Clinical Efficacy of Isatuximab Plus Carfilzomib and Dexamethasone in Relapsed/Refractory Multiple Myeloma Patients
Funding: The authors received no specific funding for this work.
ABSTRACT
Isatuximab, a novel anti-CD38 monoclonal antibody, is approved in combination with carfilzomib and dexamethasone (Isa-Kd) in relapsed/refractory multiple myeloma (RRMM) patients. Because of its recent introduction, real-world efficacy and safety are poorly reported. In this Italian multicenter real-life observational retrospective study, efficacy and safety of the Isa-Kd regimen were evaluated in a cohort of 103 RRMM patients. Overall response rate (ORR) was 85%, with stringent (sCR) or complete response (CR) in 18% of cases and very good partial response (VGPR) in 39%. Median PFS and OS were not reached within the study period, while 1-year PFS and OS were 72% and 77%, respectively. Hematological toxicities were observed in 42% of subjects, and cardiac toxicities occurred in 24% of cases. Moreover, we conducted a subanalysis on patients (N = 69) treated with Isa-Kd after one prior line of therapy, showing an ORR of 88%, with sCR + CR in 20% of subjects, VGPR in 46%, and PR in 22% of patients. In this group, median PFS and OS were not reached, while 1-year PFS and OS were 92% and 95%, respectively. In conclusions, our study confirmed Isa-Kd as an effective treatment option for RRMM with a manageable safety profile even in real-life settings.
1 Introduction
Multiple myeloma (MM), a plasma cell (PC) neoplasm, originates from the uncontrolled proliferation of clonal bone marrow PCs and from the overproduction of monoclonal immunoglobulins (M-proteins), leading to their accumulation within tissues and organs and causing end-organ damage [1]. MM predominantly affects older adults, with a median age at diagnosis of 69 years [2, 3]. Prognosis has significantly improved in recent years, due to the introduction of novel therapeutic agents, including new proteasome inhibitors (e.g., bortezomib, carfilzomib [K], and ixazomib), immunomodulatory agents (IMiDs; e.g., thalidomide, lenalidomide, and pomalidomide), and anti-CD38 monoclonal antibodies (mAbs; e.g., daratumumab and isatuximab) used in different combinatorial regimens [4].
CD38, a single-chain type II transmembrane glycoprotein, is an ideal therapeutic target due to its constitutional expression on normal and neoplastic PCs, while it is present at low surface levels on myeloid, lymphoid, and erythroid cells [5]. CD38 exerts several functions, including involvement in lymphocyte-to-endothelial cells through CD31 binding, cell migration, signal transduction, and ectoenzyme activity for regulation of CD31-mediated intracellular calcium mobilization and nicotinamide adenine dinucleotide catabolism [6]. Anti-CD38 mAbs, such as daratumumab and isatuximab, represent effective and well-tolerated anti-MM agents that have rapidly become the backbone of MM therapeutic strategies, even in early lines of treatments [7, 8]. Anti-CD38 mAbs induce tumor killing through multiple mechanisms, including antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), direct cellular apoptosis, complement-dependent cytotoxicity (CDC), and modulation of extracellular ectoenzyme activity [9].
Isatuximab, a novel anti-CD38 mAb, has been approved in combination with the second-generation proteasome inhibitor carfilzomib and dexamethasone (Isa-Kd) for the treatment of patients with refractory/relapsed MM (RRMM). From the prospective, randomized, open-label, parallel-group phase III IKEMA trial (NCT03275285) conducted on 179 patients randomly assigned to Isa-Kd arm and 123 to the control group (KD) [10], Isa-Kd has showed impressive results in terms of median progression-free survival (PFS) (median PFS, 35.7 vs. 19.2 months, Isa-Kd vs. KD) [11], including patients with poor prognosis, and rates of stringent (sCR) + complete response (CR) (44% vs. 28.5%) and negative minimal residual disease (MRD; 33.5% vs. 15.4%) are higher with Isa-Kd without unexpected toxicity [11].
To date, indications about the efficacy and safety of Isa-Kd regimen for the treatment of RRMM in real-life studies are lacking due to its recent approval. In this Italian retrospective observational multicenter study, efficacy and safety of Isa-Kd were investigated in RRMM and in a subgroup of subjects who received Isa-Kd as second-line therapy.
