Identification, diversity and host specificity of the wood-decay fungi in major sawmill depots of north-eastern Bangladesh
Abstract
The study was carried out to identify wood-decay fungi, and quantify the diversity and host preferences of the fungi in major sawmill depots in north-eastern Bangladesh. A total of 23 fungal species belonging to 15 genera in seven families were recorded and identified. The Polyporaceae was the most dominant family, while Schizophyllum commune was the most abundant species among all species recorded. Other commonly observed fungal species were Daldinia concentrica, Trametes versicolor, Trametes coccinea and Flavodon flavus. The Simpson diversity index (0.93) and Shannon–Wiener index (2.90) showed a wide distribution of the wood-decay fungi in the study areas. The species diversity index (0.036), species evenness index (0.92) and species richness index (3.40) indicated a diverse distribution of the fungal species. Two-thirds of the identified fungal species showed significant preferences for their hosts. The host vulnerability was found to be significantly affected by storage facility, duration of storage, depot yard condition, treated or non-treated wood and shade facility. The findings of this work may help sawmill owners to utilize a scientific approach to management of logs and timber stored in depots, to minimize fungal decay before incurring any economic loss.
1 INTRODUCTION
The biological degradation of wood biomass in the depots caused by wood-decay fungi is a major concern because of associated economic loss and quality degeneration (Sivrikaya & Can, 2014; Yalçin et al., 2019). After harvesting trees from the forests, logs are taken to sawmill depots for processing and trading. Usually, logs and timbers are stored outdoors for a considerable amount of time due to low input maintenance cost. The exposure of logs and timber to outdoor weather conditions and piling directly on the soil expose them to decay fungi (Pavlidis et al., 2005). Fluctuations in temperature inside the wood combined with microbial attack accelerate the deterioration processes in the sawmill depots (Hofmann et al., 2018). Degradation may be very rapid during to prolonged storage and the depots themselves become a breeding ground for spread of the wood-decay fungi (Yalçin et al., 2019). Fungal spores spread onto fresh-cut logs and timber in the sawmill depots (Kantay & Köse, 2009). The losses incurred by decay agents not only cause economic losses but also creating extra pressure on the forests due to additional requirements for wood consequently, adversely affecting the sustainability of the forests (Komut, 2011).
To prevent the losses in the depots, an extensive understanding of the nature of the damage, causal fungi and factors favouring the fungal invasion is required. Wood-decay fungi colonize logs, some even using living trees as host, causing damage in both deadwood and live standing trees (Hoff et al., 2004; Ranadive, Jagtap, et al., 2012; Shortle & Dudzik, 2012). Based on decay, the fungi are divided into three types: brown-rot fungi, soft-rot fungi and white-rot fungi (Deacon, 2006). Wood-decay fungi possess varied preferences for wood as substrates. For instance, white-rot fungi decay mostly lignin and, secondarily, cellulose from the wood structure while brown-rot fungi decay mostly cellulose and secondarily lignin; soft-rot fungi degrade mainly the carbohydrates (Srivastava et al., 2013). Decay fungi are mostly filamentous species of Basidiomycota or Ascomycota (Arnstadt et al., 2016; Swift, 1982). It is only when conditions are favourable that fungal spores can germinate, including sufficient rain or moisture, the availability of assimilable nutrients of low molecular mass and an acceptable host (Osherov & May, 2001). Fungal spores can remain viable for several years before favourable conditions are present, using mechanisms to avoid germination (Chitarra et al., 2004).
Biological degradation of woody biomass is a natural phenomenon; nevertheless, preventive interventions are required to minimize the decay and maintain the wood quality for subsequent uses by humans. If care and appropriate measures are not taken, storage sites may become hotspots for decay fungi. Several early studies showed the abundance, distribution of decay fungi and extent of damage, the rate of decay, fungal species diversity that varied from region to region, even in between sub-regions depending on the prevailing weather and climatic conditions, depot design, tree species, ages and proportions of sap and heartwood (Heinek et al., 2013; Wihersaari, 2005). Research is required, therefore to minimize the loss of wood in sawmills.
