Long-term haematological response and maintained immunological function after laparoscopic subtotal splenectomy in patients with hereditary spherocytosis
Abstract
Introduction
Subtotal or total splenectomy are recommended in severe and should be considered in intermediate forms of hereditary spherocytosis (HS). Data on laparoscopic subtotal splenectomy (LSTS) in HS patients are sparse.
Methods
Thirty three patients with HS (median age 10.7 years (yrs), range 1.8–15.5) underwent LSTS. Baseline and follow-up investigation included haematological parameters, microscopic analysis of pitted erythrocytes (pitE), and B-cell subpopulations assessed by flow cytometry. Results were compared to those of non-splenectomised HS patients, HS patients after total splenectomy (TS), and healthy individuals.
Results
After LSTS, haemoglobin levels were normalised in all patients. During median long-term follow-up of 3.9 yrs (range 1.1–14.9), only four patients presented mild anaemia. Despite re-growing of the remnant spleen none of the patients required a second surgical intervention. As compared to TS, PitE in LSTS patients were significantly lower and indicated normal to only moderately decreased spleen function. Relative but not absolute IgM memory B-cell counts were reduced in both LSTS and TS patients.
Conclusions
LSTS is effective for the treatment of patients with HS. A small remnant spleen is sufficient to provide adequate phagocytic function and to induce a pool of IgM memory B-cells.
Novelty statements
What is the new aspect of your work?
After laparoscopic subtotal splenectomy, absolute IgM memory B-cell counts are not reduced as compared to controls.
What is the central finding of your work?
Laparoscopic subtotal splenectomy (LSTS) in patients with hereditary spherocytosis is safe and leading to sustained normalization of haemoglobin and decreased relative reticulocyte counts and total serum bilirubin.
What is the specific clinical relevance of your work?
In LSTS patients, phagocytic function of the remnant spleen as assessed by analysis of ‘pitted’ erythrocytes is only mildly to moderately reduced and even normal in a minor group of patients.
1 INTRODUCTION
Hereditary spherocytosis (HS) is the most common congenital haemolytic anaemia in Central Europe; however, with a prevalence of 1:2.500 to 1:5.000, it is considered a rare disorder per definition.1 HS is caused by genetic defects in the protein network located on the inner surface of the erythrocyte membrane, which stabilises the lipid bilayer. The absence or deficiency of the erythrocyte membrane proteins ankyrin (about 50% of cases), band 3 protein or α-/β-spectrin (about 20% each), in rarer cases of protein 4.2, leads to reduced deformability and accelerated degradation of erythrocytes in the spleen.2-4
Splenectomy by removing the main site of red blood cell (RBC) destruction lengthens the RBC life span. According to published guidelines, it is recommended in severe and should be considered in intermediate forms of HS.5 In addition, although meanwhile discussed controversially for mild cases, splenectomy for patients with mild or moderate HS having an indication for cholecystectomy due to symptomatic cholecystolithiasis had been recommended in previous publications.6, 7 However, total splenectomy (TS) is associated with loss of important physiological functions leading to infectious and vascular risks.8-10 To preserve the splenic function, subtotal splenectomy (STS) has been proposed as an alternative to TS. Several series of HS patients who underwent STS have been published and showed that it reduced the haemolytic rate and increased RBC life span while maintaining efficient splenic phagocytic function.11-15 Studies vary with regard to the surgical approach, the size and subsequent growth of the remnant spleen and the need for a later second intervention. The majority of studies comprising a substantial number of patients was performed in patients undergoing open STS. Only very few data on laparoscopic STS (LSTS) had been published to date.16-18
This study in a substantial cohort of HS patients was aimed to reveal data on the efficiency and safety of LSTS, and on its potential to preserve splenic function. Since not only the phagocytic function of the spleen but also its role for B-cell development, antigen presentation and maturation of the immune system is of importance for antimicrobial defence, we assessed both phagocytic function and B-cell populations in patients after LSTS as compared to non-splenectomised HS patients, HS patients after TS and non-HS controls.
2 PATIENTS AND METHODS
2.1 Patients
This single centre study included HS patients treated in our department between 2002 and 2018. Analysis of splenic function parameters and evaluation of clinical and laboratory parameters started after approval of this study by the University ethics committee in 2012. Written informed consent was obtained from all patients and/or their legal representatives.