2 Patients and Methods
2.1 Study Cohort
A total of 103 patients from 16 Italian Hematology Units who started Isa-Kd outside clinical trials were enrolled from March 2022 to April 2024. Inclusion criteria were, age ≥ 18 years old; diagnosis of MM according to the 2016 International Myeloma Working Group (IMWG) updated criteria [12]; refractoriness or relapse from one to three previous lines of therapy. High genetic risk MM, lenalidomide refractoriness (progression during treatment or within 60 days from the last dose), and frailty were assessed according to IMWG criteria [12, 13]. This study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines [14], and protocols approved by our Ethics Committee “Campania Sud,” Brusciano, Naples, Italy (prot./SCCE no. 24988). All patients provided written informed consent.
2.2 Treatment
Patients received isatuximab 10 mg/kg intravenously (Days 1, 8, 15, and 22 of the first 28-day cycle; Days 1 and 15 of subsequent cycles), carfilzomib 20 mg/m2 intravenously (Days 1 and 2 of cycle 1) and then 56 mg/m2 (Days 8, 9, 15, and 16 of cycle 1; Days 1, 2, 8, 9, 15, and 16 of subsequent cycles), and dexamethasone 20 mg administered intravenously or orally (Days 1, 2, 8, 9, 15, 16, 22, and 23) until disease progression, unacceptable toxicity, or clinical decision. Laboratory assessments were evaluated at baseline, before starting each cycle to assess disease status and determine when treatment was permanently discontinued. Antibiotic, antiviral, and antifungal prophylaxis were administered according to international guidelines [15, 16].
2.3 Endpoints
Primary endpoint was PFS, defined as the time between Isa-Kd start and the first documented progression. Secondary endpoints were overall response rate (ORR), including sCR, CR, very good partial response (VGPR), or partial response (PR) according to IMWG criteria [17], after 1 month of therapy; best response; overall survival (OS); time to best response; and safety, assessed using the National Cancer Institute's Common Terminology Criteria for Adverse Events version 6.0 (CTCAE v6.0).
2.4 Statistical Analysis
Data were collected in spreadsheets and analyzed using R statistical software (v. 4.0.5; RStudio), SPSS (v. 25; IBM), and Python (v. 3.12.3). Differences between groups were assessed by chi-square, Fisher's, Wilcoxon signed-rank, or unpaired two-tailed t-tests. Kaplan–Meyer, log-rank, and Breslow tests were used for survival analysis, and univariate and multivariate Cox regression models were used to examine effects (hazard ratio, HR) of independent variables on survival. A p value of < 0.05 was considered statistically significant.
3 Results
3.1 Clinical Characteristics at Enrollment
A total of 103 patients (median age, 64 years) were included in this study, with a median follow-up of 12 months at data cutoff. Clinical characteristics are summarized in Table S1. High genetic risk was observed in 39 (38%) and extramedullary disease (EMD) in 17 cases (16%). According to the revised international staging system (R-ISS), 23 patients (22%) were in stage I, 38 (37%) in stage II, 20 (19%) in stage III, and 22 (21%) had no data available. Previous lenalidomide maintenance was reported in 63 patients (61%), with a median duration of 23 months (range, 2–62 months), with < 12 or < 24 months in 21 (20%) and 32 (31%) cases, respectively. Among patients who received lenalidomide, 20 (19%) and 73 (71%) were lenalidomide-exposed and lenalidomide-refractory, respectively.
3.2 Treatment Response and Discontinuation
ORR after one cycle of Isa-Kd was 69% (N = 71), with 3% sCR/CR (N = 3), 16% VGPR (N = 17), and 50% PR (N = 51). The best ORR was 87% (N = 85), with 18% sCR/CR (N = 19), 39% VGPR (N = 40), and 27% PR (N = 28), and with a median time to best response of 3 months (range, 1–20 months) and a median number of cycles of six (range, 1–24). Consolidation with autologous stem cell transplantation (ASCT) was performed in 13 patients (12%). Median PFS was not reached [95% CI, not estimable (NE)], with 1-year PFS of 72%, as well as median OS was not reached (95% CI, NE), with 1-year OS of 77%. Reasons for treatment discontinuation were disease progression (N = 30, 29%), toxicity (N = 5, 4%), death (N = 5, 4%), and consolidation ASCT (N = 6, 5%) (Table 1).
