Differences in treatment and monitoring of chronic myeloid leukemia with regard to age, but not sex: Results from a population-based study
Abstract
There are established guidelines for treatment and monitoring of chronic myeloid leukemia (CML) but little is known about routine care. Data on ICD-10 codes as well as prescribed medications were available for 10.5 million patients in the statutory health insurance system in Bavaria for the years 2010 to 2016. Also, data on the molecular and cytogenetic monitoring were integrated. A total of 1714 adult patients with CML were observed. Only 50.8% received more than 67.5 daily doses per quarter year (target: 91.5) while 18.2% did not receive any tyrosine kinase inhibitor (TKI). The median number of daily doses was at least 80 doses per quarter year for all age groups in men, but decreased to 62 doses in elderly women. With this exception, no differences between men and women were observed. The percentage of patients without any TKI increased with age. The median number of molecular examinations was 3.54 independent of age and sex. Even in a highly developed country, still a considerable number of patients with CML seem to not receive adequate treatment, whereas molecular monitoring can be considered satisfactory.
1 INTRODUCTION
For one and a half decade, tyrosine kinase inhibitors (TKIs) have been the standard treatment for Philadelphia chromosome-positive, BCR-ABL1–mutated chronic myeloid leukemia (CML). This story of success had started with the advent of imatinib, the first TKI. In Europe, there are now five different TKIs. Three of them, imatinib, dasatinib, and nilotinib, can be used as first-line treatments, while bosutinib and ponatinib are only approved as second-line treatments.
Treatment recommendations suggest the lifelong daily use of TKIs for the vast majority of patients.1 However, TKI treatment has its price; with about 40 000-70 000 Euros per patient and year, the costs are considerable. Meanwhile, patient protection for imatinib has been expired in Germany in 2016 and generic imatinib became available.
There is no evidence that elderly patients of 65 years and older should not receive TKIs. Recently, Proetel et al2 have shown in a large trial that imatinib is effective and well tolerated in all age groups and that there is no reason to withhold imatinib from the elderly. Similar results had already been published by others.3-5 Latagliata et al6 performed a retrospective study on patients older than 75 years, an age group often excluded from clinical trials. They concluded as well that imatinib should be administered to all patients in chronic phase regardless of their age. These findings are further confirmed by the prognostic EUTOS score, which does not include age as a relevant factor for predicting complete cytogenetic remission and progression-free survival.7
An obviously important requirement for an effective treatment is that the treatment is used as indicated. Although CML is a fatal disease, poor patients' compliance with TKI treatment is common and is associated with reduced effectiveness.8-11 Marin et al, using an electronic medication monitoring system, showed that proper adherence with TKI treatment is a major predictor of achieving major molecular remission (MMR) with relative risks between 11.7 and 17.6.12 A patient can however only be fully compliant if there is a sufficient supply of medication, that is, if the physician regularly hands over a prescription for TKIs.
A regular cytogenetic and/or molecular monitoring has been considered necessary for a successful treatment and a prolonged survival.13-15 However, it has been shown that at least in the United States, the number of molecular examinations is far away from the guidelines' recommendations.16
Thus, the aim of our study was to evaluate whether the prescribing of TKIs and the subsequent monitoring of patients with CML in Germany follow the current European LeukemiaNet (ELN) recommendations and whether prescribing is sufficient to allow for a satisfactory patients' compliance. Notably, we were interested in the question, if the prescribing patterns were different between older and younger patients and the sexes. Additionally, we wanted to find out whether the number of molecular and cytogenetic assessments followed the ELN recommendations.
2 METHODS
The Bavarian Association of Statutory Health Insurance Physicians (Kassenärztliche Vereinigung Bayerns, KVB) covers the outpatient care of 84% of the Bavarian population of 12.5 million. It holds claims data of all bcr-abl–positive CML diagnoses and prescribed medications within the statutory health insurance system with 10.5 million people. For our analyses, data from the years 2010 to 2016 were used consisting of datasets on a quarterly base. Only patients with at least two recordings of CML and a CML-related treatment (TKI, interferon, hydroxyurea, busulfan) in two separate quarters were included. The identification of CML cases was done via the ICD-10 code C92.1, which represents the code for bcr-abl–positive CML. Details on the identification have already been described elsewhere.17 For all these patients, information on reimbursed, that is, handed out, medication was available. As the data are intrinsically used for accounting purposes, completeness can be assumed.
