appear to affect their long-term persistence if they had originally diversified in a more productive habitat. In other words, the trajectory of diversity in a culture experiencing increasing productivity was not along the same response surface as a culture experiencing a decrease in productivity. Therefore, it is likely that the direction of environmental change can influence the length of time over which time-integrated effects can persist – environments increasing in productivity can be invaded by de novo mutants, whereas deteriorating environments may prevent such invasions via their suppression by established competitors.
In concert, these results suggest that time–energy effects manifest most strongly during early stages of diversification and persist longest in deteriorating environments. Once niches are sufficiently saturated with de novo phenotypes, competitive exclusion of similar low-frequency phenotypes sets the upper limit on richness. In the simple physicochemical habitats used in my experiments, niche saturation occurred relatively quickly and persisted indefinitely. Time–productivity effects may not have been as pronounced had cultures been allowed to remain at equilibria for a longer stretch of time prior to model fitting. However, doing so would have forced the system away from modelling natural diversity dynamics. There is currently limited consensus on whether a strict asymptotic diversification model holds for natural systems, primarily due to the fact that key evolutionary innovations and mass extinctions tend to keep diversification rates from reaching prolonged steady states (Rabosky 2013). Whether or not the observation of hyper-diverse biotas in sub-optimal habitats or refugia represents extinction debt or evolutionary acclimation requires further investigation, though it is clear from these data that if extinction debt responsible for this observation, it is occurring over a period at least three times as long as the time required for diversity to first appear. Answering this question requires long-term experiments and new methods to identify and characterise novel phenotypes.
In conclusion, high historical energy availability drove the evolution of niche specialists, which were unable to successfully establish in resource-poor environments. Low-productivity cultures inoculated with diverse assemblages from high-productivity habitats, however, did not experience extinctions, implying that a habitat's standing diversity can be decoupled from its contemporary environmental conditions. These results confirm that modern day ‘snapshot’ ecosystem metrics are at best proxies for explaining regional variation in extant diversity, particularly among endemic species. At worst, these variables can mislead analyses on drivers of biodiversity. Further, these results extend the domain of time-integration hypotheses to bacteria – organisms rarely considered bound by historical biogeographic constraints (but see Hanson et al. 2012). Going forward, these findings support the need for more historical data on both area and productivity to explain patterns of biodiversity at large spatial and temporal scales.
Acknowledgements
I thank P. Fine, T. Fukami, W. Sousa, D. Quiroz, H. Kurkjian, A. Hurlbert, two anonymous reviewers and the IB 250 graduate seminar on latitudinal gradients for inspiration, insight and feedback. Laboratory instrumentation was kindly provided by E. Simms. P. Rainey generously donated the bacterial strain used for this study. Support for this research comes from UC Berkeley's Wang Family Fellowship and the Department of Integrative Biology.
Authorship
DWA conceived this work, performed data collection and analysis and wrote the manuscript.




