Activation of the mouse odorant receptor 37 subsystem coincides with a reduction of novel environment-induced activity within the paraventricular nucleus of the hypothalamus
Abstract
Within the main olfactory system of mammals, a unique subsystem exists that is comprised of sensory neurons expressing odorant receptors (ORs) of the OR37 subfamily. These receptors are exclusive for mammals and are highly conserved across species. The mouse OR37 receptor subtypes A, B and C were shown to be activated by the long-chain aliphatic aldehydes pentadecanal, hexadecanal and heptadecanal, respectively. The search for biological sources of these compounds showed that bodily secretions from conspecifics activated the OR37A, B and C glomerulus. At the same time, the activity of cells in a target region of projection neurons from OR37 glomeruli, the paraventricular nucleus of the hypothalamus (PVN), was reduced compared with controls (clean test box). A large number of the activated cells in the PVN of mice that were placed into a clean test box were corticotropin-releasing hormone cells, indicating an induction of the stress axis due to the novel environment. The much lower number of activated cells of mice in a box enriched with bodily secretions from conspecifics indicated a reduced stress response. As bodily secretions from conspecifics activated the OR37 system and simultaneously reduced stress-induced activation of the PVN, it was tested whether the ligands for OR37 receptors could induce this effect. Indeed, a similarly reduced activity in the PVN was found in mice kept in a clean test box and exposed to a mixture of the OR37 ligands delivered via an air stream. These data indicate that the OR37 system may play a role in mediating a phenomenon called social buffering.
Introduction
A wide range of olfactory cues influences multiple behavioral interactions that are essential for survival and highly relevant for social communication, such as recognising mating partners or own offspring, as well as identifying predators and conspecifics. The recognition and discrimination of odorous signals are accomplished by the olfactory system that consists of several structurally and functionally distinct subsystems (for reviews, see Breer et al., 2006; Munger et al., 2009; Ma, 2010). Within the main olfactory system, a unique subsystem exists that is comprised of sensory neurons that express odorant receptors (ORs) of the OR37 subfamily (Strotmann et al., 1992, 1994). OR37 receptors differ from other vertebrate ORs in several aspects. Among others, they are exclusively found in mammals and are highly conserved during evolution (Hoppe et al., 2006). These aspects have led to the hypothesis that OR37 receptors may be specifically tuned to recognise chemical compounds that are particularly relevant for mammals. In the search for suitable ligands it was recently found that OR37 receptors are activated by long-chain aliphatic aldehydes (Bautze et al., 2012). These compounds are not considered to be typical odorants; they are extremely hydrophobic and were never described previously as ligands for any OR. Moreover, it was found that each of the OR37 receptor subtypes selectively responds to a different but very similar aldehyde, which differs only in chain length. Pentadecanal was found to be the best ligand for OR37A, hexadecanal for OR37B and heptadecanal for OR37C (Bautze et al., 2012). The question of whether these compounds may be released by mammals themselves was affirmed recently, demonstrating that hexadecanal, the ligand for OR37B, was present on the surface of mouse feces, apparently deposited by the anal glands (Bautze et al., 2014). The search for biological sources of ligands for OR37A and OR37C is still in progress. In addition to the unique ligand specificity of the receptors, the neuronal pathway for processing olfactory information from the OR37 subsystem is also unique. Olfactory sensory neurons that express one of the OR37 receptor subtypes send their axons to only one glomerulus within the main olfactory bulb and all glomeruli of the various OR37 subtypes seem to be grouped together within the ventral domain of the bulb (Strotmann et al., 2000). In addition, projection neurons of OR37 glomeruli are not wired to the olfactory cortex, as is typical for projection neurons from the main olfactory bulb, but rather seem to be connected to the paraventricular nucleus of the hypothalamus (PVN) (Bader et al., 2012). This finding has led to the suggestion that stimulation of the OR37 subsystem may lead to an activation of neurons in the PVN. To explore whether ligands of OR37 receptors or bodily secretions from conspecifics lead to an activation of the entire OR37 subsystem, we monitored the neuronal activation in parallel at the level of appropriate glomeruli and in the PVN. As a read-out, the expression level of the immediate early gene c-Fos was analysed.
