Correlated evolution of physiological and life history traits in phytochemical-tolerant Drosophila
Abstract
We investigated the correlated evolution of fitness-related traits in cactophilic species Drosophila buzzatii Patterson & Wheeler and D. koepferae Fontdevila & Wasserman (Drosophilidae, Drosophilini) as an indirect response to adaptation to high concentrations of phytochemicals in their host plant (Trichocereus terscheckii Briton & Rose). Specifically, we examined whether the experimental evolution of phytochemical tolerance influenced metabolic rate, fecundity, longevity, and starvation resistance. Our findings reveal that adaptation to phytochemical defenses led to correlated responses in both species, likely driven by genetic correlations and energy allocation strategies. Notably, sexual dimorphism was evident, underscoring the significance of sex-specific effects in the adaptive process. The tolerant phenotypes of each species emerged from distinct pleiotropic backgrounds, with D. buzzatii exhibiting more correlated responses than D. koepferae, suggesting a deeper genetic perturbation or response during the experimental evolution. This study demonstrates how exploiting a marginally used host can impose costs on crucial fitness-related traits, such as adult lifespan and reproductive output. Moreover, ignoring variation in tolerance to phytotoxins would overlook the diversity of responses across species. This variation is essential for understanding how different species manage to exploit toxic plants. Neglecting this aspect would result in a simplified view of insect–plant interactions, failing to account for the nuanced ways in which insects evolve strategies to detoxify or avoid harmful substances.
INTRODUCTION
Phenotypic variation is the result of a complex combination of evolutionary processes beyond the simplistic idea of adaptation modifying isolated traits (Gould & Lewontin, 1979; Lloyd, 2021). In this sense, the correlation between traits due to trade-offs, metabolic costs, and pleiotropic effects constitutes a main driver of evolution by restricting or promoting phenotypic change (Mauro & Ghalambor, 2020; Price & Langen, 1992).
Plants have evolved a variety of secondary metabolites to avoid or reduce herbivory from phytophagous insects, which has driven the dynamics of the insect–plant interaction for at least 300 million years (Gullan & Cranston, 2014; Schoonhoven et al., 2005). Simultaneously, insects have evolved several mechanisms such as enzymatic detoxification and phytochemical sequestration to counteract phytochemical defenses (Després et al., 2007; Zunjarrao et al., 2020). These detoxifying mechanisms are key for the evolution and ecology of insect lineages, shaping features such as niche breadth, degree of specialization, and potential for novel host acquisitions (Agrawal & Weber, 2015; Ehrlich & Raven, 1964; Schoonhoven et al., 2005). However, plant–insect interactions not only involve physiological adaptations and counter-adaptations but also constitute an excellent model to assess the role of correlation between traits in phytophagous insect evolution.
Drosophila species (Diptera, Drosophilidae), breeding in flowers, fruits, tree fluxes, and even cacti (Markow & O'Grady, 2008) are excellent models to investigate organismal integration during ecological diversification of host exploitation. Cactophilic Drosophila use decaying cactus tissues as feeding and breeding resources (Wasserman, 1982. This strategy implies coping with a vast array of chemical plant defenses generated by the Cactaceae family during its evolution (e.g., triterpene glycosides, sterol-diols, alkaloids, and medium-chain fatty acids; Nobel, 2002). Genomic studies have shown that several detoxifying enzymatic families are involved in phytochemical tolerance in Drosophila species (Rane et al., 2019), and transcriptomic studies demonstrated that almost 28% of coding genes of cactophilic Drosophila change their expression under host shifts (De Panis et al., 2016; Etges, 2019; Rane et al., 2019). These genes are involved in redox and carbohydrate metabolism, structural and detoxifying functions, and developmental and neurobiological processes (De Panis et al., 2016; Etges, 2019; Matzkin, 2012, 2014) among other functions.