To assess splenic function, HS patients were grouped according to their splenectomy status: Group 1: without splenectomy (NS), Group 2: after laparoscopic subtotal splenectomy (LSTS) and Group 3: after total splenectomy (TS). Because of the otherwise low number of patients, the latter group also included six affected parents of paediatric HS patients.
Forty five healthy individuals without underlying haematological disease served as controls.
2.2 Laparoscopic subtotal splenectomy
LSTS was performed based on the laparoscopic TS technique described by Schaarschmidt et al. adapted for LSTS by further experience with open partial or subtotal splenectomy19: access to the abdomen was obtained through a paraumbilical arch incision, followed by insertion of an optical trocar and two or three working trocars, after which stepwise mobilisation of the splenic inferior pole and anterior surface is performed. After careful preparation of the splenic hilum, visualisation of the entire splenic vascular tree allows determination of the optimal splenic transection plane. Once this has been defined, temporary clamping of individual lobar vessels is performed using laparoscopic vessel clamps, followed by transection of the short gastric vessels using the biclamp or LigaSure®. The central splenic vessels are then clamped and resected and finally the vessels of the superior pole are also ligated, leaving only a well-perfused inferior splenic pole. The planned splenic transection plane is then coagulated in eight individual steps using laparoscopic radiofrequency thermoablation and then resected using ultrasonic scissors or LigaSure®. The resected splenic tissue is packed into a laparoscopic bag and removed either morcellated through the umbilical access or intact through an additional abdominal incision.
In contrast to many reports on partial splenectomy, the aim of the surgical procedure described above was not to remove a fixed percentage of the spleen (60%–90% as recommended in the literature), but to preserve the smallest possible residual spleen as anatomically defined by sequential clamping of the splenic vessels. The total residual spleen volume should be less than 30 mL. We use the term ‘subtotal’ rather than ‘partial’ for this procedure because the former better reflects the aim of the procedure, as opposed to the more ‘arbitrary’ sounding latter.
2.3 Efficacy assessment
In LSTS patients, abdominal ultrasound was performed before, shortly post-intervention, between month 1 and 12 after LSTS (TP1), and at a later follow-up (TP2; median 3.9 years [yrs] after LSTS; details shown in Table S2). Each spleen remnant was measured in three dimensions, and volume was calculated using the prolate ellipsoid formula (L × H × W × 0.523). Spleen volume was considered normal or increased according to previously published data.20, 21
The effect of LSTS on haemolysis was assessed at these different time points by measuring haemoglobin levels, absolute and relative reticulocyte counts and total serum bilirubin concentration.
2.4 Splenic function
The assessment of residual spleen function included the evaluation of both the phagocytic and the cellular immunological function of the remnant spleen. We assessed the splenic function at different time points (BL = baseline, TP1 = within the first year, TP2 = at last follow-up; details shown in Table S1).
To determine phagocytic function we analysed the presence of pitted erythrocytes in the blood smear (pitE) using differential interference contrast microscopy with Nomarski optics.22
- <2% PitE: normal
- 2%–4% PitE: borderline (marginally increased)
- >4% PitE: clearly increased
- >15% PitE: severe hyposplenia/asplenia
To gain information concerning cellular immunological function of the remnant spleen, we analysed marginal zone (MZ)-like B-cells (CD19+, CD20+, CD27+, IgM+, IgD+) as the spleen specific major fraction of so called IgM memory B-cells by flow cytometry (for details see supplemental methods). For ease of understanding, we use the term IgM memory B-cells in this article, which is used synonymously with MZ-like B-cells in most publications.
Since the composition of B-cell populations and the absolute number of B-cells in the peripheral blood changes with age, we evaluated results for three different age groups (age group 1: ≥5–<10 yrs, age group 2: ≥10–<16 yrs, age group 3: ≥16 yrs).
Selective spleen scintigraphy26 was used in three LSTS patients for the adjustment of blood count and ultrasound results.
Data were evaluated as median with corresponding minimum and maximum values. If two or K independent samples were to be tested, the Mann–Whitney U test or the Krustal–Wallis test was used. All tests have been tested at the significance level alpha p < .05.
Flow cytometry results on IgM memory B-cells obtained in HS patients were compared to those of healthy controls. Since they were not normally distributed, empirical quantiles were calculated from the available data to determine the quantile range comprising 68% of the observed data lies (i.e., 16%—quantile to 84%—quantile).