All analyses on daily doses of TKIs are based on the dosages as approved by the European Medicines Agency (EMA). These recommended doses 18-22 which are identical to the ELN1 recommended doses can be found in Table 1. These standard dosages are the dosages that are reimbursed by the sickness funds without any problems. As the dosage for children is calculated according to their body surface area,23 we excluded persons below 20 years (n = 4) from the analysis.
| Recommended dose | |
|---|---|
| Imatinib | 400 mg/d |
| Dasatinib | 100 mg/d |
| Nilotinib | 2 × 300 mg/d |
| Bosutinib | 500 mg/d |
| Ponatinib | 45 mg/d |
As there were carried out a few TKI discontinuation trials after 2012, we performed a sensitivity analysis to account for this. It was not possible to identify those patients; thus, the median number of TKI doses per quarter year was recalculated after randomly removing 20 percent of the quarter years without any TKI admission.
Data on the frequency of cytogenetic evaluations, FISH, and PCRs were provided by the Munich Leukemia Laboratory (MLL). The MLL is the largest diagnostic laboratory in the field of leukemia and lymphomas in Germany and has information on the monitoring of about 5000 patients with CML. Data were used from 2010 to 2016 too. The MLL holds data on all analyses performed there in this time frame as well as the date of diagnosis and death, allowing to estimate the monitoring frequency. It has to be stated that while the KVB data represent a complete survey of the Bavarian population within the statutory health system, the MLL data represent a sample of patients being examined at a specific laboratory. Due to data privacy regulations, it was not possible to merge both datasets or to link them to any survival data. As, for example, patients without any examination can naturally not be part of the MLL dataset, it might be seen as a positive selection. Therefore and because of the large sample size, no significance tests, that is, no P-values, were calculated.
All Analyses were performed with R 3.1.0 or SAS 9.4, and figures were created with SPSS 22.
3 RESULTS
3.1 Tyrosine kinase inhibitors
In total, 2013 different CML patients were identified between 2010 and 2016 in the KVB data. Of those, 1714 patients were observed for more than four quarter years. The number of patients grew from 1067 in 2011 to 1351 in 2015, indicating a rising prevalence due to considerably longer survival times.
During the 6 years analyzed, 1 887 181 daily doses of TKIs, as defined in Table 1, had been handed out by pharmacists to patients of age 20 and older. The majority of TKI doses were imatinib with 1 379 515 daily doses (73%), followed by nilotinib with 315 321 daily doses (17%) and dasatinib with 185 662 daily doses (10%). These drugs were available for the complete observation period. For ponatinib and bosutinib, only 4133 resp. 2550 daily doses were recorded within the 6 years observed. The low numbers for both are not surprising, since both had not been approved before 2013 and are second-line TKIs, only to be prescribed if first-line TKI had failed.
At first, we analyzed the number of doses prescribed per day. By restricting this analysis to patients whose CML was reported for at least four subsequent quarter years, we ensured that newly diagnosed patients who were still in their pre-TKI run-in phase were not considered. Further, this restriction reduces the influence of short-term effects.
A total of 1714 patients fulfilled this criterion and were observed between 2010 and 2016. We identified 311 patients with CML (18%) who did not get any TKIs. A total of 135 patients (8%) received TKIs but less than 22.5 daily doses per quarter year on average, about 25% of the recommended dose. A total of 168 patients (10%) received between 22.5 and 45 daily doses and 227 (13%) more than 45 but less than 67.5 daily doses per quarter. Only 873 patients (51%) got more than 67.5 daily doses, thereof 61 (4%) with more than 100 daily doses per quarter year. The latter are likely to be patients that obtained a high-dose (imatinib) therapy, receiving, for example, 600-800 mg/d. The maximum were 160 daily doses imatinib per average quarter year. The average daily doses per quarter year are shown in detail in Figure 1. A total of 652 patients received any second-generation TKI (38.0%). Out of the 311 patients that did not receive any TKI, 288 (92.6%) were treated with hydroxyurea, and only eight patients (2.6%) received interferon.
