Impairments in gait kinematics and postural control may not correlate with dopamine transporter depletion in individuals with mild to moderate Parkinson's disease
Abstract
Parkinson's disease (PD) is a progressive neurodegenerative disorder caused by the loss of dopamine, an important neurotransmitter involved in regulating movement. Nuclear medicine imaging methods such as single-photon emission computed tomography (SPECT) combined with radiotracers can obtain the density of this neurotransmitter. This reduced density leads to classic PD symptoms, such as bradykinesia, tremor and stiffness, consequently affecting walking and postural control. The aim of this study was to verify the correlation between disorders of gait kinematics and postural instability with dopamine depletion in individuals with mild to moderate PD. This is a descriptive, observational cross-sectional study. Subjects were assessed for spatiotemporal gait parameters by a three-dimensional motion capture system, for postural control by stabilometry on a force plate. Dopamine depletion was verified through 99mTc-TRODAT-1 (SPECT-CT) examination. The subjects were in the off-stage of levodopa in all analysis. We evaluated 71 individuals, 32 with mild to moderate PD (HY 2 and 2.5) and 39 healthy individuals matched for gender, age, and height. There was a significant difference between the groups regarding the spatiotemporal variables of gait, as well as in the stabilometric variables. However, there was no correlation between these disturbances and the uptake values of 99mTc-TRODAT-1. The results indicate that there is no correlation between gait impairments and postural instability of individuals with mild to moderate PD and the dopaminergic depletion measured through the 99mTc-TRODAT-1 (SPECT-CT).
Abbreviations
-
- COP
-
- Center of pressure
-
- DAT
-
- Dopamine transporters
-
- H&Y
-
- Modified Hoehn and Yahr Scale
-
- MDS-UPDRS III
-
- Motor Examination of Movement Disorder Society Unified Parkinson Disease Rating Scale
-
- MoCA
-
- Montreal cognitive assessment
-
- PD
-
- Parkinson's disease
-
- ROI
-
- Regions of interest
-
- SPECT
-
- Single-photon emission computed tomography
1 INTRODUCTION
Parkinson's disease (PD) is a progressive neurodegenerative disorder caused by dopamine depletion, an important neurotransmitter involved in movement control (Shulman et al., 2008). Injury of the nigrostriatal dopaminergic pathway leads to the classic neurological symptoms of PD, such as rest tremor, muscle stiffness and bradykinesia (Perez-Lloret & Barrantes, 2016).
Gait impairments represent an important cause of disability in PD (Allen, Schwarzel, & Canning, 2013). Step length, velocity and lower limbs range of motion decrease, steps timing is irregular and asymmetrical, and the upper limbs swing decrease or even turn absent (Grabli et al., 2012; Kelly, Eusterbrock, & Shumway-Cook, 2012; Yogev et al., 2005).
The subjects with PD also present significant postural instability (Kim, Allen, Canning, & Fung, 2013), due to the inability to properly balance the center of mass on the support base, predisposing these individuals to falls (Morris, Iansek, Smithson, & Huxham, 2000). Many factors are attributed to the occurrence of postural instability in PD. These include postural reflex loss (Williams, Watt, & Lees, 2006); inability to integrate visual, vestibular, and proprioceptive inputs (Hwang, Agada, Grill, Kiemel, & Jeka, 2016) and limited trunk flexibility due to axial stiffness (Carpenter, Allum, Honegger, Adkin, & Bloem, 2004).
An accurate diagnosis of PD can be challenging, remaining complex to differentiate PD from other parkinsonian syndromes (Uyama et al., 2017). Recently, molecular imaging techniques using single-photon emission computed tomography (SPECT) have been widely employed in the identification of dopamine deficit, assessing the dopamine transporters (DAT) depletion (Brooks, 2010). DAT SPECT represent a valuable tool for the differential diagnosis of PD, disease progression and pharmacological treatment efficacy (Bajaj et al., 2014; Jiménez-Jiménez, Alonso-Navarro, García-Martín, & Agúndez, 2012; Kägi, Bhatia, & Tolosa, 2010; Shih et al., 2006; Shinto et al., 2014). Studies using DAT SPECT also indicate the correlation between dopaminergic dysfunction, anxiety and depression (Moriyama et al., 2011; Wu, Lou, Huang, & Shi, 2011). However, there is no evidence in the current literature regarding the possible correlations between DAT density, gait impairments and postural instability in PD. The purpose of this study was to verify the correlation between disorders in spatiotemporal parameters of gait and stabilometric impairments of PD subjects with dopamine depletion assessed through 99mTc-TRODAT-1 (SPECT-CT).