3 RESULTS
3.1 Patients and procedures
A total of 60 HS patients were included into the study. Group 1 (NS) comprised 13 HS patients without splenectomy (median age 14.1 yrs, range 5.2–27.4). Thirty-three patients underwent LSTS (Group 2; Table 1). Group 3 (TS) consisted of 14 HS patients after total splenectomy (median age 25.6 yrs, range 12.5–55.3).
| BL | TP1 | TP2 | |
|---|---|---|---|
| Median (range) | Median (range) | Median (range) | |
| Age (yrs) | 10.7 (1.8–15.5) | 11.0 (2.3–15.8) | 14.6 (5.4–25.7) |
| Sex (m/f) | 19/14 | ||
| HS Severity | |||
|
9 | ||
|
22 | ||
|
2 | ||
| LSTS + CCO | 13 | ||
| LSTS + CCE | 9 | ||
| Haemoglobin (g/dL) | 10.8 (5.7–13.8) | 14.2 (12–18.5) | 14.1 (11.4–17.6) |
| Relative reticulocyte count (%) | 10.6 (0.7–34.4) | 2.2 (0.8–10.9) | 3.3 (0.8–16.9) |
| Total bilirubin (μmol/L) | 65 (27–194) | 25 (10–82) | 44 (10–147) |
| Spleen volume (mL) | 450 (161–1108) | 30.5 (2–182) | 116 (1–666) |
- Note: HS severity was assessed according to Eber S and Lux SE.4
- Abbreviations: CCE, cholecystectomy; CCO, cholecystotomy; HS, hereditary spherocytosis; LSTS, laparoscopic subtotal splenectomy.
Median follow-up time after LSTS was 3.9 yrs (range 1.1–14.9). Overall, 13/33 LSTS patients had additional cholecystotomy whereas additional cholecystectomy was performed in nine LSTS patients (Table 1). Before intervention, the majority of LSTS patients presented with moderate or severe HS including two patients requiring regular transfusion. Nine patients underwent LSTS despite displaying only mild HS. In those patients, symptomatic cholecystolithiasis resulting from relevant haemolysis led to the need of surgical intervention which then was performed as combined procedure including LSTS (Table 1).
For anatomical reasons, one patient had to shift to laparoscopic TS during the procedure. After LSTS, one patient became secondary asplenic due to inadequate perfusion of the remnant whereas one patient suffered an abdominal trauma with an infarcted splenic haematoma 7 years after LSTS. These patients with secondary spleen loss were evaluated together with TS patients (Group 3) in further analysis of phagocytic and immunological splenic function.
One LSTS patient and one patient with secondary spleen loss suffered from portal vein thrombosis occurring within the first 2 months after intervention. Both patients presented additional prothrombotic risk factors, one had a heterozygous prothrombin 20210G > A mutation, the other a heterozygous factor V Leiden (1691G > A) mutation.
3.2 Efficacy
At presentation within the first year post-intervention (p.i.; TP1; median 3.2 months, range 1–12), all LSTS patients presented with normal haemoglobin (Figure 1). The median haemoglobin increase from baseline was 3.95 g/dL (range 0.8–6.3). In general, haemoglobin was kept stable during long-term follow-up (TP2; median 3.9 yrs p.i., range 1.1–14.9) with a median haemoglobin difference (TP2−TP1) of −0.05 g/dL (range −3.3 to 2.0). At TP2, only 4/31 patients (12.9%) showed haemoglobin mild anaemia with of 0.5–0.9 g/dL below the lower limit of the age-related normal range.
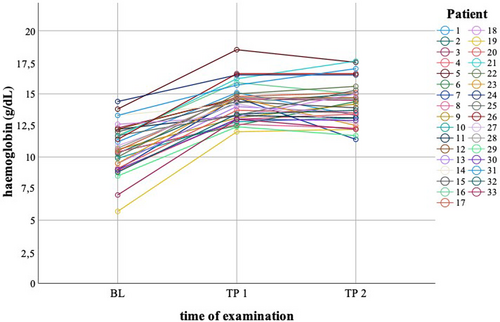
Relative reticulocyte counts were normal (<1.4%) or only mildly elevated (<4.5%) in 25/28 LSTS patients (89.2%) in the first year after LSTS. At late follow-up, 20/31 patients (64.5%) presented normal or mildly elevated reticulocyte counts, 9/31 patients (29%) moderate reticulocytosis (≥4.5–≤9%), and 2/31 patients (6.5%) had high relative reticulocyte count above 9%.