Understanding diversity or organisms provides important information on a species community as well as the interactions influencing ecosystems. The environmental conditions present in sawmills influence the fungal community and can be used when identifying the species responsible for decay, giving an insight into the possible amounts of damage caused by the wood-decay fungi. The total diversity of fungi is estimated to be 0.7–5.1 million species (Blackwell, 2011; Hawksworth & Lucking, 2017), but to date only about 1.9% (97,330 species) have been described so far (Abrahao et al., 2019). Approximately 538 genera and 6384 species of wood-decay Agaricomycetes are known worldwide (Kirk et al., 2008). However, how many decay-causing Agaricomycetes are present in Bangladesh is known, as there have been few studies conducted in Bangladesh on macrofungal diversity (Aminuzzaman & Das, 2017; Das et al., 2017; Das & Aminuzzaman, 2017; Marzana et al., 2018; Rahaman et al., 2016; Rubina et al., 2017; Rumainul et al., 2015; Rumainul & Aminuzzaman, 2016; Shayesta & Rahman, 1992; Tanjim et al., 2019). To our knowledge, no publications exist on fungal–host interactions and host vulnerability in the storage conditions commonly occurring in sawmill depots. The aim of the work reported here was to identify decay fungi colonizing stored logs and timber in sawmill storage depots in Bangladesh, plus determine distribution of the fungi, host specificity and factors affecting host vulnerability.
2 MATERIALS AND METHODS
2.1 Study area
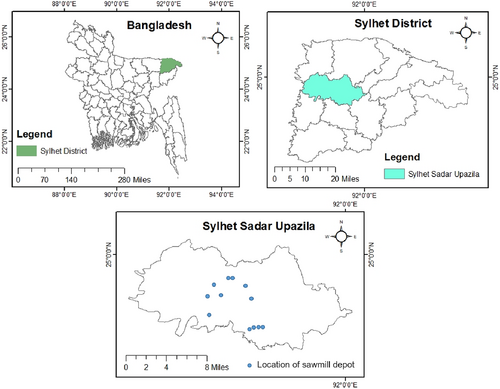
The study was conducted in Sylhet Sadar Upazila (sub-district) of Sylhet district located between 24°52′N and 25°02′N latitudes and 91°01′E and 91°40′E longitudes in north-eastern Bangladesh (Figure 1). Sylhet has a typical tropical monsoon climate with a distinct hot and rainy season and cooler dry weather. The hot rainy season is from April to October and the cold dry season is from November to February (Figure 2). The average annual temperature is 25°C, whereas the average annual rainfall is 3334 mm, relative humidity (RH) varying from 60% to 88% (Islam et al., 2017).

2.2 Sampling of sawmill depots and fungal specimen collection
Twelve sawmill depots were selected randomly for the study, and each depot was considered as an individual sampling unit or plot (Figure 1). Surveys were carried out from June to December 2020: Each sawmill depot was surveyed three times at an interval of 2 months. Logs and deadwood stored in sawmill depots were examined for fruiting bodies of wood-decay fungi. Fruiting body samples were separated carefully from the hosts using a sharpened knife. Following photographing at the study sites using a Xiaomi Note 7 Pro camera, samples were collected into polythene bags and fully labelled with locations and collection date and host log species. Samples were returned to the laboratory, carefully dried at 40–45°C for 2 and 3 days to prevent deterioration (Yalçin et al., 2019) and finally stored in polythene bags for further studies.
2.3 Identification of fungal samples
The fungal specimens were examined carefully under a microscope in the laboratory for macroscopic and microscopic features (cap size and shape, cap colour, gills, spores, cystidia, form of hyphae and mycelia). Body sections of the fruiting bodies were also examined under a light microscope. Specimens were identified by comparing their morphological features against reports in books (Ginns, 2017; Ostry et al., 2011), scientific papers (Abrahao & Gugliotta, 2012; Arya et al., 2008; Cui et al., 2019; Das & Aminuzzaman, 2017; Din & Mukhtar, 2019; Nnagadesi & Ayar, 2015; Patil, 2019; Rumainul & Aminuzzaman, 2016; Schwarze, 1994; Tanjim et al., 2019; Verma et al., 2018), web information (https://www.mushroomexpert.com/; https://firstnature.com/; https://www.indiabiodiversity.org/) and personal communication with experts.