2 MATERIALS AND METHODS
Descriptive, observational cross-sectional study was approved by the Research Ethics Committee of the Federal University of Health Sciences of Porto Alegre (UFCSPA; CAAE: 51522915.0.0000.5345). All subjects signed an informed consent form.
2.1 Subjects and clinical assessment
We recruited participants from the Neurologic Service at Irmandade Santa Casa de Misericórdia de Porto Alegre (ISCMPA). Inclusion criteria were: diagnosis of idiopathic PD according to the criteria of Queen Square Brain Bank (Hughes, Daniel, Kilford, & Lees, 1992); Classified according to the Modified Hoehn and Yahr Scale (Goetz et al., 2004) in the stages 2 and 2.5; Exclusion criteria were: previous history of stroke or cranioencephalic trauma, early dementia, Deep brain stimulation, or any musculoskeletal/neurological pathology. We also recruited healthy participants without any musculoskeletal disorder, as a reference group for gait and postural control analysis.
Subjects with PD were characterized according to the Modified Hoehn and Yahr Scale (H&Y), Motor Examination of Movement Disorder Society Unified Parkinson Disease Rating Scale (MDS-UPDRS III), and the Montreal Cognitive Assessment (MoCA). After sample characterization, all subjects were assessed regarding gait kinematics through a three-dimensional motion capture system, postural control through stabilometry on a force plate and dopamine transporter imaging through 99mTc-TRODAT-1 (SPECT-CT). All participants with PD were under antiparkinsonian medication treatment but they performed all the analysis with a medication withdrawal period of at least 12 hr.
The H&Y classifies PD as: 0 – No signs of disease; 1 – Symptoms are very mild; unilateral involvement only; 1.5 – Unilateral and axial involvement; 2 – Bilateral involvement without impairment of balance; 2.5 – Mild bilateral disease with recovery on pull test; 3 – Mild to moderate bilateral disease; some postural instability; physically independent; 4 – Severe disability; still able to walk or stand unassisted; 5 – Wheelchair bound or bedridden unless aided (Goetz et al., 2004).
The MDS-UPDRS III assesses the motor symptoms of PD encompassing speech, facial expression, rigidity, finger tapping, hand movements, pronation-supination movements of hands, toe-tapping, leg agility, arising from chair, gait, freezing of gait, postural stability, posture, global spontaneity of movement, and tremor (Goetz et al., 2008). The cut-off points are: Mild/Moderate – 32/33, Moderate/Severe 58/59 (Martínez-Martín et al., 2015).
The MoCA assesses cognitive function, presenting a greater sensitivity to cognitive deficits in PD in comparison to other cognitive instruments, such as the well-known Mental State Mini Exam (Hanna-Pladdy et al., 2010). MoCA encompasses executive functions, immediate and late recall, fluency, orientation, calculation, abstraction, visual perception, naming, and attention (Nasreddine et al., 2005). The total score ranges from 0 to 30. Scores <10 indicate severe cognitive deficit, between 10 and 17 moderate deficit, between 18 and 26, mild deficit and >26 the cognition is normal (Trzepacz, Hochstetler, Wang, Walker, & Saykin, 2015).
2.2 Kinematic gait analysis
The kinematic analysis was performed in the Movement Analysis and Rehabilitation Laboratory of the UFCSPA. Reflective markers were placed at specific anatomical landmarks, according to Davis, Õunpuu, Tyburski, and Gage (1991) protocol (Davis et al., 1991; See in Appendix S1).
Kinematic data were acquired by a synchronized system composed of six infrared cameras (BTS SMART DX 400 System) with resolution of 1 Megapixel, accuracy <0.3 mm (calibration volume of 4 × 3 × 3 m), acquisition rate of 100 Hz, synchronized with two Digital videocameras (BTS eVIXTA) 1.9 Megapixel with acquisition rate up to 60 Hz.
Subjects were instructed to walk independently, barefoot, at a self-selected speed along an 8 m flat walkway. An experienced evaluator chose 3 from 10 trials according to the consistency of the protocol procedures.