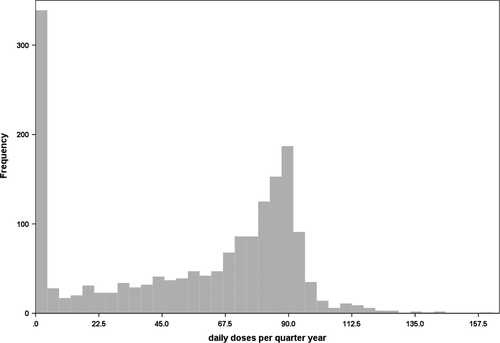
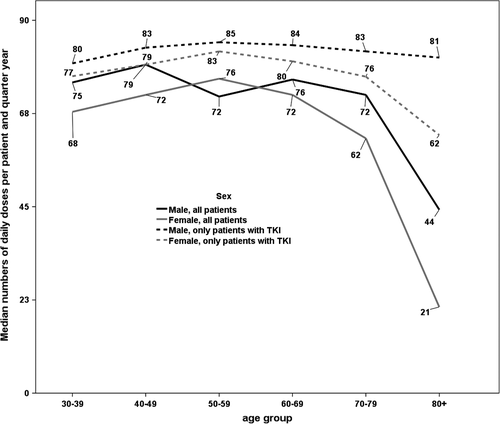
Table 2 provides an overview across the age groups. When considering only those patients that actually had received any TKIs in that quarter year, the median number of daily doses was quite constant for males with 80 to 85 doses. In contrast, for women we observed a slight increase until 50-59 years from 75 to 83 doses per quarter year and a decrease afterward. Women of 80 years and above received only 62 daily doses at median per quarter year. The details can be found in Figure 2. When taking all patients into account, the decrease in the elderly was much stronger.
| Number of patients in this age group | Observed quarter years in this age group | Number of daily doses (total) in this age group | ||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| 20-29 | 31 | 29 | 354 | 334 | 24 482 | 17 551 |
| 30-39 | 65 | 54 | 849 | 618 | 60 900 | 36 537 |
| 40-49 | 153 | 149 | 1810 | 1673 | 122 716 | 110 021 |
| 50-59 | 219 | 212 | 2772 | 2470 | 198 515 | 156 543 |
| 60-69 | 271 | 277 | 3088 | 3473 | 195 611 | 216 579 |
| 70-79 | 345 | 325 | 4075 | 4070 | 247 533 | 224 495 |
| 80 and older | 168 | 198 | 1669 | 2150 | 75 368 | 78 963 |
Note
- Naturally, the sum of the patient numbers is larger than the actual number of patients, since some patients changed age groups.
The percentage of patients who did not receive any TKI increased with age with only slight differences between sexes. Between 9% and 14% of the patients in the age groups between 20 and 59 did not get any TKI, while in the age group 60 to 69 years, already 17% of the males and 19% of the females were not treated with any TKI.
This percentage rose to 24% and 25%, respectively, in males and females aged between 70 and 79 years, rising further to 31% and 41% in the oldest age group of 80 years and above.
Looking in detail on the second-generation TKIs, we found a trend toward a more common use over time, while the general use of TKI was fairly constant. The percentage of imatinib dropped from about 90% of the prescribed TKI doses to 54% in 2016. We did not find any differences between men and women regarding the use of second-generation TKI. However, the percentage of patients receiving nilotinib decreased with age. While 32% respectively 35% of the male resp. female patients between 20 and 29 years received nilotinib, this was only the case in 11% resp. 15% of the oldest patients. For dasatinib, we observed a similar pattern with a maximum of 26% resp. 22% of the patients 40 to 49 years and a minimum in the oldest age group above 80 years, with only 10% resp. 9%.
Additionally, we performed a sensitivity analysis to account for the potential effect of TKI discontinuation trials. The percentage of patients participating in these trials is unknown and cannot be estimated from the data. Thus, we identified all those quarter years where patients did not receive any TKI or hydroxyurea, but had already been receiving TKI before. Per random sampling, 20 percent of those quarter years were removed. The average dose was corrected for those patients. However, the impact on the results was only minor. The median increased from 69 to 72 doses per quarter year, when considering all patients, and from 77 to 79 doses per quarter year, when only patients with any TKI admission were considered.
3.2 Cytogenetic and molecular monitoring
Based on the MLL data, we estimated the median number of cytogenetic, FISH, and PCR examinations per patient and year. Only patients with at least two examinations and a minimal observation time of 1 year were considered. As the differences between Bavaria and the rest of Germany were negligible, we report the results for both areas taken together. The ELN guidelines recommend that PCRs are conducted every 3 months until an MMR has been achieved and then every 3 to 6 months. Cytogenetic analyses should be done every 3-6 months until a complete cytogenetic remission has been achieved and once per year afterward. Data on 2104 patients were used for this analysis.