Materials and methods
Experimental animals
In the present study, adult wild-type mice (C57BL6), OR37 transgenic mice that carry a targeted mutation of IRES-taulacZ at the mOR37A, mOR37B, or mOR37C locus (Strotmann et al., 2000), and transgenic mice expressing the red fluorescent protein variant tdTomato in corticotropin-releasing hormone (CRH)-positive cells were employed. Expression of tdTomato in CRH cells was achieved by breeding CRH-IRES-Cre mice (Taniguchi et al., 2011) to Ai9 reporter mice (Madisen et al., 2010). Mice were housed under a 12 h light/dark cycle in groups or individually at the Central Unit for Animal Research at the University of Hohenheim and had access to food and water ad libitum. All mice were transferred to the experimental room at 2–5 days prior to the experiment. During this time, odor donor mice were housed either individually or in groups of three mice, whereas all recipient mice were housed individually. Mice were killed by cervical dislocation and subsequent decapitation. Experiments were carried out in accordance with the Council Directive 2010/63EU of the European Parliament and the Council of 22 September 2010 on the protection of animals used for scientific purposes. The work was approved by the Committee on the Ethics of Animal Experiments at the Regierungspräsidium Stuttgart (V311/14 Phy) and the University of Hohenheim Animal Welfare Officer (T42/10 Phy, T125/14 Phy and T126/14 Phy).
Collection of bodily secretions
Odor donor mice (both sexes) were placed into a closed plastic box (approximately 11.5 cm height × 14 cm width × 29 cm length), either individually or in a group of three (same gender) for 30 min. Single odor donor mice were placed into the box either only once, or they were first habituated to the box six times for 30 min each, twice a day. After 30 min the mice were removed and transferred back to their home cages. All bodily secretions, including urine, feces and others were kept inside the box after the last placement. Subsequently a recipient mouse was placed into the box.
Exposure to novel environment in the presence of bodily secretions
Recipient mice (either males or females) were placed into an unfamiliar closed plastic box (approximately 11.5 cm height × 14 cm width × 29 cm length) for 90 min, either together with a conspecific or alone. When mice were alone, the box was either clean or enriched with bodily secretions from unknown conspecifics. After exposure, mice were killed and dissected for immunohistochemistry.
Exposure to novel environment in the presence of odorants
Recipient mice (either males or females) were placed into an unfamiliar closed plastic box (approximately 9 cm height × 15.5 cm width × 24 cm length) with a connector at one side that allowed connection to compressed air through a plastic tube and small holes on the opposite side that allowed the flow of charcoal-filtered air through the box. Different odorants were used, i.e. pentadecanal, hexadecanal and heptadecanal, the ligands of OR37A, B and C, respectively (TCI Europe). Odorants were dissolved in 1,2-propandiol (Roth) and warmed to 37 °C. A 4 μm solution was prepared to obtain a solution that contained ~10 ng of the aldehydes in 10 μL. Therefore, a 4 μm solution was also employed for control experiments using the odorant benzaldehyde (Sigma). For additional controls, mice were exposed to the solvent alone. The test solutions (10 μL) were spotted on a piece of filter paper (1.5 × 1 cm) that was placed into the plastic tube connected to the test box. After 90 min, the mice were killed and dissected for immunohistochemistry.