Total serum bilirubin was not analysed in all patients and at every time point. In addition, we excluded patients with Gilbert syndrome from the analysis: for the remaining patients, the course of median bilirubin paralleled that of relative reticulocyte counts (Table 1).
During follow-up, three patients after combined LSTS and cholecystotomy and one patient after isolated LSTS were diagnosed with new cholecystolithiasis. One of the patients after previous combined procedure underwent a second cholecystotomy and decided to combine this with TS despite normal spleen remnant size and normal haemoglobin at that time. Like the patients with secondary spleen loss this patient was included into Group 3 for the assessment of spleen function.
Before LSTS (BL), all except two patients presented with splenomegaly with a median spleen volume of 478.5 mL (range 161–1108). The median remnant spleen volume p.i. (TP0) was 30.5 mL (range 2–182) with less than 30 mL in 15 patients (Figure 2; Table S2). At first follow-up p.i. (TP1; ≤ 12 months p.i.) the remnant spleen was stable in the majority of patients. Secondary spleen loss occurred in two patients (Figure 2: patient 18, 24). During the later follow-up (TP2; median 3.9 yrs, range 1.1–14.9), the median residual spleen volume was 116 mL (range 1–666). Eight patients presented relevant splenomegaly at last follow-up visit (median 10.3 yrs, range 5.1–14.9; Figure 2: patient 5, 8, 11, 12, 13, 27, 29 and 33). So far, none of these patients required secondary TS (or LSTS) because of haemolysis, and only two of these patients presented with mild anaemia (Table S3, patient 8, 12).
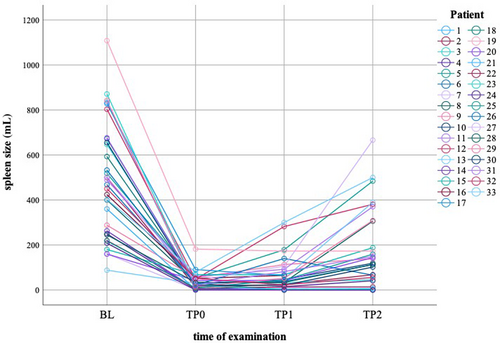
In three patients, selective spleen scintigraphy was performed and showed a homogeneous accumulation of marked RBCs in the remnant spleen. Duplex ultrasound confirmed a corresponding adequate spleen perfusion. All patients showed age-appropriate Hb values at the time of analysis.
3.3 Spleen function
3.3.1 Analysis of pitE
As expected, median pitE of HS patients at late follow-up (TP2) after LSTS or TS were higher as compared to healthy controls or HS patients without splenectomy (NS; p < .001; Figure 3A).
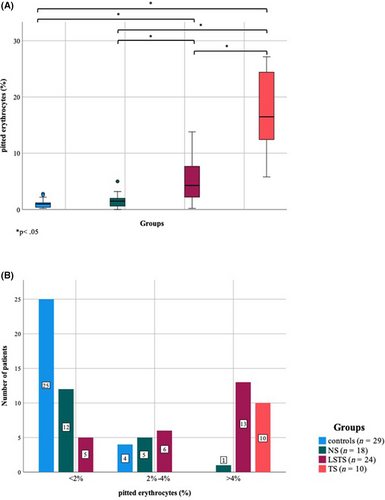
All control individuals and all except one NS patient presented pitE <2% (Figure 3B).
Five LSTS patients (20.8%) had less than 2% pitE, 6/24 patients (25%) showed pitE from 2% to 4% and 13/24 patients (54.2%) presented pitE above 4%, but <15%. In contrast, all TS patients showed pitE above 4% with 6/10 patients (60%) presenting >15% pitE. Patients who also underwent selective spleen scintigraphy had pitE values of 0.2%, 3.9% and 5.3%.
3.3.2 Analysis of IgM memory B-cells
Surprisingly, the absolute number of IgM memory B-cells (MZ-like B-cells) at late follow-up did not differ significantly among the four different groups (Figure 4B).