2.3 Postural control analysis
Postural control was assessed on a force plate with subjects in orthostatic position, opened eyes, barefoot, two feet apart in a comfortable position, with the eyes fixed on a target 1 m away. Individuals were instructed to stay as quiet as possible in three acquisitions. Each test lasts 30 s, the first 5 s were discarded for adaptation in the force plate and the last 5 s discarded to avoid fatigue interferences (Błaszczyk, 2016).
The force platform signals were sampled at 100 Hz, and a cut-off frequency low-pass filter was chosen after a residual analysis. A low-pass fourth order Butterworth digital filter at 10 Hz was applied. The variables were normalized to participant's height in meters.
For the analysis of stabilometric data, an algorithm was developed in the Matlab Software (Mathworks Inc., Natick, USA) to filter the original data and calculate the descriptive center of pressure (COP) in time and frequency domain. The COP expresses the location of the vector resulting from the ground reaction force on a force plate (Duarte & Freitas, 2010). The COP displacements were computed in the anteroposterior (AP) and medio-lateral (ML) directions in the following variables:
- Total displacement of sway (DOT): length of COP trajectory on the base of support [mm] (Duarte & Freitas, 2010).
- Root mean square for AP (RMSap) and ML (RMSml) directions: range of COP displacement from the mean position during an interval [mm]. High RMS indicates large internal perturbation and greater need for postural adjustments (Duarte & Freitas, 2010).
- 80% Power Frequency (F80%): Frequency analysis provides a general view of the spectrum of frequencies that can be decomposed from a COP signal. The 80% of the power frequency is the best value to characterize modifications in postural control. Higher frequencies indicate a postural control with faster and smaller adjustments (Baratto, Morasso, Re, & Spada, 2002).
2.4 Dopamine transporter imaging
The Dopamine Transporter Imaging was assessed through 99mTc-TRODAT-1 (SPECT-CT), performed at the Nuclear Medicine Service of the ISCMPA. TRODAT was labeled with 99mTc (technetium). The vial containing the saline solution (“Cold” Kit) was labeled with 50 mCi of pertechnetate. Chemical quality control tests were performed prior to the administration of the radiopharmaceutical. The radiochemical (colloid, free pertechnetate) and radionuclide (marking power) purities were determined by chromatography. Only flasks with radiochemical purity greater than 90% were released for injection.
No preparation was required prior to the injection of the radiopharmaceutical. The administered dose was 20 mCi of 99mTc-TRODAT by peripheral vein. The time required between dose administration and the onset of imaging was 4 hr.
No oral or intravenous contrast material was used. SPECT images were acquired on a Siemens SPECT-CT (Symbia T2)/dual-head SPECT/CT system with low-energy, high-resolution collimators. A step-and-shoot protocol was acquired in a 128 × 128 matrix size through 360° rotation at 3° intervals of 20 s for a total of 60 views per camera head was used. Immediately after SPECT acquisition, CT was performed. The parameters included a current of 2.5 mA, a voltage of 140 kV, and 10-mm slices reconstructed; the CT rotated at 2.6 rotations per minute. Acquisition required 7–9 min.
Transverse, coronal, and sagittal SPECT images were generated using a Hann prefilter and a Butterworth postprocessing filter with two ordered-subset expectation maximization (OSEM) iterations and a maximum of 10 OSEM subsets; the attenuation correction factor was 0.6. Transverse, coronal, and sagittal SPECT/CT images were generated using a Butterworth prefilter and postprocessing filter with 4 OSEM iterations for a maximum of 8 OSEM subsets. Images were reviewed on a Siemens workstation/Leonardo.
For the analysis of striatal 99mTc-TRODAT-1 binding, regions of interest were drawn. Binding potential (BP) was estimated to evaluate DAT density/affinity by measuring the mean specific activity in the basal ganglia region, calculated by subtracting the mean counts per pixel in the striatum (STR) from the mean counts per pixel in the occipital as background (BKG) and dividing the result by the mean counts per pixel in the background (STR–BKG/BKG). This method has been previously validated in several studies and has been used in several studies from our group (Bressan et al., 2009; Shih et al., 2007). All images were examined by two observers.
Three regions of interest (ROI) were analyzed: Caudate, anterior and posterior putamen. ROI were generated from the anatomical image of each subject, using the structural limit as reference. Uptake values >1.19 were assumed as normal (Silva et al., 2014).