| Characteristics | Overall cohort, N = 103 |
|---|---|
| ORR after one cycle, n (%) | 71 (69) |
| sCR + CR | 3 (3) |
| VGPR | 17 (16) |
| PR | 51 (50) |
| Best response, n (%) | 87 (85) |
| sCR + CR | 19 (18) |
| VGPR | 40 (39) |
| PR | 28 (27) |
| Time to best response, months, median (range) | 3 (1–20) |
| Isa-Kd cycles, median (range) | 6 (1–24) |
| Ongoing therapy, yes, n (%) | 57 (55) |
| Consolidation with ASCT, n (%) | 13 (12) |
| Carfilzomib dose reductions, n (%) | 23 (22) |
| Reasons for treatment discontinuation, n (%) | |
| Progression | 30 (29) |
| Toxicity | 5 (4) |
| Death | 5 (4) |
| ASCT | 6 (5) |
| Number of MM progressions, n (%) | 30 (29) |
| Progression-free survival, median, months (95% CI) | Not reached |
| 1-year PFS (%) | 72 |
| Number of deaths, n (%) | 23 (22) |
| Overall survival, median, months (95% CI) | Not reached |
| 1-year OS (%) | 77 |
- Abbreviations: ASCT, autologous stem cell transplantation; CR, complete response; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
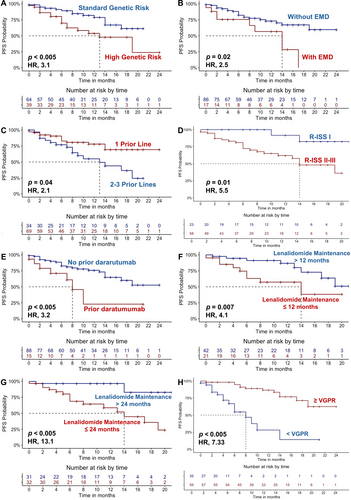
Median PFS was significantly shorter in high genetic risk (13 months, 95% CI, 7.2–18.7) compared with nonhigh genetic risk patients (not reached; 95% CI, NE; HR: 3.1; 95% CI, 1.3–7.1; p < 0.005), as well as in subjects with EMD (14 months [95% CI, 8.5–19.4] vs. not reached [95% CI, NE]; HR, 2.5; 95% CI, 1.1–5.8; p = 0.02), or those treated with Isa-Kd after ≥ 2 lines of therapy versus one prior line (13 months [95% CI, 6.2–21.9] vs. not reached [95% CI, NE]; HR, 2.1; 95% CI, 1.1–4.5; p = 0.04). Similarly, patients with R-ISS stages II and III had shorter PFS compared with stage I (14 months [95% CI, 9.1–18.9] vs. not reached [95% CI, NE]; HR, 5.5; 95% CI, 1.3–23.5; p = 0.01), in patients who previously received daratumumab (8 months [95% CI, 5.4–10.7] vs. not reached [95% CI, NE]; HR, 3.2; 95% CI, 1.3–7.7; p < 0.005) or who started Isa-Kd after MM progression within 12 months after initiation of lenalidomide maintenance (11 months [95% CI, NE] vs. not reached [95% CI, NE]; HR, 4.1; 95% CI, 1.4–11.7; p = 0.007), or within 2 years (14 months [95% CI, 8.1–19.9] vs. not reached [95% CI, NE]; HR, 13.1; 95% CI, 1.8–101.5; p < 0.005). Furthermore, achieving a response < VGPR was associated with very poor outcomes (median PFS, 8 months [95% CI, 4–11.9]; HR, 7.3; 95%CI, 3.2–17; p < 0.005) (Figure 1). In contrast, no differences were observed for patients with severe renal dysfunction (p = 0.7), those undergoing previous ASCT (p = 0.57), lenalidomide-treated versus lenalidomide-naïve subjects (p = 0.93), lenalidomide-refractory versus lenalidomide-exposed patients (p = 0.67), or for patients undergoing a subsequent consolidation with ASCT (p = 0.32).