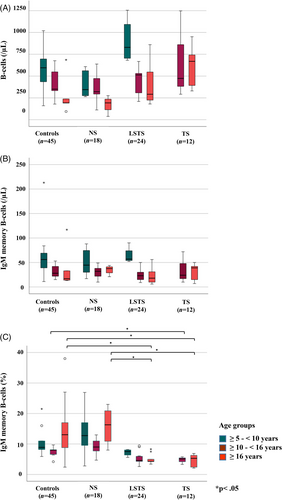
However, different results were obtained regarding the relative percentage of IgM memory B-cells particularly in older age groups (Table 2, Figure 4C).
| Age group | Absolute IgM memory B-cell count (cells/μL) | Relative IgM memory B-cell count (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | NS | LSTS | TS | pa | Controls | NS | LSTS | TS | pa | |
| Age group 1 (≥5–<10 yrs) | ||||||||||
| n | 9 | 8 | 4 | 9 | 8 | 4 | ||||
| Median (min–max) | 56 (11–213) | 44.5 (17–88) | 57 (52–90) | .585 | 8.9 (5.9–21.5) | 12.8 (2.8–26.9) | 7.6 (5.6–8.2) | .055 | ||
| 16% Q | 6.380 | |||||||||
| 84% Q | 18.200 | |||||||||
| Age group 2 (≥10–<16 yrs) | ||||||||||
| n | 9 | 6 | 11 | 3 | 9 | 6 | 11 | 3 | ||
| Median (min–max) | 28 (15–53) | 32 (10–49) | 23 (9–50) | 24 (10–72) | .532 | 8 (4.2–9.7) | 9 (4.7–13) | 4.6 (2.6–9.4) | 5.1 (3.3–5.8) | .019* |
| 16% Q | 5.520 | |||||||||
| 84% Q | 9.460 | |||||||||
| Age group 3 (>16 yrs) | ||||||||||
| n | 18 | 4 | 9 | 9 | 18 | 4 | 9 | 9 | ||
| Median (min–max) | 17 (13–117) | 37.5 (21–44) | 18 (6–56) | 39 (7–50) | .454 | 13.1 (2.4–38) | 16.3 (8–23) | 4.3 (3.4–8.3) | 7.1 (2.6–9.2) | <.001* |
| 16% Q | 6.768 | |||||||||
| 84% Q | 19.880 | |||||||||
- a Kruskal–Wallis-test.
- * p < 0.05 (significant).
In LSTS patients at age ≥ 10–<16, median relative IgM memory B-cell count did differ significantly from controls and NS patients with 6/11 patients (54.5%) presenting relative values below the 16% quantile (Figure 4C). Relative values in TS patients in this age group were also significantly lower than those of controls with 2/3 values below the 16% quantile (Figure 4C).
In patients ≥16 years, both LSTS and TS patients presented a significantly lower relative IgM memory B-cell count as compared to controls and NS patients (Figure 4C). Accordingly, 7/9 LSTS patients (77.7%) and all examined TS patients had relative IgM memory B-cell values below the 16% quantile. In parallel to the decrease of relative IgM memory B-cells counts, the total number of B-cells after LSTS and TS increased (Figure 4A). This was associated with an increased fraction of Transitional and Naive B-cells (details shown in supplemental Figure S1).
3.3.3 PitE and IgM memory B-cells
In the group of LSTS patients, 5/13 patients with relative IgM memory B-cells below the 16% quantile presented pitE 2%–4%, with additional 7/13 showing pitE >4% (Table S4). Thus, 92.3 percent of patients with relative IgM memory B-cells below the 16% quantile had borderline or increased pitE. In the TS group, all patients with relative IgM memory B-cells below the 16% quantile presented pitE >4% including the six patients with pitE >15%. There were two patients in whom only relative IgM memory B-cell analysis but not pitE measurement was performed, in one of them relative IgM memory B-cell count was <16 Q.
Five of seven LSTS patients with pitE 2%–4% and 7/12 patients with pitE >4% had relative IgM memory B-cells below the 16% quantile (Table S4). Thus, 63.1 percent of LSTS patients with borderline or increased pitE presented relative IgM memory B-cells below the 16% quantile. Among TS patients (all with pitE >4%) only one patient did present normal relative IgM memory B-cells.