Immunohistochemistry
For sectioning, all bones surrounding the olfactory bulb and the nasal turbinates were excised. Specimens were immersed in fixative (4% paraformaldehyde in 150 mM phosphate buffer, pH 7.4, 4 °C) for 15 min on ice. Subsequently, the tissue was cryoprotected by incubation in 25% sucrose in phosphate-buffered saline (PBS) (0.85% NaCl, 1.4 mM KH2PO4, 8 mM Na2HPO4, pH 7.1) overnight at 4 °C. Finally, the tissue was embedded in Tissue Freezing Medium (Leica Microsystems, Bensheim, Germany) and frozen on dry ice. Sections (12 μm) were generated using a CM3050S cryostat (Leica Microsystems) and mounted onto microscope slides (Superfrost slides, Menzel, Braunschweig, Germany). Sections were air dried for 30 min and rinsed in PBS for 10 min at room temperature (approximately 22 °C). All primary antibodies were diluted in PBS/0.3% Triton X-100 containing 10% normal goat serum (Dianova, Hamburg, Germany) for olfactory bulb sections and 10% normal donkey serum (Dianova) for PVN sections and incubated overnight at 4 °C. For immunohistochemistry on olfactory bulb tissue, mouse anti-beta-galactosidase (Promega, Mannheim, Germany) (1 : 2000) and an antibody specifically recognising the N-terminus of c-Fos [rabbit anti-c-Fos; c-Fos(4) SC-52; Santa Cruz Biotechnology, Santa Cruz, CA, USA] (1 : 600) were used. For immunohistochemistry on brain tissue (PVN) we used rabbit anti-c-Fos (1 : 200), mouse anti-vasopressin-associated neurophysin (1 : 50) and mouse anti-oxytocin-associated neurophysin (1 : 100) antibodies (kindly provided by Dr Harold Gainer, NIH, Bethesda, USA). After three rinses for 5 min in PBS, the bound primary antibodies were visualised by incubating appropriate secondary antibodies conjugated to Alexa 488, Alexa 568 (Invitrogen, Karlsruhe, Germany) or Cy3 (Jackson ImmunoResearch) diluted in PBS/0.3% Triton X-100 containing 10% normal goat serum/normal donkey serum for 2 h at room temperature. After washing three times for 5 min, the sections were counterstained with 4′,6-diamidin-2-phenylindole (DAPI) (1 μg/mL, Sigma Aldrich, Schnelldorf, Germany) for 3 min at room temperature, rinsed with H2O and mounted in MOWIOL [33% glycerin, 13% polyvinylalcohol 4-88 (Sigma) in 0.13 M Tris, pH 8.5].
Microscopy and photography
Sections were analysed using a Zeiss Axiophot microscope (Carl Zeiss MicroImaging, Jena, Germany). Fluorescent images were captured using an AxioCam MRm (Carl Zeiss Microscopy, Göttingen, Germany) and the program AxioVision SE64 Rel. 4.9 (Zeiss).
Quantitative analyses
Quantitative analyses of OR37 glomeruli were performed according to Bautze et al. (2012, 2014). For cell counts, the sections were examined with a 40× objective. An OR37 glomerulus was defined as a region of β-galactosidase-immunoreactive neuropil delimited by DAPI-stained juxtaglomerular cells. The c-Fos-immunoreactive and DAPI-stained cells that were immediately adjacent to the OR37 glomerulus (maximum of four nucleus widths from the outer boundary of the axon fibers within the glomerulus) were counted on serial sections. The c-Fos signals were counted when their signal intensity was above background and the signal was colocalized with a DAPI-stained nucleus. The percentage of c-Fos-immunoreactive cells out of the number of DAPI-stained cells surrounding the glomeruli was determined and given as mean ± SD.
For quantitative analyses of the PVN, images of every third section through the structure (a representative series is shown in Fig. S1) were captured using a 20× objective. The extent of the PVN on each section could be determined by DAPI staining and marked with a line using the AxioVision program. The images were exported with this mark and the green channel (c-Fos) only. Because the operator analysing the c-Fos-labeled cells was not blind to the experimental design, an automated counting method using the program ImageJ was employed. A noise tolerance of 20 was used for the automated cell counting. The cell numbers of all analysed sections were summed up and multiplied by 3. For double-labeling experiments with c-Fos and CRH, vasopressin or oxytocin, double-labeled cells were counted and the percentage of vasopressin/oxytocin/CRH-positive cells out of the number of c-Fos-immunoreactive cells was determined and given as mean ± SD. Statistical significance was determined by the Mann–Whitney U-test. Group comparisons were conducted by Kruskal–Wallis anova with post-hoc Mann–Whitney U-tests. Statistical tests were performed by using the SPSS software version 21 (IBM). Statistical significance was quoted by asymptotic P-values and set at P ≤ 0.05 (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