Similar to PFS, median OS was significantly lower in high genetic risk (18 months [95% CI, 9.7–27] vs. not reached [95% CI, NE]; HR, 2.6; 95% CI, 1.1–6.2; p = 0.02), in patients who had received a previous daratumumab-based treatment (10 months [95% CI, NE] vs. not reached [95% CI, NE]; HR, 2.5; 95% CI, 0.9–6.8; p = 0.04), who achieved a poor-quality response (10 months [95% CI, 6.1–14] vs. not reached [95% CI, NE]; HR, 5.9; 95% CI, 2.5–14.4; p < 0.005). Conversely, subjects with severe renal dysfunction (GFR < 40 mL/min) had a worse outcome (9 months [95% CI, 2.8–15.2] vs. not reached [95% CI, NE]; HR, 4; 95% CI, 1.6–9.9; p < 0.005) (Figure S1). No significant differences were observed for patients receiving previous ASCT (p = 0.06) or consolidation with ASCT (p = 0.4), who received lenalidomide (p = 0.09), who were lenalidomide-exposed versus lenalidomide-refractory (p = 0.07), or those who were treated with Isa-Kd after one versus ≥ 2 previous therapy lines (p = 0.85).
3.3 Toxicity and Infections
Hematological toxicity was observed in 43 patients (42%) with grades I and II in 23 (22%) and grades III and IV in 20 (19%) cases (Table 2). Cardiac toxicity included tachyarrhythmias (N = 4, 3%), grades I and II hypertension (N = 12, 11%), grades III and IV hypertension (N = 3, 2%), heart failure (N = 3, 2%), dyspnea (N = 1, 1%), and pulmonary embolism (N = 3, 2%) (Table 2). Upper airway infections were reported in 14% of cases (N = 15), pneumonia in 13% (N = 14), and bacteremia in 2% of subjects (N = 3). Guideline-based dose reduction of carfilzomib was opted in 22% of patients (N = 23), without impacting clinical efficacy (data not shown).
| Characteristics | Overall cohort, N = 103 |
|---|---|
| Hematological toxicity, n (%) | 43 (42) |
| Grades I and II | 23 (22) |
| Grades III and IV | 20 (19) |
| Cardiac toxicity, n (%) | 25 (24) |
| Tachyarrhythmias | 4 (3) |
| Hypertension, n (%) | 15 (14) |
| Grades I and II | 12 (11) |
| Grades III and IV | 3 (2) |
| Heart failure | 1 (1) |
| Dyspnea | 3 (2) |
| Pulmonary embolism | 1 (1) |
| Upper airway infections, n (%) | 15 (14) |
| Pneumonia, n (%) | 14 (13) |
| Bacteremia, n (%) | 3 (2) |
3.4 Clinical Characteristics and Outcomes of Patients Treated With Isa-Kd as Second-Line Therapy
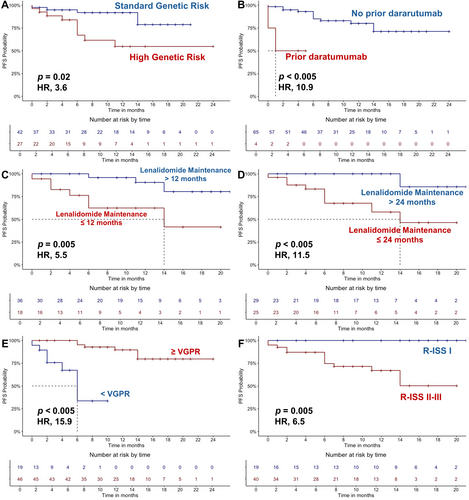
Next, a subgroup analysis of RRMM patients treated with Isa-Kd as second-line therapy (N = 69) was conducted, and clinical and treatment characteristics are detailed in Table S2. In this cohort, ORR after one cycle was 77% (N = 53), with 3% of sCR + CR (N = 2), 23% VGPR (N = 16), and 51% PR (N = 35). The best ORR was 88% (N = 61), with 20% sCR + CR (N = 14), 46% VGPR (N = 32), and 22% PR (N = 15), and with a median time to best response of 2 months (range, 1–20 months) (Table 3). Toxicities are summarized in Table 4. Median PFS and OS were not reached (95% CI, NE), with one-year PFS of 92% and 1-year OS of 95% (Table 3). In this group, high genetic risk negatively influenced PFS, with 1-year PFS of 55% versus 82% in standard genetic risk patients (HR, 3.6; 95% CI, 1.1–11.8; p = 0.02). Prior daratumumab exposure was also significantly associated with worse outcomes (median PFS, 1 month [95% CI, NE] vs. not reached [95% CI, NE]; HR, 10.9; 95% CI, 2–60; p < 0.005), like MM progression within 12 months (median PFS, 14 months [95% CI, 0.1–28.92] vs. not reached [95% CI, NE]; HR, 5.5; 95% CI, 1.4–21.5; p = 0.005) or 2 years (median PFS, 14 months [95% CI, NE] vs. not reached [95% CI, NE]; HR, 11.5; 95% CI, 1.4–91; p < 0.005) after initiating lenalidomide maintenance. Furthermore, poorer PFS was observed in patients who did not achieve a deep response (≥ VGPR) (median PFS, 6 months [95% CI, 3.9–8] vs. not reached [95% CI, NE]; HR, 15.9; 95% CI, 3.9–66; p < 0.005), or with R-ISS stages II and III (median PFS, not reached [95% CI, NE] vs. not reached [95% CI, NE]; 1-year PFS, 67% vs. 100%; HR, 43.7; 95% CI, 1.1–5051; p = 0.005) (Figure 2). No differences in PFS were observed for patients with previous ASCT (p = 0.74), who received a consolidation ASCT (p = 0.67), who were lenalidomide-treated (p = 0.96) or refractory (p = 0.48), or with EMDs (p = 0.9).
| Characteristics | N = 69 |
|---|---|
| ORR after one cycle, n (%) | 53 (77) |
| sCR + CR | 2 (3) |
| VGPR | 16 (23) |
| PR | 35 (51) |
| Best response, n (%) | 61 (88) |
| sCR + CR | 14 (20) |
| VGPR | 32 (46) |
| PR | 15 (22) |
| Time to best response, months, median (range) | 2 (1–20) |
| Isa-Kd cycles, median (range) | 7 (1–24) |
| Ongoing therapy, yes, n (%) | 46 (67) |
| Consolidation with ASCT, n (%) | 10 (15) |
| Carfilzomib dose reductions, n (%) | 18 (26) |
| Reasons for treatment discontinuation, n (%) | |
| Progression | 14 (20) |
| Toxicity | 3 (5) |
| Death | 2 (3) |
| ASCT | 4 (6) |
| Number of MM progressions, n (%) | 14 (20) |
| Progression-free survival, median, months (95% CI) | Not reached |
| 1-year PFS (%) | 92 |
| Number of deaths, n (%) | 14 (20) |
| Overall survival, median, months (95% CI) | Not reached |
| 1-year OS (%) | 95 |
- Abbreviations: ASCT, autologous stem cell transplantation; CR, complete response; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
| Characteristics | Overall cohort, N = 103 |
|---|---|
| Hematological toxicity, n (%) | 32 (46) |
| Grades I and II | 18 (26) |
| Grades III and IV | 14 (20) |
| Cardiac toxicity, n (%) | 18 (26) |
| Tachyarrhythmias | 2 (3) |
| Hypertension, n (%) | 10 (15) |
| Grades I and II | 9 (14) |
| Grades III and IV | 1 (1) |
| Heart failure | — |
| Dyspnea | 2 (6) |
| Pulmonary embolism | — |
| Upper airway infections, n (%) | 7 (10) |
| Pneumonia, n (%) | 10 (20) |
| Bacteremia, n (%) | 1 (1) |
OS was negatively impacted by several factors, including high genetic risk (median OS, 18 months [95% CI, 8.9–27] vs. not reached [95% CI, NE]; HR, 3.9; 95% CI, 1.2–12.4; p = 0.01), previous daratumumab-based treatment (median OS, 5 months [95% CI, NE] vs. not reached [95% CI, NE]; HR, 9.9; 95% CI, 1.9–52; p < 0.005), failure to achieve a ≥ VGPR (median OS, 8 months [95% CI, 3.6–12.3] vs. not reached [95% CI, NE]; HR, 11.1; 95% CI, 3.2–39; p < 0.005), R-ISS stages II and III (median OS, 18 months [95% CI, NE] vs. not reached [95% CI, NE]; HR, 6.5; 95% CI, 1.2–50.9; p = 0.04), and severe renal impairment (median OS, 6 months [95% CI, 0.84–9.1] vs. not reached [95% CI, NE]; HR, 2.2; 95% CI, 1.1–5.9; p < 0.005) (Figure S2). No significant differences were observed in patients with EMD (p = 0.24), previous ASCT (p = 0.64), consolidation with ASCT (p = 0.41), lenalidomide-treated (p = 0.27) or refractory (p = 0.27), and lenalidomide maintenance duration < 12 (p = 0.39) or < 24 months (p = 0.24).