During the observation period, none of the patients suffered from severe infection by encapsulated bacteria. All patients were regularly vaccinated according to guideline recommendations.
4 DISCUSSION
For decades, splenectomy has been established as standard of care for patients with moderate and severe HS. Since total splenectomy (TS) is associated with loss of important physiological functions leading to infectious and vascular risks, the role of partial, subtotal or near-total splenectomy has increased remarkably during the last two decades. So far, only very few studies on the use of a laparoscopic approach for subtotal splenectomy (LSTS) exist. However, particularly in patients undergoing parallel cholecystectomy this approach may have major advantages considering the necessary surgical access route, subsequent wound healing, and mobilisation after the intervention. This may be even more important for paediatric patients who tend to be physically very active soon after surgical procedure. In addition, subtotal splenectomy is advantageous in the case of an indication before the age of 6 years.
One major concern regarding partial or subtotal splenectomy is the possible need for later secondary TS or second STS. Such intervention may be necessary if a growing remnant spleen leads to loss of haematological response or other problems like adhesive ileus, abdominal pain or severe discomfort. In previous studies using open surgical access for STS the rate of secondary TS reached up to 40%.15, 27-29 It has been shown that growth of the remnant and the need for secondary intervention depends on the remnant spleen size after STS. This also explains the excellent results of open near-total splenectomy with a very low secondary resection rate.30 Thus, any surgical intervention for STS has to aim to reach a low secondary resection rate by minimising the remnant spleen after STS. Fortunately, despite remnant growth in all patients in our cohort, none required secondary intervention for clinical reasons. Considering that eight patients presented with remarkable splenomegaly at last follow-up it cannot be excluded that any of them may require secondary TS in the future. Nevertheless, LSTS results in our cohort regarding remnant growth are at least comparable or even better than those previously reported in patients with open STS.17, 18, 26-29 Fittingly, in another study on a cohort of 16 patients, none developed splenic regrowth within a mean follow-up period of 34.6 months after LSTS.16
This positive outcome is also reflected by the overall efficacy of LSTS. It was successful with regard to haemoglobin normalisation and reduction of haemolysis in all patients. During follow-up, only four patients developed very mild anaemia which was not compromising daily activities of the patients. Four of 23 patients who had not been cholecystectomised were diagnosed with new cholecystolithiasis during follow-up with one of them undergoing a second cholecystotomy in combination with TS. Thus, the rate of cholecystolithiasis after LSTS is lower than that reported in the largest published cohort of STS patients with up to 36 percent of patients being affected by this long-term complication.27
Particularly in the background of the need to keep the remnant spleen as small as possible, the major objective of performing splenectomy not in total—that is, preservation of immune function—is of particular interest. Actually, in near-total splenectomy with the smallest remnant after the procedure, the assessment of pitted erythrocytes showed a remarkably high percentage of pitE up to values usually observed after TS in a substantial number of patients.30 In contrast, in the largest cohort of ‘classic’ open STS with a remnant volume around 30 mL or higher all examined patients presented with normal pitE.27, 31 In our cohort, almost half of the LSTS patients presented normal (<2%) or borderline (2%–4%) pitE values, and none of the patients had pitE >15% indicating severe hyposplenia.23 Thus, relevant phagocytic function is preserved in LSTS patients.
MZ B-cells are formed and stored in the marginal zone of the spleen. Weller et al.32 were able to demonstrate that MZ-like B-cells in the periphery correspond to the MZ B-cells in the spleen. Kruetzmann et al.33 showed that circulating IgM memory B-cells mirror the presence of functional splenic tissue. MZ-like B-cells as the major fraction of total IgM producing B-cells are primarily generated in the spleen.31, 33 Accordingly, after subtotal or total splenectomy a specific reduction in IgM memory B-cells was demonstrated.33 For this reason, we used the IgM memory B-cells as marker for the immunological function of the spleen. Surprisingly, we did not find a difference in absolute IgM memory B-cell counts between different patient groups and controls. One possible explanation are other possible storage locations for those cells outside the spleen. Cameron et al.34 showed that after splenectomy these cells initially decrease with a latency of 6 months, but then remain stable at a low level. As also described by Good-Jacobson and Tarlinton,35 a slow turnover rate and thus a long lifespan in the periphery are possible explanations for this phenomenon. In our study this seems particularly relevant for the group of patients aged 5–10 years (age group 1) in whom B-cell population analysis was performed rather early during follow-up. Regarding patients after TS it has also to be considered that this group included affected parents of paediatric HS patients. These adult patients had not been systematically examined for the exclusion of ectopic splenic tissue, that is, ancillary spleens.