The Drosophila buzzatii cluster comprises seven closely related species exploiting different cactus genera (Manfrin & Sene, 2006; Ruiz & Wasserman, 1993). D. koepferae Fontdevila & Wasserman and D. buzzatii Patterson & Wheeler have been extensively studied (Fanara et al., 1999; Hasson et al., 1992; Soto et al., 2012, 2014). D. koepferae is tightly associated with columnar cacti of the Trichocereus and Cereus genera in the mountain plateaus of northwest Argentina and southern Bolivia, and marginally exploits prickly pears, cacti of the Opuntia genus. In contrast, D. buzzatii has a wider distribution, originally including northern Argentina, Paraguay, Bolivia, and Brazil, although it also colonized Australia, Europe, and Africa as a consequence of anthropic activities (Barker et al., 2013; Manfrin & Sene, 2006). It is mostly associated with the Opuntia genus, but it can also exploit other cacti (Hasson et al., 1992). These species are sympatric in most of D. koepferae's distribution, where hosts such as T. terscheckii and O. sulphurea coexist (Soto et al., 2012). The nutritional and toxicological compositions of these cacti differ significantly. T. terscheckii represents a highly challenging host, being nutritionally poorer than O. sulphurea while containing a much higher concentration of secondary metabolites (e.g., medium-chain fatty acids, phenylethylamine alkaloids) acting as herbivory deterrents (Carreira et al., 2014; Padró & Soto, 2013; Reti & Castrillón, 1951). Alkaloids of T. terscheckii have a detrimental effect on fitness-related traits of both D. koepferae and D. buzzatii (Corio et al., 2013; Padró et al., 2014; Soto et al., 2014), although D. koepferae displayed a greater natural tolerance (Soto et al., 2014). These differences in host exploitation and chemical composition may even explain the historically observed interspecific variation in life history and morphological traits (Bouzas et al., 2021; Padró et al., 2014).
Experimental evolution assays have developed strains of both species with high tolerance to T. terscheckii's phytochemicals. These experimental populations of D. buzzatii and D. koepferae evaluated here maximized larval survival in artificial breeding media containing extremely high concentrations of these phytochemicals (Padró et al., 2018). Adaptive responses were species-specific, with D. koepferae showing greater inherent tolerance capacity (Padró et al., 2018). A recent study showed that detoxifying systems such as P450 enzymes play a role alongside other detoxification pathways in the adaptation to T. terscheckii phytochemicals (Carreira et al., 2022). As the adaptation of these Drosophila strains probably constitutes a complex and polygenic process during the adaptive acquisition of phytochemical tolerance, other traits may also evolve via pleiotropy and linkage disequilibrium (Mauro & Ghalambor, 2020; Price & Langen, 1992). An increased phytochemical tolerance might also include a metabolic cost of producing and maintaining specialized detoxification machinery, which could, in turn, have a detrimental effect on other fitness-related traits (e.g., Bouzas et al., 2021). For instance, changes in the reproductive output (e.g., the number of eggs produced in a certain amount of time) determined by a host shift could critically affect organismal fitness (Novoseltsev et al., 2003). Additionally, given that in nature, the food availability varies, the evolution of lifespan in the presence and absence of food is a key determinant of reproductive success (Goenaga et al., 2012). In Drosophila melanogaster, exposure to various phytochemicals has been shown to reduce macromolecular oxidative damage and susceptibility to oxidative stress, improve locomotor performance, and decrease oxygen consumption rates, thereby affecting metabolic rate (Leonov et al., 2015). Thus, studying the effects of exploiting a marginally used host on these traits can shed light on how life history and physiological traits are shaped in phytophagous insects. In this context, genetic correlations affecting fitness-related traits and energy allocation may be significant drivers of phenotypic change, ultimately leading to speciation through host specialization.
Our objective was to evaluate whether the adaptation process for tolerance to high concentrations of phytochemicals resulted in correlated evolution of other fitness-related traits. Specifically, we expected that tolerant strains of D. buzzatii would exhibit a greater reduction in metabolic rate, fecundity, longevity, and starvation resistance compared to control strains, whereas D. koepferae, already adapted to chemically complex hosts, would show lesser reductions. Given the ecological background of both species, we expected that the adaptive challenge would be more demanding for D. buzzatii than for the specialized D. koepferae, leading to higher costs in fitness-related traits in the former.
MATERIALS AND METHODS
Experimental stocks
The experimental stocks were laboratory strains derived from wild-caught flies subjected to a protocol of artificial selection to increase tolerance to different concentrations of T. terscheckii's secondary metabolites during larval rearing. Wild-caught flies were sampled in 10 locations of northwestern Argentina (see Padró et al., 2018 for coordinates of sampling locations) during the austral summer of 2013, and the progeny of wild-caught inseminated females were used to establish isofemale lines. A total of 43 isofemale lines of D. buzzatii and 46 of D. koepferae were established and maintained under controlled conditions (L12:D12 photoperiod at 25 ± 1°C) in Drosophila standard rearing medium (hydrated starch and killed commercial yeast, Padró et al., 2018).