Results
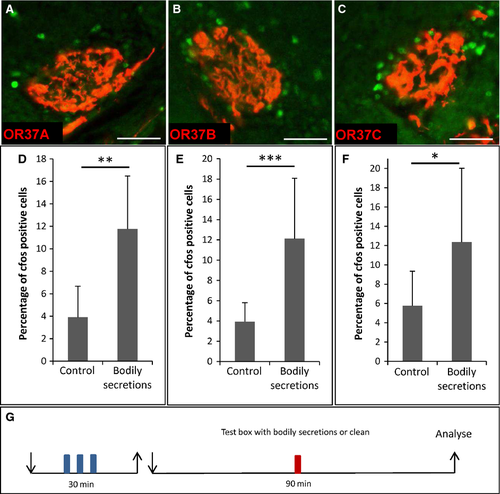
Exposure of mice to bodily secretions from conspecifics activated the OR37A, B and C glomerulus
In order to examine whether mice produce chemicals that activate receptors of the OR37 subfamily (OR37A, B and C), a group of three mice was placed inside a closed plastic box for 30 min (odor donor mice). Subsequently, the odor donor mice were removed and an OR37 transgenic test mouse was placed in this box for 90 min (recipient mouse). During this period the mouse had direct contact with all types of bodily secretions left behind by the conspecifics, such as urine, feces, skin secretions, saliva, etc. To analyse whether exposure to bodily secretions from conspecifics led to an activation of OR37 neurons in the recipient mouse, we employed the previously established activity-measure paradigm, monitoring the upregulation of c-Fos expression in juxtaglomerular cells at receptor-specific glomeruli (Bautze et al., 2012, 2014). It was found that, after 90 min in the ‘contaminated’ box, the number of c-Fos-positive cells surrounding the glomeruli of all three receptor subtypes, i.e. OR37A (Fig. 1A), OR37B (Fig. 1B) and OR37C (Fig. 1C), was increased. This observation was substantiated by determining the number of c-Fos-positive cells of mice exposed to bodily secretions from conspecifics compared with control mice exposed to a clean plastic box. The results are depicted for OR37A in Fig. 1D (bodily secretions: 11.8 ± 4.7%, n = 14; control: 3.9 ± 2.8%, n = 7; P = 0.002; U = 7.5), for OR37B in Fig. 1E (bodily secretions: 12.1 ± 6.0%, n = 25; control: 3.9 ± 1.9%, n = 11; P = 0.0003; U = 24.5) and for OR37C in Fig. 1F (bodily secretions: 12.4 ± 7.6%, n = 11; control: 5.8 ± 3.6%, n = 11, P = 0.035; U = 28.5). These results indicate that the mice produced chemical compounds that activated the three OR37 receptor types OR37A, B and C.
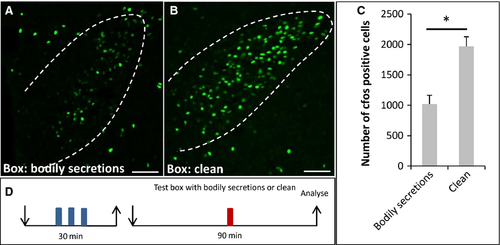
Exposure to bodily secretions from conspecifics induced less c-Fos expression in the paraventricular nucleus of the hypothalamus than an exposure to a clean box
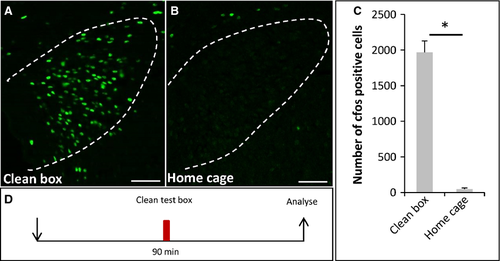
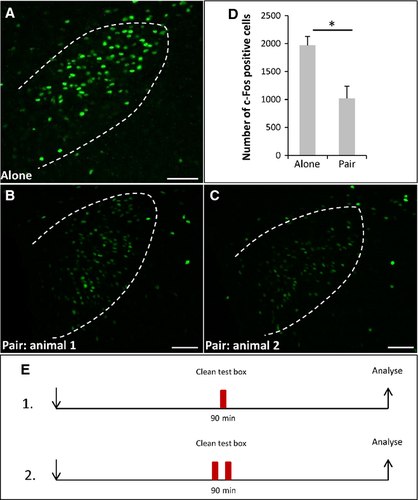
So far, it remained completely elusive as to what functional implication an activation of the OR37 subsystem may have. Therefore, as a next step we examined whether, in recipient mice, a target area in the higher brain centers of projection neurons from OR37 glomeruli was activated. It was previously shown that defined OR37 glomeruli are connected to the PVN (Bader et al., 2012). Examination of the PVN employing c-Fos immunohistochemistry revealed that, in mice exposed to the secretions of conspecifics, c-Fos-positive signals were visible (Fig. 2A). Interestingly, in control mice that were exposed to a clean plastic box without any secretions, the number of c-Fos-positive cells was much higher (Fig. 2B). Quantitative analyses substantiated this observation (Fig. 2C) (bodily secretions: 1020 ± 144.8, n = 4; clean: 1969 ± 159.4, n = 3; P = 0.034; U = 0). This unexpected finding led to the idea that the exposure to a novel, unfamiliar surrounding (termed novel environment or novelty) may be stressful for the mouse and thus induce an activation of neurons in the PVN. The bodily secretions and even the scent from conspecifics may have the potential to mitigate this stress level. A similar phenomenon, termed social buffering, has recently been described for rats (Kiyokawa et al., 2009; Takahashi et al., 2013). If this concept holds true, one would expect that the level of c-Fos expression would be particularly low in mice that stayed in their familiar home cages. Indeed, hardly any c-Fos-positive cells could be detected within the PVN of home cage mice (Fig. 3B; 46.5 ± 17.23, n = 4, Fig. 3C) compared with the novel clean box (Fig. 3A; 1969 ± 159.4, n = 3; P = 0.032; U = 0, Fig. 3C).