4 Discussion
Clinical history of MM is characterized by remitting–relapsing periods necessitating frequent therapy switch without curative results, even after ASCT. Introduction of novel targeted therapies has significantly improved outcomes of MM patients; however, on the other hand, multidrug resistance and refractoriness have markedly increased as well [6]. Isatuximab is a novel anti-CD38 mAb and has been only recently approved in combination with the second-generation proteasome inhibitor carfilzomib and dexamethasone for treatment of RRMM. To date, real-life data on efficacy and safety of Isa-Kd are lacking, while they are of great interest, as real-world data can give insights on real efficacy of drugs in difficult-to-treat patients who are usually underrepresented in clinical trials. Indeed, inclusion criteria of the IKEMA trial are very strict, as patients with refractory MM to previous carfilzomib-based treatment, serum free-light chain measurable disease only, and ECOG PS > 2, had a contraindication to treatment with dexamethasone, and severe renal or cardiac impairment has not been included in the study [10]. In our real-world study, we confirmed clinical efficacy and safety of Isa-Kd in RRMM patients, with substantial ORR across diverse groups of patients, including those with high genetic risk, EMD, prior exposure/refractoriness to lenalidomide, and exposure to anti-CD38 mAbs. Despite the presence of a difficult-to-treat MM population, our observed ORR of 85% was similar to that reported in the IKEMA trial, reinforcing the robustness of Isa-Kd in a real-world setting, as the high response rates (sCR + CR in 18%, and VGPR in 39%) and a rapid onset of action (median time to best response of 3 months) are indicative of its efficacy even beyond controlled clinical trials. Survival times in our population were shorter than those reported in the 1-year follow-up period of the IKEMA trial, while in line with those reported in longer follow-ups (1-year PFS, 72% vs. > 80%, respectively; and OS, 77% vs. > 90% vs. 59.7%, our study 1-year OS vs. 1-year IKEMA OS vs. 4-year IKEMA OS) [10, 11, 18]. This discrepancy could be related to the inclusion in our study of a larger group of patients with adverse genetic risk, early relapse, EMD, and/or several frailties, compared with selected patients enrolled in clinical trials.
As in the IKEMA study population, we enrolled RRMM patients who received Isa-Kd after one to three prior lines of therapy. Of note, we conducted an analysis on a subgroup of patients treated with Isa-Kd as second-line therapy, to add evidence for the need of redefine therapeutic algorithms in MM, especially in fit patients who had previously undergone ASCT and lenalidomide maintenance. The proportion of patients with only one prior line of therapy was higher in our cohort compared with the IKEMA trial (67% vs. 44%), although the absolute number was similar (69 vs. 79 patients, respectively). As expected, patients with fewer prior treatments experienced greater benefits from Isa-Kd in terms of median PFS compared with those who had undergone 2–3 previous lines of therapy; however, we did not observe a similar benefit in OS, likely due to the impact of subsequent therapies administered after disease progression.
Another important difference with the IKEMA trial is the great clinical heterogeneity of our cohort that included subjects from several Italian centers who were underrepresented or absent in the phase III IKEMA trial. Indeed, a high representation of patients previously exposed to daratumumab, with reduced renal function (GFR < 40 mL/min), short duration of lenalidomide maintenance, who were lenalidomide-exposed or refractory, with EMD, and high genetic risk (including chromosome 1 gain/amplification) were included in this observational retrospective study. Moreover, we confirmed the efficacy of Isa-Kd in MM patients with severe renal impairment [19]. Therefore, our observed efficacy of Isa-Kd in this very heterogeneous while representative of real-life MM population highly confirmed the clinical potential of this combinatorial regimen not only in RRMM but likely also in newly diagnosed MM with poor prognostic factors, as shown (with the addition of lenalidomide) in the Phase III ISKIA trial [20].