2.4 Diversity of the wood-decay fungi
Frequency (F) is the per cent proportion of sample plots in which a species is represented. Relative frequency (RF) is per cent ratio of the frequency of a given species to the total frequencies of all species. Density (D) is the number of individuals of a given species as a proportion of the total number of sample plots studied. Relative density (RD) is the number of individuals of a given species expressed as a percentage of the total number of individuals of all species. Abundance (A) is the number of individuals of a given species as a proportion of the number of sample plots in which the species occurred.
A diversity index is a quantitative measure that reflects how many different types there are in a dataset and simultaneously considers how evenly they are distributed (Okpiliya, 2012). Diversity indices used for data analyses were Shannon–Wiener index (H) (Equation 1; Shannon & Wiener, 1949), Simpson's diversity index (D) (Equation 2; Simpson, 1949), species diversity index (SDI) (Equation 3), species richness index (R) (Equation 4) and species evenness index (E) (Equation 5). Species richness index and species evenness index were calculated using Margalef's formula (Margalef, 1958). One-way analysis of variance (ANOVA) and Duncan multiple range test were carried to determine the difference in diversity indices among the host plants.
2.5 Determination of the host preferences
To determine host fungal association, the preferences of wood-decay fungi for host trees were determined following Eom et al. (2000). The data set of individual fungus was transformed to rank cases to normalize the data before analysis. Rank case transformation was done in ascending order, and mean rank of tied values was used for ties. One-way ANOVA was carried out to determine the preference of each fungus for a host (dependent variable: rank cases transformed individual of wood-decay fungi; independent variable: host species) and preference of each host for fungal species (dependent variable: rank cases transformed individual of wood-decay fungi; independent variable: wood-decay fungi). One-way multivariate analysis was used to evaluate host effect on overall fungal communities (dependent variable: rank cases transformed individual of wood-decay fungi, wood-decay fungi; fixed factor: host species).
where Y = vulnerability (0 = not vulnerable, 1 = low vulnerability, 2 = moderate vulnerability, 3 = high vulnerability), Xp = different dependent variable such as storage duration (dummy: 1 = less than 1 year, 0 = more than 1 year), shade (dummy: 1 = yes, 0 = no), treatment (dummy: 1 = seasoned, 0 = not seasoned), storage condition (dummy: 1 = stacked not directly on ground, 0 = stacked directly on ground), yard condition (dummy: 1 = well drained, 0 = not well drained), harvest period (dummy: 1 = dry season, 0 = wet season), host age at felling (dummy: 1 = less than 25 years old, 0 = more than 25 years old), βo = constant or intercept, and βp = regression coefficient of each dependent variable, ε = error term.
2.6 Statistical analyses
Data were analysed using Microsoft Excel Office (version 2013) and IBM SPSS (version 23.0). Microsoft Excel was used to determine the qualitative features and diversity indices of the wood-decay fungi. IBM SPSS was used for rank cases transformation of data, to evaluate the host preferences of wood-decay fungi and determine the vulnerability of hosts to fungi. The relationship network among the hosts and fungi was determined by using Cytoscape (version 3.8.0). A sampling map was created using ArcGIS (version 10.5).