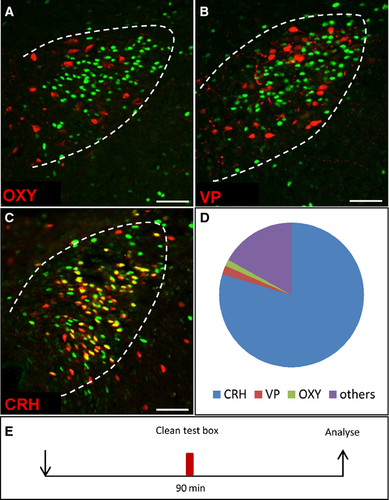
After exposure of mice to a novel box the majority of c-Fos-positive cells in the paraventricular nucleus of the hypothalamus were corticotropin-releasing hormone-expressing cells
An activation of neurons in the PVN is supposed to indicate an increased stress response (Kiyokawa et al., 2007; Takahashi et al., 2013), because the PVN plays a central role in the processing of stress via the hypothalamic-pituitary-adrenal axis. This effect is mainly mediated via CRH. However, in addition to the CRH cells there are several other neuron types in the PVN. Therefore, it was necessary to identify the cell type that was activated by exposure to a novel environment, and double-labeling experiments were performed. Different neuron types of the PVN produce CRH, oxytocin and vasopressin. Oxytocin- and vasopressin-producing cells were visualised by immunohistochemistry with specific antibodies. This was not possible for CRH-expressing cells and therefore these cells were identified in a transgenic mouse line that expresses tdTomato in CRH cells. The results depicted in Fig. 4 clearly indicated that the induced c-Fos signals were rarely colocalized with oxytocin-immunoreactive (Fig. 4A; 1.4 ± 0.3%, n = 3, Fig. 4D) or vasopressin-immunoreactive (Fig. 4B; 2 ± 0.7%, n = 3, Fig. 4D) cells. The great majority of c-Fos-positive cells was found to express CRH (Fig. 4C; 79.7 ± 1.6%, n = 3, Fig. 4D). Furthermore, during the stay in an unknown plastic box, 90.8 ± 1.8% (n = 3) of all CRH cells were activated. These data indicate that an exposure to a novel environment leads to an activation of CRH neurons and suggest an increased activity of the hypothalamic-pituitary-adrenal axis as a stress response.
The presence of conspecifics during exposure to a novel box led to a reduction of c-Fos immunoreactivity within the paraventricular nucleus of the hypothalamus
To investigate whether conditions that are considered as social buffering may affect the stress induced by the novel environment in an unknown box, two mice were placed together in such a box. It was found that in both mice the number of c-Fos-immunoreactive cells (Fig. 5B,C; 1017 ± 219.5, n = 4, Fig. 5D) was reduced by about 52% compared with control mice that were exposed to the novel environment alone (Fig. 5A; 1969 ± 159.4, n = 3; P = 0.034; U = 0). These results imply that conditions of social buffering were active on novel environment-induced stress and suggest that the bodily secretions from conspecifics may be sufficient to elicit this effect.