2.5 Statistical analysis
Data are expressed as frequency (%), mean ± SD and mean and 95% confidence intervals. We performed associations between categorical variables using the Chi-square tests. For comparing continuous variables between groups, Student t or Mann–Whitney tests were used, depending on their distribution. Spearman's correlation tests were used to correlate quantitative variables. We assumed the cut-off values of r, 0–0.19 as very weak, 0.2–0.39 as weak, 0.40–0.59 as moderate, 0.6–0.79 as strong and 0.8–1 very strong (Altman, 1991). Statistical significance was accepted at p < 0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences (PASW Statistics for Windows, Version 23.0. Chicago: SPSS Inc).
Since no previous study has correlated DAT density data with gait and postural control variables, we estimated the sample size using the G-Power 3.1 software. Looking for a large effect size (ρ = 0.5; Cohen, 1969), two-tailed tests with 80% power and significance of 5% resulted in a minimum sample size of 26 subjects.
3 RESULTS
Seventy-one subjects were evaluated: 32 in the PD group and 39 in the reference group. The groups were homogeneous with respect to gender (p = 0.914), age (p = 0.974) and height (p = 0.056).
Table 1 presents a comparative analysis of the clinical, demographic and imaging findings in both groups. On average the UPDRS Motor Exam was 28.68 ± 14.21, MoCA was 25.25 ± 3.22 and the 99mTc-TRODAT-1 uptake ratio was 0.54 ± 0.25 in the caudate, 0.32 ± 0.14 in the anterior putamen and 0.19 ± 0.11 in the posterior putamen. Brain images in transverse views of a PD subject, 64-year-old, H&Y 2 are shown in Figure 1, demonstrating the asymmetrical dopaminergic uptake pattern found in this population.
| Variables | Reference group (n = 39) | Parkinson group (n = 32) | p Value |
|---|---|---|---|
| Male (%) | 61.5 | 65.6 | 0.914 |
| Age, years | 59 ± 11 | 60 ± 11 | 0.974 |
| Height, meters | 1.68 ± 9.5 | 1.63 ± 10 | 0.056 |
| Modified Hoehn and Yahr Scale | |||
| Stage 2 | – | 25 (78.1) | |
| Stage 2.5 | – | 7 (21.9) | |
| MDS-UPDRS Motor Exam | – | 28.68 ± 14.21 | |
| Montreal Cognitive Assessment | – | 25.25 ± 3.22 | |
| 99mTc-TRODAT-1 uptake | |||
| Caudate | – | 0.54 ± 0.25 | |
| Anterior Putamen | – | 0.32 ± 0.14 | |
| Posterior Putamen | – | 0.19 ± 0.11 | |
- Data are presented as frequency (%) and mean ± SD. MDS: Movement Disorder Society; UPDRS: Unified Parkinson's Disease Rating Scale.
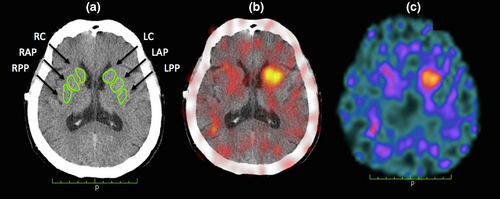
Table 2 shows the comparison of kinematic gait analysis between PD and reference group. There was a statistically significant difference between the groups in the spatiotemporal parameters and lower limbs range of motion. Comparison between reference and PD group shows a significant reduced swing phase (p = 0.049), reduced mean velocity (p < 0.001), cadence (p = 0.001), stride length (p < 0.001), step length (p < 0.001), step width (p = 0.002), shorter hip flex-extension (p = 0.012), knee flex-extension (p < 0.001) and shorter ankle flex-extension (p = 0.029).