Anti-CD38 agents are highly effective in MM because of their direct specific cytotoxic effects against neoplastic PCs and their ability to modulate tumor microenvironment. In physiological conditions, CD38 is expressed at low levels by macrophages, neutrophils, and T cells, and contributes to interferon γ-driven immune suppression, vascular endothelial growth factor receptor 2-mediated angiogenesis, T regulatory cells recruitment, and endothelial adhesion of myeloid-derived suppressor cells and lymphocytes [6, 21]. Conversely, CD38 is expressed at high levels in MM cells. Therefore, pharmacological targeting of this antigen causes direct cytotoxic effects on CD38-expressing neoplastic PCs, and also to modifications in tumor microenvironment and angiogenesis, especially in combination with other inhibitors of angiogenic pathways, such as erlotinib, leading to a synergistic blockade of tumorigenesis [22]. Moreover, isatuximab could downregulate constitutive and inducible T regulatory cells while increasing tumor-specific cytotoxic T cells, suggesting enhanced immunological surveillance against MM cells and promoting microenvironment modulations [23].
Given the high efficacy of anti-CD38 therapies in MM, it is crucial to explore the feasibility of retreatment in patients who have previously received anti-CD38 agents. However, the clinical efficacy of retreatment with different anti-CD38 drugs, such as isatuximab, is still poorly investigated, even though CD38 can be re-expressed on neoplastic PCs after treatment interruption, although at reduced levels [24]. Notably, daratumumab-treated patients are not present in the Phase III IKEMA trial, while they are included in part B of the Phase I and II prospective trial (NCT02514668), and they have received isatuximab as monotherapy, resulting in unpromising outcomes (53.1% of stable disease with a median PFS of 1.6 months) [25]. In particular, patients in this study are heavily pretreated (median of seven prior therapy lines), with their last dose of daratumumab within 24 weeks before starting isatuximab treatment [25]. In contrast, in our cohort, patients were exposed to daratumumab (not necessarily refractory) with the last dose at least 6 months before starting isatuximab in combination with carfilzomib and dexamethasone, and they were not heavily pretreated. Moreover, we showed that prior daratumumab exposure was an independent negative prognostic factor for disease progression and survival. However, further prospective studies with larger sample sizes are needed to clarify the role of anti-CD38 retreatment in patients exposed or refractory to this class of anti-MM agents.
High genetic risk, including chromosome 1 abnormalities, is an independent negative prognostic factor in MM [26]. In the IKEMA trial, high-genetic risk patients are less represented (23.5% of cases), although chromosome 1 abnormalities are 41.9% of patients, and high-risk cytogenetics is associated with PFS disadvantage in KD compared with Isa-Kd-treated patients (HR, 0.724; 95% CI, 0.361–1.451) [27]. In our real-life study, high-genetic risk patients represented 39% of cases, and specifically had a shorter median PFS of 13 months compared with standard risk. Interestingly, we suggested that the presence of EMD could be a confounding factor, by masking the true negative prognostic factor of high genetic risk, often associated with EMD [28]. However, our real-life data highlight that, despite new therapies that have markedly improved clinical outcomes in MM patients, current drugs still do not specifically target genetic abnormalities in neoplastic PCs, thus not modifying pathogenetic mechanisms of disease development and progression. Our findings underline the need for novel therapeutic strategies and earlier intervention with T-cell reconditioning therapies, such as bispecific antibodies or chimeric antigen receptor (CAR)-T cells, and for more specific targeted therapies.
High functional risk MM is defined as an early disease progression within 18 months from treatment initiation and/or within 12 months after frontline ASCT [29], together with a short ineffective lenalidomide treatment, and is associated with poorer outcomes, also after ASCT [30-32]. According to published studies, our high functional risk MM also showed worse outcomes compared with those subjects who did not relapse within the first year after ASCT despite lenalidomide maintenance (p < 0.005; HR, 3.4; data not shown), supporting the evidence that high functional risk MM is an independent prognostic factor. In the IKEMA study, efficacy of Isa-Kd in lenalidomide-exposed/refractory patients with different duration of treatment, including maintenance, has not been reported, while we showed a significant reduction in PFS for patients with diminished sensitivity to lenalidomide maintenance, particularly those with maintenance durations < 12 or 24 months before progression. Conversely, our patients with excellent lenalidomide sensitivity displayed high benefits from Isa-Kd treatment after progression, and no differences were observed between lenalidomide exposure and refractoriness. Finally, the safety profile of Isa-Kd was consistent with those reported in the IKEMA trial, with manageable rates of hematological and cardiac toxicities.