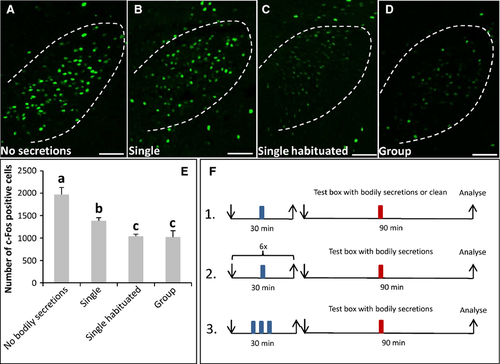
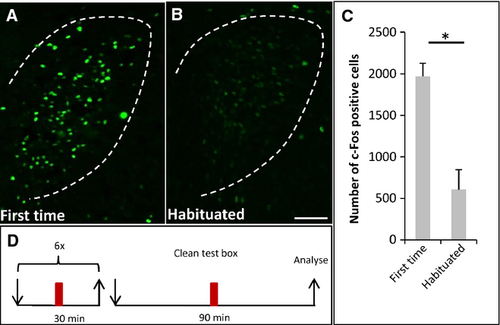
In the previous experiments, the effect of bodily secretions from a group of conspecifics was analysed. This raised the question of whether a group of mice was necessary to release secretions in the right amount and composition to elicit social buffering, or whether one odor donor mouse was sufficient. The results of corresponding experiments revealed that the exposure to bodily secretions from a single odor donor mouse resulted in a 1.4-fold lower number of c-Fos-positive cells (Fig. 6B; 1385 ± 71.6, n = 3, Fig. 6E) than exposure to no secretions (Fig. 6A; 1969 ± 159.4, n = 3; P = 0.05; U = 0, Fig. 6E). There was also a significant difference in the number of c-Fos-positive cells between recipient mice that were exposed to bodily secretions from a single mouse (Fig. 6B; 1385 ± 71.6, n = 3, Fig. 6E) or from a group of conspecifics (Fig. 6D; 1020 ± 144.8; n = 4; P = 0.034; U = 0, Fig. 6E). Thus, bodily secretions from a single odor donor mouse seemed not to be sufficient to induce a reduction of activity in the PVN of the recipient mouse as strongly as bodily secretions from a group of mice. It is conceivable that this was due to the different amounts of compounds produced by one mouse compared with three. Alternatively, a single mouse that is placed into the box for the first time may have a higher stress level than individuals from a group, and may not secrete the crucial compounds. To test this hypothesis, we reasoned that a single mouse that has been habituated to the novel environment several times may begin to secrete the compounds. To prevent an accumulation of secretions and crucial components, the box was cleaned between the different habituation sessions. After the last session, a recipient mouse was exposed to the novel environment with bodily secretions from the habituated single odor donor mouse (Fig. 6C). It was found that the number of c-Fos-positive cells was reduced to a similar extent (1040 ± 46.9, n = 3) as in mice exposed to bodily secretions from a group (Fig. 6D; 1020 ± 144.8; n = 4; P = 1; U = 6, Fig. 6E). This result showed that, even though a single odor donor mouse apparently produced fewer secretions than a group, the bodily secretions from a single habituated odor donor mouse had the potential to reduce c-Fos activity in the PVN of recipient mice similarly. Thus, it seems conceivable that a single mouse that entered a completely novel environment for the first time did not secrete the crucial components, probably because of its elevated stress level. In fact, the number of c-Fos-positive cells in the PVN of single odor donor mice exposed to the plastic box the first time (Fig. 7A; 1969 ± 159.38, n = 3, Fig. 7C) was 3.3-fold higher than in odor donor mice that were habituated to the box (Fig. 7B; 606 ± 239.64, n = 3; P = 0.05; U = 0, Fig. 7C). This result supports the notion that an individual mouse exposed to a novel environment for the first time exhibits a higher stress level than an individual mouse that was familiarised with the box. This difference may contribute to the ability of the respective bodily secretions to reduce the PVN activity.