| Variables | Reference group (n = 39) | Parkinson group (n = 32) | p Value |
|---|---|---|---|
| Temporal parameters | |||
| Stance phase (%) | 62.35 [61.54–63.16] | 62.67 [60.68–64.63] | 0.913 |
| Swing phase (%) | 38.07 [37.33–38.81] | 36.25 [34.92–37.57] | 0.049a |
| Double support phase (%) | 12.27 [11.58–12.95] | 14.25 [12.57–15.93] | 0.111 |
| Mean velocity (m/s) | 1.19 [1.13–1.25] | 0.90 [0.82–0.99] | <0.001b |
| Cadence (steps/min) | 112.10 [109.70–114.49] | 102.77 [98.41–107.12] | 0.001b |
| Spatial parameters | |||
| Stride length (m) | 1.27 [1.22–1.33] | 1.03 [0.94–1.11] | <0.001a |
| Step length (m) | 0.65 [0.58–0.72] | 0.51 [0.47–0.56] | <0.001a |
| Step width (m) | 0.12 [0.08–0.15] | 0.08 [0.07–0.09] | 0.002a |
| Lower limbs range of motion | |||
| Hip flex-extension (degrees) | 45.59 [43.42–47.76] | 40.86 [38.12–43.59] | 0.012a |
| Knee flex-extension (degrees) | 57.21 [55.47–58.95] | 52.30 [49.61–54.99] | <0.001b |
| Ankle flex-extension (degrees) | 30.18 [28.74–31.61] | 27.99 [25.83–30.15] | 0.029a |
- Data are presented as mean [95%IC].
- a Significant data (p ≤ 0.05) in between-groups comparison – Mann–Whitney test.
- b Significant data (p ≤ 0.05) in between-groups comparison – Student t test.
Table 3 presents the comparison of postural control data between PD and reference subjects. DOT, RMSap and F80% were statistically different between the groups (p < 0.001, p = 0.005 and p < 0.001, respectively).
| Variables | Reference group (n = 39) | Parkinson group (n = 32) | p Value |
|---|---|---|---|
| DOT | 46.92 [40.11–53.72] | 65.88 [58.85–72.91] | ≪0.001a |
| RMS, ml | 0.20 [0.19–0.22] | 0.28 [0.16–0.40] | <0.401 |
| RMS, ap | 0.40 [0.25–0.55] | 0.45 [0.39–0.50] | <0.005a |
| F80% | 1.33 [1.26–1.40] | 1.85 [1.59–2.11] | <0.001a |
- Data are presented as mean [95%IC]. AP: antero-posterior direction; DOT: total displacement of sway; F80%: 80% of power frequency; ML: medio-lateral direction; RMS: root mean square.
- a Significant data (p ≤ 0.05) in between-groups comparison – Mann–Whitney test.
Table 4 shows the Pearson's Correlations between kinematic and stabilometric analysis and quantitative values of 99mTc-TRODAT-1 uptake in subjects with PD. Only two weak correlations were statistically significant: Ankle Flex-Extension and Anterior Putamen (r = −0.538, p = <0.001); Ankle Flex-Extension and Posterior Putamen (r = −0.398, p = <0.001).
| Variables | 99mTc-TRODAT-1 uptake | |||||
|---|---|---|---|---|---|---|
| Caudate | Anterior Putamen | Posterior Putamen | ||||
| r | p | r | p | r | p | |
| Kinematic gait analysis | ||||||
| Stance Phase (%) | 0.065 | 0.73 | −0.206 | 0.26 | 0.095 | 0.61 |
| Swing Phase (%) | −0.272 | 0.13 | −0.051 | 0.78 | 0.070 | 0.70 |
| Double Support Phase (%) | 0.271 | 0.14 | 0.039 | 0.83 | −0.077 | 0.67 |
| Mean Velocity (m/s) | −0.133 | 0.47 | 0.107 | 0.56 | −0.019 | 0.92 |
| Cadence (steps/min) | −0.218 | 0.23 | −0.150 | 0.42 | −0.187 | 0.31 |
| Stride Length (m) | −0.072 | 0.70 | 0.119 | 0.52 | −0.077 | 0.67 |
| Step Length (m) | −0.045 | 0.81 | 0.196 | 0.29 | 0.098 | 0.60 |
| Step Width (m) | 0.333 | 0.06 | 0.081 | 0.66 | 0.028 | 0.88 |
| Hip Flex-Extension (degrees) | −0.156 | 0.40 | 0.029 | 0.87 | 0.082 | 0.66 |
| Knee Flex-Extension (degrees) | −0.113 | 0.54 | −0.322 | 0.07 | −0.276 | 0.13 |
| Ankle Flex-Extension (degrees) | −0.236 | 0.20 | −0.538 | <0.001a | −0.398 | <0.001a |
| Postural control analysis | ||||||
| DOT | −0.027 | 0.88 | −0.190 | 0.30 | −0.116 | 0.53 |
| RMSap | 0.169 | 0.36 | −0.108 | 0.56 | 0.139 | 0.45 |
| RMSml | 0.256 | 0.16 | −0.068 | 0.71 | 0.164 | 0.37 |
| F80% | −0.066 | 0.72 | −0.062 | 0.73 | −0.027 | 0.88 |
- AP: antero-posterior direction; DOT: total displacement of sway; F80%: 80% of power frequency; ML: medio-lateral direction; RMS: root mean square.