3 RESULTS
3.1 Identification of the wood-decay fungi
A total of 23 wood-decay fungi species (Figure 3) representing 15 genera in seven families were identified (Table S1). The families were Polyporaceae, Hymenochaetaceae, Hypoxylaceae, Irpicaceae, Fomitopsidaceae, Stereaceae and Schizophyllaceae, and the genera identified were Trametes, Daedaleopsis, Ganoderma, Spongipellis, Datronia, Earliella, Hexagonia, Microporus, Fomes, Trichaptum, Daldinia, Flavodon, Daedalea, Stereum and Schizophyllum (Table 1). The families identified in five orders: Polyporales (families, Polyporaceae, Irpicaceae, Fomitopsidaceae), Hymenochaetales (family Hymenochaetaceae), Russulales (family Stereaceae), Agaricales (family Schizophyllaceae) and Xylariales (family Hypoxylaceae). Among the orders recorded, the Polyporales, Hymenochaetales, Russulales, Agaricales are in the class Agaricomycetes, phylum Basidiomycota and Xylariales was in the class Sordariomycetes of the Ascomycota phylum. The Polyporaceae was the most abundant family with 17 species, whereas the remaining families had one species each. Trametes was the most prevalent genus with eight species (Table 1), while Ganoderma was represented by two species; each remaining genus was represented by one species (Table 1). Variations in morphology (Figure 3) of the wood-decay fungi between the study sites were noted, and basic morphological features summarized in Table S1.

| Species | Family | F (%) | RF (%) | D | RD (%) | A | Host specificitya | |
|---|---|---|---|---|---|---|---|---|
| F-value | p-value | |||||||
| Daldinia concentrica | Hypoxylaceae | 50.00 | 7.41 | 5.92 | 11.01 | 11.83 | 7.11 | .020 |
| Daedaleopsis confragosa | Polyporaceae | 33.33 | 4.94 | 2.33 | 4.34 | 7.00 | 15.00 | .012 |
| Datronia mollis | Polyporaceae | 41.67 | 6.17 | 2.00 | 3.72 | 4.80 | 4.99 | .047 |
| Daedalea quercina | Fomitopsidaceae | 25.00 | 3.70 | 2.67 | 4.96 | 10.67 | 5.40 | .045 |
| Earliella scabrosa | Polyporaceae | 33.33 | 4.94 | 2.58 | 4.81 | 7.75 | 6.53 | .034 |
| Flavodon flavus | Irpicaceae | 50.00 | 7.41 | 1.92 | 3.57 | 3.83 | 9.60 | .036 |
| Fomes fomentarius | Polyporaceae | 16.67 | 2.47 | 1.42 | 2.64 | 8.50 | 0.51 | .622 |
| Ganoderma applanatum | Polyporaceae | 33.33 | 4.94 | 2.25 | 4.19 | 6.75 | 6.41 | .035 |
| Ganoderma curtisii | Polyporaceae | 33.33 | 4.94 | 0.83 | 1.55 | 2.50 | 0.143 | .742 |
| Hexagonia tenuis | Polyporaceae | 16.67 | 2.47 | 1.08 | 2.02 | 6.50 | 0.08 | .793 |
| Microporus xanthopus | Polyporaceae | 16.67 | 2.47 | 2.50 | 4.65 | 15.00 | 5.55 | .027 |
| Schizophyllum commune | Schizophyllaceae | 25.00 | 3.70 | 7.83 | 14.57 | 31.33 | 11.19 | .003 |
| Spongipellis delectans | Polyporaceae | 25.00 | 3.70 | 1.33 | 2.48 | 5.33 | 11.25 | .044 |
| Stereum hirsutum | Stereaceae | 16.67 | 2.47 | 1.50 | 2.79 | 9.00 | 8.21 | .029 |
| Trametes sp. | Polyporaceae | 8.33 | 1.23 | 0.50 | 0.93 | 6.00 | 3.00 | .333 |
| Trametes betulina | Polyporaceae | 25.00 | 3.70 | 1.33 | 2.48 | 5.33 | 13.71 | .021 |
| Trametes cingulate | Polyporaceae | 25.00 | 3.70 | 1.75 | 3.26 | 7.00 | 15.00 | .030 |
| Trametes coccinea | Polyporaceae | 50.00 | 7.41 | 5.42 | 10.08 | 10.83 | 12.86 | .001 |
| Trichaptum fuscoviolaceum | Hymenochaetaceae | 16.67 | 2.47 | 0.83 | 1.55 | 5.00 | 0.25 | .667 |
| Trametes gibbosa | Polyporaceae | 33.33 | 4.94 | 1.42 | 2.64 | 4.25 | 9.96 | .025 |
| Trametes lactinea | Polyporaceae | 25.00 | 3.70 | 1.08 | 2.02 | 4.33 | 16.00 | .005 |
| Trametes suaveolens | Polyporaceae | 25.00 | 3.70 | 1.25 | 2.33 | 5.00 | 0.314 | .605 |
| Trametes versicolor | Polyporaceae | 50.00 | 7.41 | 4.00 | 7.44 | 8.00 | 4.76 | .035 |
- Abbreviations: A, abundance; D, density; F, frequency; RD, relative density; RF, relative frequency.