Exposure to a distinct amount of pentadecanal, hexadecanal and heptadecanal in a novel environment resulted in a low c-Fos level in the paraventricular nucleus of the hypothalamus
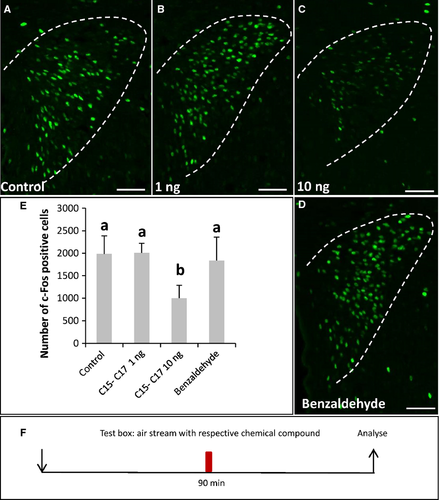
As the bodily secretions that induced a reduction of c-Fos expression in the PVN under a stressful situation also led to an activation of OR37A, B and C glomeruli, the question arose as to whether compounds identified as ligands for OR37 receptors may elicit a reduction of PVN activity. In previous studies we have shown that the OR37 receptor subtypes A, B and C are activated by long-chain aliphatic aldehydes. Each of the three subtypes was found to respond preferentially to an aldehyde with a particular chain length (A, pentadecanal; B, hexadecanal; C, heptadecanal) (Bautze et al., 2012). To examine a possible role of the OR37 subsystem, we exposed recipient mice to the novel environment together with an odor mixture of pentadecanal, hexadecanal and heptadecanal. The odors were applied by air flow so that the mice had no direct contact with the odor source. As controls, the solvent alone and a randomly chosen odorant (benzaldehyde) were applied. Analysis of the c-Fos activity in the PVN revealed that, in mice that were exposed to the aldehyde mixture consisting of 1 ng of each odorant (Fig. 8B), no differences were seen compared with control mice (Fig. 8A).The number of c-Fos-positive cells (Fig. 8E) (control: 1987.7 ± 398.1, n = 7; C15, C16, C17, 1 ng: 2007 ± 213.9, n = 4; P = 1; U = 14) was quite similar. In mice exposed to the 10 ng aldehyde mixture (10 ng of each odorant) in the novel environment, a 50% reduction in the number of c-Fos-positive cells (Fig. 8C; control: 1987.7 ± 398.1, n = 7; C15, C16, C17, 10 ng: 1001.3 ± 287.2, n = 4; P = 0.008; U = 0, Fig. 8E) was observed. After exposure to the control odorant benzaldehyde in a novel environment, the number of c-Fos-positive cells was not reduced as much as after exposure to the 10 ng aldehyde mixture (Fig. 8E; C15, C16, C17, 10 ng: 1001.3 ± 287.2, n = 4, benzaldehyde: 1837 ± 519.2, n = 3; P = 0.034; U = 0). These results indicate that the ligands activating OR37 receptors can reduce an elevated level of activity in the PVN, suggesting that the OR37 subsystem may play a role in social buffering of a novel environment stress.
Discussion
The results of the present study demonstrate that olfactory sensory neurons expressing the receptor types OR37A, B and C are activated by bodily secretions from conspecifics, implying that mice produce compounds that activate the OR37 subsystem. Hexadecanal, the best-known ligand for OR37B, has recently been shown to be deposited together with feces (Bautze et al., 2014). The present study now indicates that pentadecanal and heptadecanal are also produced by mice and released into their environment.
The functional impact of activating the OR37 system is still elusive. However, as mice themselves produce compounds that activate OR37 receptors, it is conceivable that the OR37 system is involved in sensing compounds that are relevant for social communication. This notion is supported by the facts that OR37 receptors are specific for mammals (Hoppe et al., 2006) and that OR37 glomeruli are clustered in a small ventral domain of the olfactory bulb (Strotmann et al., 2000), a region that is thought to play a role in processing socially relevant cues (Schaefer et al., 2001, 2002; Lin et al., 2007).
Although the OR37 glomeruli were strongly activated upon exposure of mice to bodily secretions from conspecifics, a target area for projection neurons of OR37 glomeruli, notably the PVN (Bader et al., 2012), showed only very weak c-Fos expression. Surprisingly, in control mice that were kept in a clean plastic box for the same period, a much higher level of c-Fos expression was found, especially compared with mice kept in their home cage. Thus, a stay in an unknown environment apparently elicited an activation of the PVN that often leads to a stimulation of the hypothalamic-pituitary-adrenal axis as response to a stressful situation. In fact, the level of c-Fos activity within the PVN has recently been used as a measure for the stress level in rats (Kiyokawa et al., 2007; Takahashi et al., 2013).