- a Significant data (p ≤ 0.05) at Spearman's Test.
4 DISCUSSION
The aim of this study was to verify the correlation between dopamine depletion and disturbances in gait and postural control in subjects with mild to moderate PD. We found few weak to moderate correlations between these variables. Subjects who were investigated had mild to moderate PD symptoms, and were classified into HY stages 2 and 2.5. In comparison with healthy age and height-matched subjects, they demonstrated significant disturbances in gait spatiotemporal parameters, consistent with prior research (Grabli et al., 2012; Kelly et al., 2012; RoizRe et al., 2010). The same was observed regarding the stabilometric data (Beretta et al., 2015; Schlenstedt et al., 2016).
Although the kinematic gait impairments and postural instability were evident, these disturbances may not correlate with the dopamine depletion, suggesting the involvement of non-dopaminergic pathways. A study states that antiparkinsonian medications enhance performance of certain gait tasks, but substantial impairments still remain (McNeely, Duncan, & Earhart, 2012). Similarly, another study claim that dopaminergic treatment can improve some axial signs but usually does not improve postural instability significantly (Maurer et al., 2003).
A multisystem degeneration and deficiencies in other neurotransmission systems are probable contributors to gait and postural control impairments in PD, like acetylcholine, serotonin, norepinephrine (Maillet, Pollak, & Debû, 2012) and cholinergic dysfunctions (Bohnen & Albin, 2011; Rochester et al., 2012). Bohnen et al. (2013) investigated dopaminergic and cholinergic correlation of gait speed in PD to test the hypothesis that gait disturbances in PD may result from multisystem degeneration. They concluded that cortical cholinergic denervation is a more robust marker of slowing gait in PD than nigrostriatal denervation alone. Another study from Karachi et al. (2010), found that bilateral lesioning of the cholinergic part of the pedunculopontine nucleus (PPN) induced gait and postural deficits in non-dopaminergic lesioned monkeys, revealing that cholinergic neurons of the PPN play a central role in controlling gait and posture. Besides that, gait disturbances relate to the “executive-attention” network. The tissue loss in regions involved in executive function and reduced connectivity in those networks may lead to a dysfunction in the premotor area, inferior frontal gyrus, precentral gyrus and parietal lobe (Herman, Giladi, & Hausdorff, 2013).
Moreover, even though the aim of this study was not to evaluate the upper limbs kinematics, we noticed that subjects included in this study presented disturbances of the upper limbs during gait analyses. Ponsen, Daffertshofer, Wolters, Beek, and Berendse (2008) suggest that in the early stages of PD the upper limbs are more affected than the lower limbs (Ponsen et al., 2008), maybe due to a more expressive dopamine deficiency in the initial stages of the disease.
Our study has certain limitations. The subjects performed the analysis in the off medication state, in a laboratory setting, which may limit the ability to generalize to other contexts. However, to the best of our knowledge, this was the first study to investigate the relationship of PD gait and postural disturbances and DAT density through 99mTc-TRODAT-1 (SPECT-CT). Further research exploring the correlation between gait and postural control impairments with other neurotransmitters, like acetylcholine, and investigating other brain regions are still necessary. Additionally, future studies should include subjects in different stages of the disease, including more severe stages.
In conclusion, kinematic gait impairments and postural instability of individuals with mild to moderate PD may not correlate with dopaminergic depletion assessed through the 99mTc-TRODAT-1 (SPECT-CT).
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST STATEMENT
There are no conflicts of interest to report.
DATA ACCESSIBILITY STATEMENT
Raw data and analysis scripts are freely available from the corresponding author (FC).
AUTHORS CONTRIBUTIONS
All authors have made significant contributions to this article. In particular, research design, analysis and manuscript writing were done by MEPC, AdSP, ASdP, CRdMR, GPS, LHTF, NdSJ and FC; participant recruitment and data collection were done by MEPC, ACDA, ABF, BTP, PSC.