- a One-way ANOVA indicates the preference of each wood-decay fungi for host species. Level of significance was p < .05.
3.2 Diversity of the wood-decay fungi
Schizophyllum commune was the most dominant wood-decay fungi species having an abundance of 31.33, while Ganoderma curtisii appeared to be of the lowest abundance 2.50; Microporus xanthopus, Daldinia concentrica, Trametes coccinea and Daedalea quercina were the co-dominant species with abundances of 15.00, 11.83, 10.83 and 10.67, respectively (Table 1). D. concentrica, T. coccinea, Trametes versicolor and Flavodon flavus had the highest frequencies (50%) and relative frequencies (7.41%; Table 1). Trichaptum fuscoviolaceum, Hexagonia tenuis, Fomes fomentarius, Stereum hirsutum and M. xanthopus were noted with lower frequencies (16.67%) and relative frequencies (2.47%). Trametes sp. showed the lowest frequency (8.33%) and relative frequency (1.23%; Table 1). Density and relative density for S. commune were 7.83% and 14.57%, respectively. Trametes sp. showed the lowest density and relative density, 0.50% and 0.93%, respectively.
The diversity indices such as the Simpson index (D) and Shannon–Wiener index (H) were 0.93 and 2.90, respectively (Table 2). The species richness index was also high (R = 3.40). The evenness index (E) was 0.92 (Table 2) indicated that the wood-decay fungi were almost evenly distributed. Taking into consideration the values of the indices, the findings indicated a rich diversity of the wood-decay fungi identified in the study sites (Table 2).
| Overall diversity indices of wood-decay fungi | |||||
|---|---|---|---|---|---|
| D | H | SDI | R | E | |
| 0.93 | 2.90 | 0.036 | 3.40 | 0.92 | |
| Diversity of wood-decay fungi for each host species | |||||
| D | H | SDI | R | E | |
| Host plant | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) |
| Albizia procera | 0.72 (0.017)a | 1.23 (0.046)a | 0.22 (0.022)a | 1.37 (0.076)a | 0.92 (0.014)a |
| Artocarpus heterophyllus | 0.56 (0.019)b | 0.67 (0.023)b | 0.35 (0.044)ab | 1.31 (0.092)a | 0.90 (0.021)a |
| Samanea saman | 0.69 (0.021)a | 1.20 (0.061)a | 0.18 (0.017)a | 1.49 (0.068)a | 0.88 (0.025)a |
| Swietenia mahagoni | 0.74 (0.018)a | 1.27 (0.088)a | 0.28 (0.030)ab | 1.34 (0.092)a | 0.94 (0.046)a |
| Tectona grandis | 0.55 (0.028)b | 0.61 (0.016)b | 0.43 (0.056)b | 1.21 (0.115)a | 0.89 (0.023)a |
| Sig. (p-value)* | <.001 | <.001 | .022 | .670 | .424 |
- Abbreviations: D, Simpson index; E, species evenness index; H, Shannon–Wiener index; R, species richness index; SDI, species diversity index; SE, standard error.
- * One-way ANOVA indicates the difference in wood-decay fungi diversity indices among the host plants. Different letter(s) in the same column are significantly (p < .05) different according to Duncan multiple range test.