Most of the cells in the PVN (Swanson & Kuypers, 1980; Biag et al., 2012) that were activated under these conditions were CRH-producing cells; only very few oxytocin cells or vasopressin cells were labeled. Moreover, 90% of the CRH cells were c-Fos-positive, supporting the idea that the elevated c-Fos expression indicates a response to a stressful situation. This view is in line with previous studies, arguing that exposure of mice to a novel environment can cause moderate stress (Abel, 1991; Papa et al., 1993; Emmert & Herman, 1999). Our finding that an exposure to an unknown environment enriched with bodily secretions from conspecifics resulted in weaker c-Fos activity is indicative of a reduced stress response. Evidence of a reduced stress level in the presence of conspecifics has been described previously, e.g. for guinea pigs kept in a novel cage, whose blood level of cortisol was much lower when a conspecific was present (Hennessy et al., 2008). This phenomenon is called ‘social buffering’ (Hennessy et al., 2009) and, as excretions and glandular secretions often contain semiochemicals that animals use for chemical communication (for review, see Apps, 2013), our results indicate that such a social buffering effect is mediated via olfactory cues. This is also in line with previous studies demonstrating that olfactory signals released by conspecifics are sufficient to reduce the typical freezing response of rats to a fear conditioned stimulus (Takahashi et al., 2013), and that social buffering of conditioned fear responses is abolished in rats with a lesioned main olfactory epithelium (Kiyokawa et al., 2009).
At present, it is unknown which chemical compounds emitted from bodily secretions of conspecifics are responsible for social buffering effects. Our observation that bodily secretions from conspecifics in the novel environment reduce activity in the PVN and simultaneously induce a higher activity in the OR37 glomeruli raises the possibility that ligands activating the receptors OR37A, B and C may be able to mediate this effect. In fact, the level of c-Fos in the PVN of mice that are kept alone in a clean box was significantly reduced when the mixture of the OR37 ligands pentadecanal, hexadecanal and heptadecanal was delivered via air flow. Dose–response experiments revealed that as little as 10 ng of each aldehyde exposed to the air stream was sufficient to elicit this effect, demonstrating the extreme sensitivity of the system. The specificity of the effect was supported by the finding that a randomly chosen odorant did not show any effect on c-Fos expression in the PVN. Based on our previous finding that one of the compounds, hexadecanal, is also present in the anal gland secretion of dog, the data imply that the context in which the substances appear is important. This is conceivable when they are detected in combination with species-specific molecules and by this means trigger distinct physiological reactions or behavioral outputs.
The question of how an activation of the OR37 receptors may affect the CRH-expressing neurons in the PVN is currently elusive. We have recently shown that projection neurons originating from the OR37C glomerulus are wired to vasopressin-expressing cells in the PVN (Bader et al., 2012). It is conceivable that their activation triggers a local release of vasopressin that may subsequently act on neighboring cell types. In fact, it is well established that vasopressin-producing cells release this compound locally (Landgraf, 1995; Ludwig et al., 2005). Furthermore, receptors for vasopressin have been shown to be expressed in the PVN (Young et al., 2006). However, it is not yet clear whether they are present on CRH-expressing cells.
Nevertheless, this is the first time that a reaction that seems to be related to a social buffering effect was found to be elicited by identified chemical compounds and this indicates that the OR37 subsystem may play a role in mediating social buffering phenomena.?>
Conflict of interests
The authors declare no conflicts of interest, financial or otherwise.
Acknowledgements
The authors would like to thank Dr Harold Gainer (NIH, Bethesda, USA) for kindly providing the mouse anti-neurophysin–vasopressin and anti-neurophysin–oxytocin antibodies. We thank Professor Johannes Steidle (Institute of Zoology, University of Hohenheim) for his support with the statistical analyses. This study was supported by the Deutsche Forschungsgemeinschaft (grant STR619/5-1).
Abbreviations
-
- CRH
-
- corticotropin-releasing hormone
-
- DAPI
-
- 4′,6-diamidin-2-phenylindole
-
- OR
-
- odorant receptor
-
- PBS
-
- phosphate-buffered saline
-
- PVN
-
- paraventricular nucleus of the hypothalamus