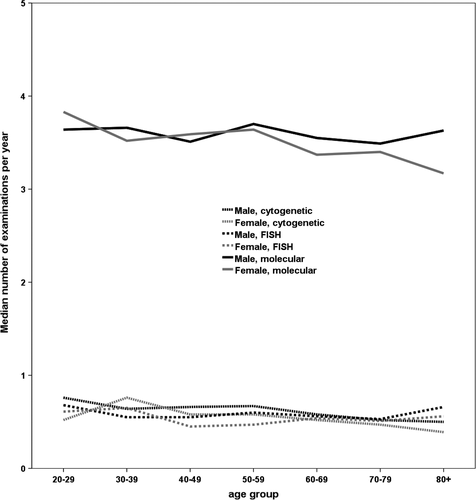
The median number of cytogenetic examinations in the dataset was 0.58 examinations per patient and year, with a slightly higher number for male than for female patients (0.61 vs 0.53). When considering age groups, we found a trend of decreasing examinations with age: While the median in the group 30-39 years was 0.72 examinations per year, only 0.55 were done in patients aged 60-69 and 0.44 in the oldest age group (Figure 3). We were not able to find any calendar time trend. While the median for patients from office-based physicians (OBP) was 0.50 cytogenetic analyses per year, patients from municipal hospitals (MH) were examined 0.65 times and patients from teaching hospitals (TH) 1.02 times. This difference was observed for all age groups.
For FISH, the median per patient and year was 0.54 examinations, with almost no differences between men and women. In contrast to cytogenetics, we did not find any age or other trend as well. The differences between treatment centers were smaller, with medians of 0.48, 0.62, and 0.74 for OBP, MH, and TH respectively.
The number of PCRs was considerably higher than for both the other tests with a median of 3.53 per patient and year. With medians of 3.59 and 3.47, we found no considerable differences between men and women (Figure 3). 15.6% of the patients received less than two PCRs per year, which is the bottom line according to the ELN recommendations. When investigating a potential time trend, we had to focus on the first year after diagnosis, as the number of examinations depends on the remission status. Here, we found a steady increase with the mean number of PCRs rising from 3.00 in 2010 to 3.65 in 2015.
For PCR, the treatment center did not seem to play a role, as office-based physicians had even a slightly higher median (3.60) than municipal (3.45) and teaching hospitals (3.41).
4 DISCUSSION
Based on the KVB data, our analyses provide a representative, population-based insight into the current TKI treatment situation. That is an important strength of our study. It is alarming that even in the most prosperous part of a highly developed country with an extensive statutory health insurance system, still a considerable number of patients did not receive the adequate treatment for a potentially fatal disease. We found that especially elderly patients were often treated with hydroxyurea only. But even the patients with CML that received TKIs, received on average (at least 30% of the population) a too little supply of TKI, did not administer the recommended dose of 400 mg imatinib per day. Molecular monitoring was found to be done regularly in the majority of patients in all age groups.
The comparison of the numbers estimated from our data to the population-based registry of the European Treatment and Outcome Study (EUTOS) 24 for CML is quite interesting. While we found over 18% of patients not treated with any TKI, this was only the case in 3% of the patients from the registry. In the EUTOS registry, 80% of patients received imatinib as a first-line treatment and 28% of those switched treatment subsequently—mostly to second-generation TKI. This difference may be explained by the year of registration as the EUTOS data were collected between 2008 and 2013 when second-generation TKIs were not yet very common and some had not been licensed yet. In the EUTOS data, no significant differences in the treatment of males and females were found; the reason may be that the EUTOS registry did only ask for prescribed treatment and not for actual dosage used. As in our data, the use of TKIs in the EUTOS patients was decreasing in the older age groups.
The percentage of patients not receiving any TKI is considerably higher than that published for Sweden,25 where about 94% of the patients below 70 years and more than 80% of those above 70 years received any TKI in the first year. In contrast to our data, the Swedish as well as the EUTOS data refer to newly diagnosed patients only. This might at least in part explain the differences.