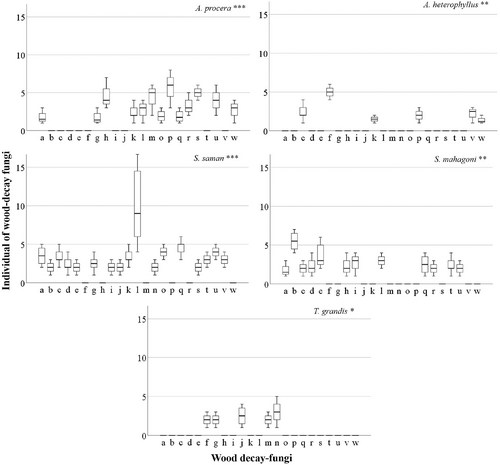
Among the host species, the highest fungal diversity was found for Swietenia mahagoni (H = 1.27), followed by Albizia procera (H = 1.23), Samanea saman (H = 1.20), Artocarpus heterophyllus (H = 0.67) and Tectona grandis (H = 0.61; Table 2). However, the highest species richness was found for S. saman (R = 1.49) followed by A. procera (R = 1.37), S. mahagoni (R = 1.34), A. heterophyllus (R = 1.31) and T. grandis (R = 1.21) (Table 2), while species evenness index (E) for S. mahagoni, A. procera, A. heterophyllus, T. grandis and S. saman was 0.94, 0.92, 0.90, 0.89 and 0.88, respectively (Table 2). For each host plant, wood-decay fungi diversity indices differed significantly Simpson index p < .001, Shannon–Wiener index p < .001, SDI p < .05, while species richness index (p > .05) and species evenness index (p > .05) did not vary significantly (Table 2). The Duncan multiple range test showed that neither the species richness index (R) nor species evenness index (E) for each host plant differed significantly (p > .05); by contrast, Simpson index (D), Shannon–Wiener index (H) and SDI varied significantly (p < .05) (Table 2).
3.3 Host effect and network relationships between the hosts and fungi
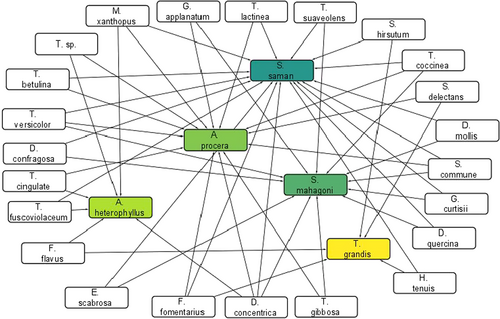
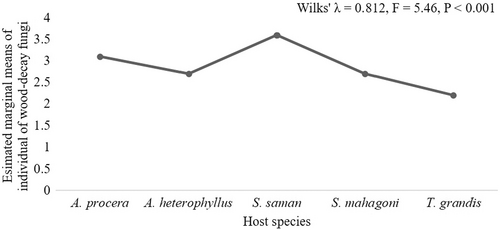
The wood-decay fungi identified in this work showed a wide range of host preferences. Significant variation (p ≤ .05) in host preference was apparent for two-thirds of the fungi identified (Table 1). The highest number of species occurred on S. saman (17), followed by A. procera (13), S. mahagoni (12), A. heterophyllus (6) and T. grandis (5) (Figure 4). D. concentrica was found to occur on four hosts. M. xanthopus, S. commune, F. fomentarius, T. coccinea and T. versicolor were identified on three hosts, while the remaining identified decay fungi occurred on two hosts (Figure 4). When host preferences of the fungi were analysed, all hosts except T. grandis showed significant relationships with several decay-causing fungi (Figure 5). Trametes cingulata was most abundant on A. procera, followed by F. flavus on A. heterophyllus, S. hirsutum on T. grandis, Daedaleopsis confragosa on S. mahagoni and S. commune on S. saman (Figure 5). Multivariate analysis revealed a significant host effect on overall fungal composition (Wilks' λ = .812, F = 5.46, p < .001) (Figure 6).