In contrast to the results on absolute IgM memory B-cell counts, relative IgM memory B-cell fractions in LSTS and TS patients ≥10 years were lower as compared to NS patients and controls.
These results might be explained by an increased B-cell production and release from the bone marrow in response to spleen size reduction or removal. Then, subsequent maturation of a certain proportion of these B-cells in remnant spleen tissue leads to a normal total count of these cells although this proportion of maturating cells is lower than normal due the reduced splenic germinal centre and marginal zone capacity. Indeed, in accordance with this possible mechanism, the percentage of Transitional and Naive B-cells is increased in patients after TS and LSTS.
Finally, it remains unclear whether the observed differences in relative IgM memory B-cells counts have any physiological relevance. Nevertheless, the presence of a substantial and even normal absolute number of such cells in patients after LSTS indicate preserved cellular immune function.
4.1 Limitations
The major objective of this study was to demonstrate that LSTS is an adequate approach to treat patients with HS with regard to efficacy and safety.
To obtain data for such a relevant cohort of HS patients undergoing spleen surgery, a very long period of time was necessary. This has the advantage for rather long follow-up for many of these patients but, on the other hand, leads to the corresponding, profoundly varying follow-up times.
LSTS was performed as part of routine clinical care for HS patients but not in the framework of a systematic clinical trial aimed to investigate LSTS in comparison to any other surgical method.
To evaluate parameters reflecting splenic function, we had to use available cohorts of HS patients without splenectomy or after total splenectomy as comparators. Since LSTS was part of our general treatment strategy in HS patients, the number of paediatric patients with TS was limited. Therefore, we also included affected parents who themselves underwent TS many years before. This, of course, leads to a limited direct comparability between the LSTS and TS group considering the very different patients' age. However, with healthy control individuals for all ages it was possible to evaluate splenic function parameters in all treatment groups as compared to controls.
As this was not a planned clinical trial, all follow-up visits were performed as part of the regular clinical care for the patients. Therefore, the exact time point at which any of the examinations was performed depended on many influencing factors including such from the patients' and families' side.
For the analysis of splenic function parameters, another confounder might be the fact that these analyses were implemented only about 10 years after the first LSTS. Therefore, the time point of these analyses after LSTS varied depending on the time of intervention. This, of course, also concerned the majority of TS patients.
Other potential advantages of LSTS as compared to open access surgery like for example faster recovery and wound healing after the procedure, and shorter stay in the hospital could not be addressed. The majority patients underwent LSTS in a paediatric surgery centre situated in a very long distance from their hometown. The fact that after discharge they had to move home will potentially have influenced the length of the stay in the clinic and, on the other hand, hampered a systematic follow-up evaluation until full recovery by the responsible surgeon.
Despite these limitations, the results allow and support the conclusion that LSTS for the treatment of patients with moderate and severe HS is effective and safe, and it can be combined successfully with cholecystectomy. According to the results of pitE and IgM memory B-cell analysis, a relevant part of splenic function may be preserved using this treatment approach. Long-term data are necessary to confirm sustainability and potential positive effects on the reduction of vascular risks associated with TS.
AUTHOR CONTRIBUTIONS
Alica L. Münch, Eva-Maria Jacobsen, Ansgar Schulz, Lisa Schiefele and Holger Cario contributed substantially to the design of the work, data acquisition and analysis. Wolfgang Loichinger, Tobias Wowra, Julia Elsner, Mike-Andrew Westhoff, Alexandre Serra, Gabriele Strauss and Klaus Schaarschmidt contributed substantially to data acquisition and analysis. Alica L. Münch and Holger Cario drafted the manuscript. All authors critically revised the manuscript and finally approved the version to be published. They agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
This study was supported in part by research funding from a MLP Medical Excellence Grant to Alica L. Münch. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to disclose.
INFORMED CONSENT
Informed consent was obtained from all participants included in the study.
Open Research
DATA AVAILABILITY STATEMENT
Requests for background and details of clinical and laboratory data underlying the results of this study can be addressed to the corresponding author.