The withholding of TKI treatment to elderly patients can have different reasons. Unfortunately, claims data do not allow analyzing causal relations. Elderly patients suffer more frequently from other comorbidities, which in some cases can prevent TKI treatment. Another reason might be that physicians do not consider it worthwhile to treat elderly patients with therapies as expensive as TKIs. This is sometimes based on a common misunderstanding regarding the life expectancy of elderly persons. According to the data of the German Federal Statistical Office,26 the current life expectancy of a newborn German is 77.72 years for a male and 82.80 years for a female. However, if we consider, for example, a 75-year-old man, the remaining average life expectancy is not just 2.72 but 10.54 years. Even an 85-year-old man has got a remaining life expectancy of 5.38 years, a time span that can usually not be reached with a hydroxyurea treatment. One may argue that these are values for the normal population, but many studies have shown that today patients with CML achieve a life expectancy similar to the normal population.2, 27-29
The concept of treatment-free remission might also be responsible for some cases that did not receive a TKI any longer. This discontinuation of treatment should still be done within prospective clinical studies only at the moment, and a few TKI discontinuation trials were running in Germany during the time of our study. It is probable that in some cases, a discontinuation has been started outside clinical trials on the patient's request. It was not possible to identify these patients precisely from our data however. But we doubt that there were many, in particular not in the early years of our dataset, as the first results of the observational STIM study were published late in 2010 only.30 When we performed a sensitivity analysis assuming a very high discontinuation rate of 20 percent, results were rather similar. Furthermore, the number of patients that did not receive any TKI should remain unaffected.
For many drugs, differences regarding the pharmacological response between men and women are known.31, 32 It has been shown for other drugs that women are more likely to have adverse drug reactions when getting the same dosage as men due to their lower average body weight. Although there are no recommendations for weight-related dose adjustments with regard to TKIs, the differences observed here may at least in part be explained by an unintended dose adjustment or by a higher tendency to pause the treatment after adverse events in women.
However, as recent research showed that actually higher doses of imatinib like 600 or 800 mg per day lead to a higher percentage of patients in MMR after 12 months of therapy it is alarming that many patients actually receive a much lower dose.33, 34 In summary, we think that our findings re-access to and intensity of TKI treatment deserve further investigations to make sure that all CML patients receive optimal treatment.
We found that molecular monitoring was done according to the guidelines in the vast majority of patients. Due to their inferior sensitivity, cytogenetic and FISH analyses have been replaced by molecular analyses during the past years. This might be a reason why the observed number of cytogenetic analyses is lower than recommended. These results are in accordance with the work of Geelen et al15
Some years ago, we had reported differences in survival between teaching hospitals, municipal hospitals, and office-based physicians.35 Here we found differences with regard to cytogenetic but not with regard to molecular evaluations. As study compliance may be more respected in teaching hospitals, the number of a complete examination of bone marrow including chromosome banding analysis may be slightly higher in patients referred from those. Further, these hospitals might see more higher risk patients that need detailed diagnostics.
We assume that the results can be generalized to other parts of Germany. As Bavaria is one of the most prosperous parts of Germany, we do not expect the prescription of TKI to be inferior. Further, monitoring frequencies were comparable between Bavaria and the rest of Germany.
Like all analyses based on routine data, our study has some limitations. Naturally, we were not able to see why, for example, a patient received a lower dose. In particular, the quarter year structure of the data is responsible for small inaccuracies on the individual patient's level. However, this should be compensated by the large size of the dataset and the long observation period.
The analysis of the monitoring frequencies only covers patients whose doctors sent their samples to a specialized laboratory and might therefore represent a positive selection. Furthermore, as we included only patients that were at least observed for 1 year, we missed all those patients that were only monitored at diagnosis. Thus, we are aware that our figures will probably present an overestimation.
As it has been repeatedly shown that too low doses of treatment with TKIs are accompanied by less therapeutic success, patients and physicians need to be motivated to take more care for using the required daily dosages. As data indicate, this may even be cost-effective as poor adherence with TKI treatment results in higher healthcare costs.36
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
AUTHOR CONTRIBUTIONS
ML designed the research, checked, analyzed, and interpreted the data. RG provided, processed, and interpreted the data. WW provided, processed, and interpreted the data. TH provided, processed, and interpreted the data. MT provided, processed, and interpreted the data. JH analyzed and interpreted the data and designed the research. VH analyzed and interpreted the data. All authors contributed to the manuscript and approved the final version.
ETHICAL APPROVAL
For this type of study formal consent is not required. This article does not contain any studies with human participants or animals performed by any of the authors.