3.4 Factors influencing vulnerability of the hosts to wood-decay fungi
Several factors such as storage duration, shade, treatment, depot yard condition, harvesting period and host age at felling were correlated with the vulnerability of hosts to wood-decay fungi. Regression analysis of aforesaid factors showed an estimated F-value as 28.42, while R2 and adjusted R2 were greater than 0.5. These data implied that all the variables used in this study were important and could explain the vulnerability of hosts (Table 3). Except for the harvest period and age of host at felling, all other factors showed a significant correlation with increasing vulnerability of the hosts to fungal invasion (Table 3). Logs harvested less than 1 year prior to sampling were found to be less infected, whereas increasing the duration of storage resulted in higher level of infection. Similarly, logs stacked and stored on the ground and in the open air as well as poor drainage in the depots showed a higher amount of decay. By contrast, a relatively low invasion was observed for logs that were air-dried before storage. Harvesting period and log age did not play a significant role in host vulnerability (Table 3). However, it was noticed that immature trees and logs harvested during wet season were liable to rapid invasion by wood-decay fungi.
| Dependent variables | Coefficient | SE | p-value |
|---|---|---|---|
| Intercept | 2.71 | 0.16 | <.001 |
| Storage duration | −0.62 | 0.21 | .006 |
| Shade | −0.58 | 0.19 | .005 |
| Treatment | −0.43 | 0.18 | .021 |
| Storage condition | −0.57 | 0.24 | .024 |
| Depot yard condition | −0.43 | 0.20 | .037 |
| Harvest period | −0.17 | 0.18 | .346 |
| Host species age | −0.03 | 0.21 | .886 |
| R 2 | 0.79 | ||
| Adjusted R2 | 0.76 | ||
| F-value | 28.42 | ||
| p-value | <.001 |
- Abbreviation: SE, standard error.
4 DISCUSSION
Wood-decay fungi are among the major agents of bio-deterioration that can invade and cause decay on both living and deadwood (Gonthier & Nicolotti, 2007). In Bangladesh, studies on the wood-decay fungi causing decay of deadwood logs and timber are limited (Rahman, 1990; Shayesta & Rahman, 1992). Identification of 23 species of wood-decay fungi representing 15 genera reflected the wide diversification of decay fungi present in sawmill depots, probably resulting from favourable environmental conditions in the context of rainfall, temperature and relative humidity. An investigation on macrofungi by Tanjim et al. (2019) on the tropical evergreen and semi-evergreen forests of Sylhet and Moulvibazar districts in Bangladesh identified 25 macrofungi species in 15 genera and 13 families. Another report on macrofungi by Marzana et al. (2018) reported 20 species under in 17 genera and 15 families from the forests of Rangamati Hill Tracts District of Bangladesh. Similar findings were also reported (Bhattacharjee et al., 2015; Rahaman et al., 2016; Rashid et al., 2016; Rumainul & Aminuzzaman, 2016), where various species of macrofungi were recorded in several genera and families.
Fungi causing wood decay are mainly in the phyla Basidiomycota or Ascomycota. In the present study, we have identified 23 species, of which only one species was Ascomycota, with the remaining 22 species being Basidiomycota. The publication by Shayesta and Rahman (1992) provided a list of wood-decay fungi, most of which were also Basidiomycota and Ascomycota. In our study, 22 species of fungi were in the class—Agaricomycetes of the Basidiomycota; the one Acomycota was in the class—Sordariomycetes of the Ascomycota. Approximately 21,000 species of Agaricomycetes are known, as saprotrophs, pathogens and fungal components in mycorrhizal symbioses, most of the saprotrophs in the Agaricomycetes appear to be wood-decay fungi (Krah et al., 2018). In the work described here, fungi were in the orders Polyporales, Hymenochaetales, Russulales, Agaricales and Xylariales, among which the Polyporales putatively included 19 species in three families, 11 genera; the remaining four orders each included only one species. The publication of Kirk et al. (2001) suggested that most wood-decay fungi were primarily Agaricales, Hymenochaetales, Polyporales or Russulales in the Agaricomycetes, except for the Ascomycota class, which includes wood-decay species containing orders such as the Xylariales, which is consistent with the present findings. The main wood-inhabiting species in the Basidiomycota is commonly known as the polypores even though they were miss-ordering cases. Surveys conducted on polypores reported 704 species in 132 genera, the majority of which, accounting for 65% of the total polypore community known in China, were Polyporales (Dai, 2011). The findings of these surveys are compatible with the results described in this paper, where most of the wood-decay fungi identified were in the order Polyporales.