Our study has some limitations: (i) its retrospective real-life nature, limiting the ability to establish clear causality between the Isa-Kd regimen and observed outcomes, despite completeness of recorded data and inclusion of consecutive RRMM patients; (ii) lack of randomization, for controlling all potential confounding variables; (iii) single-country Italian cohort study, limiting generalizability of our findings in countries and regions following other clinical guidelines and with different patients' features and genetic backgrounds; (iv) short follow-up period, as a longer observation could provide more information on late-onset effects, long-term toxicities, and disease progression; (v) assays to eliminate isatuximab interference on conventional serum protein electrophoresis (SPEP) and immunofixation electrophoresis (IFE) were not available in all centers, leading to increased false positive results and lower rates of sCR and CR in favor of VGPR rates [33]; (vi) small sample size for specific subgroup analysis, such as those with EMD or prior treatment with anti-CD38 agents; (vii) evaluation of bone marrow MRD is not available because, outside clinical trials, systematic monitoring is not yet recommended in real-life settings in Italy; and (viii) real-world data, that can underlie some variabilities in treatment administration, patients' compliance, adverse event reporting, and follow-up visits, despite all the centers adhered to Italian and international guidelines in MM management.
In conclusions, our multicenter study corroborates, for the first time, clinical utility of Isa-Kd in RRMM patients in a real-life setting, regardless of clinical characteristics at treatment initiation. This promising efficacy of Isa-Kd poses the question of whether these effective treatments could be used earlier in MM treatment strategies, and whether it is essential to redefine treatment algorithm from the second line of therapy onwards. This rapid onset of efficacy with short time to best response and increasingly high attrition rates with subsequent lines of therapy in MM suggest the use of Isa-Kd already in earlier therapy lines to maximize PFS [34]. However, high genetic risk, previously anti-CD38 exposed patients and EMD could limitedly benefit from this regimen, thus suggesting that a more specific targeted therapy is needed for this group of MM subjects. Moreover, patients with a strong sensitivity to lenalidomide maintenance, such as those who have received VTD induction, could greatly benefit from Isa-Kd combination, thus representing a promising alternative salvage treatment [35]. Future research should focus on integrating newer modalities, such as CAR-T-cell therapy and bispecific antibodies, to further enhance clinical outcomes, and additional prospective real-world studies, including large cohorts of specific patient categories, such as those exposed or refractory to daratumumab, early relapsed MM, adverse genetic risk, and EMD, are essential to validate these findings and optimize therapeutic strategies for RRMM patients.
Author Contributions
D.D.N., C.B., and C.S. designed the study. D.D.N. and V.G. wrote the statistical analysis plan. D.D.N., D.D., D.V., R.F., R.D.P., S.P., F.A., F.R., E.M., E.G., D.R., L.M., M.L.B., G.C., D.E., A.L., G.D.C., B.S., D.M., M.P., E.U., F.F., M.R., M.A., F.T., S.R., A.L., R.B., F.S., A.I., M.S., P.T., M.G.R., M.D.P., A.P.F., L.M., M.C., G.S., M.A., C.M., and F.F. provided the patient data. F.F., M.A., C.C., G.S., A.M.C., G.M., F.P., A.M.R., C.B., and C.S. supervised the study. D.D.N. and V.G. wrote the manuscript. All authors have critically revised the manuscript and approved the manuscript for publication.
Acknowledgments
This research was supported by the Intramural Program of the Department of Medicine, Surgery and Dentistry, University of Salerno, Italy. Open access publishing facilitated by Universita degli Studi di Salerno, as part of the Wiley - CRUI-CARE agreement.
Consent
Patients received informed consent obtained in accordance with the Declaration of Helsinki (World Medical Association 2013) and protocols approved by local ethic committee (Ethics Committee “Campania Sud,” Brusciano, Naples, Italy; prot./SCCE no. 24988).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.