Trametes was the most dominant genus found, whereas Ganoderma was co-dominant. Das and Aminuzzaman (2017) recorded Ganoderma as dominant, and Trametes and Inonotus as co-dominant decay fungal species from the mangrove forests of Bangladesh. In the present study, the highest frequency (50%) and relative frequency (8.82%) were for T. versicolor and D. concentrica, respectively, and maximum density (7.75) and relative density (15.82%) were recorded for S. commune in these study sites. S. commune was the most abundant species. The findings of the present study are consistent with Tanjim et al. (2019) and Tapwal et al. (2013).
In the present study, wood-decay fungi had broad host ranges on angiospermic trees. Most of the identified fungi showed significant host specificity. A wide host range and host specialization were both also reported for wood-decay fungi elsewhere in the tropics (Iqbal et al., 2017; Lindblad, 2000). In India, Kamble et al. (2012) indicated that almost all wood-decay fungi occurred on angiosperm plants, which was also consistent with our present findings of host preference. Host specialization of such fungi was also reported in other studies (Gilbert et al., 2008; Gilbert & Sousa, 2002; Krah et al., 2018; Ranadive, Joshi, et al., 2012).
Wood-decay fungi require suitable food materials and favourable environmental conditions for growth and survival (Srivastava et al., 2013). However, the optimum temperature for growth of wood-decay fungi can vary between 20 and 40°C and wood moisture content ranges from 35% to 70% (Verbist et al., 2019). Prevailing temperature and relative humidity in the study sites examined in the present work were clearly suitable for the growth and development of several wood-decay fungal species, as also demonstrated elsewhere (Yalçin et al., 2019). During the surveys, sawmill depots used unscientific management protocols, storing logs in outdoor environments, including in damp position that could also be contribute to the high abundance of fungal species. In our study, prolonged retention of logs (>1 year) in the depots led to increase invasion by decay fungi. A strong correlation between the holding time of logs in sawmill depots and fungal abundance were also reported by Yalçin et al. (2019). Earlier studies in other countries have reported similarly that temperature, relative humidity, wood contact with soil and damp ground in the depots influence the abundance of wood-decay fungi and the severity of decay (Mueller et al., 2004; Talley et al., 2002; Yalçin et al., 2019). Fungal abundance and species variations have been reported as host-specific in several other studies (Deflorio et al., 2008; Malakani et al., 2014; Yalçin et al., 2019), corroborating the findings in the current work. Variations in vulnerability of different timbers/logs to decay fungi were suggested to be result from the different compositions of sapwood and heartwood, particularly content of phenolic compounds (Harju et al., 2003; Umezawa, 2001; Windeisen et al., 2002).
In conclusion, the host log and decay fungus interaction revealed a broad host range network for many decay-causing species, with a significant preference of fungi for a specific host log. S. saman was highly susceptible to fungal attack, as compared to other species sampled in the study. The vulnerability of the hosts was significantly affected by storage retention time in depots, pre-drying treatment, shade facilities depot yard and storage conditions. The data suggest a short log storage time, good depots floor conditions and pre-seasoning of logs before storage might minimize decay.
ACKNOWLEDGEMENTS
The authors are thankful to the Research Centre, Shahjalal University of Science and Technology (SUST), Bangladesh, for allocating funds to conduct the study (grant number: FES/2020/1/7). We also express deep gratitude to the owners and staff of the sawmill depots.
Open Research
DATA AVAILABILITY STATEMENT
Availability of data: Data openly available in a public repository that issues datasets with DOIs.