To generate the initial experimental population with the highest genetic variation possible, three generations of mass-breeding (~2000 adults) of each species were performed, pooling all available isofemale lines. From these mass-breeding populations, 200 inseminated females were transferred into independent rearing cages corresponding to one of the following regimes: Control (100% standard medium) composed of hydrated starch (Maizena) and killed commercial yeast (Levex), Selection regime 1 (S1, 50/50% standard/T. terscheckii's chlorenchyma powder medium) or Selection regime 2 (S2, 25/75% standard/T. terscheckii's chlorenchyma powder medium). For the chlorenchyma powder medium, we extracted the layer of chlorenchyma tissue rich in allelochemicals from the stems of T. terscheckii. This tissue was homogenized in a blender, dehydrated (78hs oven-dry at 50°C), sieved to a fine powder, and stored dry. This tissue was used for the selection regimes given that it contains the higher phenylethylamine alkaloid and medium-chain fatty acid concentrations of the plant (Reti & Castrillón, 1951). Given that larval survival is a major fitness-related trait, only strains maximizing larval survival under selection regimes were considered to be adapted to the high concentrations of T. terscheckii chlorenchyma (Padró et al., 2018). After 50 generations, D. buzzatii's S1 and D. koepferae's S2 maximized larval survival (and evolved longer developmental times) in comparison with Control strains. Hence, only these “Tolerant (T)” strains, along with the Control (C) strains, were assessed in the present study; the low viability of the D. buzzatii S2 strain prevented it from being maintained in laboratory conditions in the long term. Population size was kept at 200 breeding individuals per selection treatment to avoid bottleneck effects and maintained in discrete generations under uncrowded larval conditions for 50 generations. The experimental populations were held in a controlled photoperiod (L12:D12) and constant temperature (25 ± 1°C). Experiments were set right after the experimental evolution process to avoid confounding effects of genetic drift and under the same (rearing) environmental conditions.
Common-garden rearing
To ensure comparable conditions for phenotypic comparisons between Control (C) and Tolerant (T) strains, 50 first instar larvae from each selection strain were transferred to vials containing 5 g of a common rearing medium (50/50% w/w standard and chlorenchyma medium). Upon emergence, individuals were separated by sex prior to sexual maturity and maintained under uncrowded conditions with ad libitum food until the start of the experiments.
Metabolic rate
Three days after emergence, metabolic rate was measured in 9–10 mature virgin individuals of each Strain × Sex combination as follows:
Respirometry
We utilized a high-resolution TR-2 system from Sable System International (SSI; Las Vegas, NV, USA), with a Li-Cor (LI-6251) CO2 infrared analyzer (Lincoln, NE, USA; resolution 0.1 ppm CO2). To begin, air that was free of CO2 and H2O was drawn through low-permeability Bev-A-Line tubing at a flow rate of approximately 80 mL min−1 STP using a SS4 sub-sampler from SSI. The analog outputs from the CO2 analyzer and air flow rate measuring devices were connected to an A/D converter (SSI UI-2, with 16-bit basic accuracy = 0.05%). For a detailed description of the setup, see Rolandi et al. (2014). The data were acquired at 1 Hz, digitized, and processed using the SSI ExpeData data acquisition and analysis software. Prior to conducting the measurements, the CO2 analyzer was calibrated with gaseous nitrogen to define zero and a standard CO2 concentration of 97 ± 5 ppm (Linde Gas S. A. Group, Buenos Aires, Argentina).
Experimental procedure
We utilized a closed system with the dynamic injection technique for constant volume respirometry (for description, see Bartholomew et al., 1985; Lighton, 2018; Schilman, 2017). Briefly, we used 6-mL syringes with a three-way valve and a drilled hole very close to the outermost limit of the fully withdrawn plunger as the chamber (see Figure S1A). An array of 8 chambers was first washed with CO2-free air and water at a high flow rate, all of them with flies inside except for one that was empty (control). Next, each syringe was disconnected from the air current, and the plunger was pushed to the 4 mL mark of the syringe (so that the hole is outside the chamber), and the three-way valve was closed to seal the chamber with a fly inside (Figure S1B). Each closed chamber was then placed inside an SSI PTC-1 Peltier Effect cabinet at 25 ± 0.2°C, which was controlled by an SSI Pelt-5 temperature controller for a period of time ranging from 1.5 to 3 h. After this period, a 2 mL air sample from the syringe was injected into a stream of H2O/CO2-free air flowing at 80 mL per minute and then analyzed using an IRGA-CO2 analyzer, and the enclosure time of each fly was calculated to determine the individual rate of CO2 production.
Data transformation and analysis
Then, the measured CO2 from the empty chamber (control), which was about two orders of magnitude lower than the measured values (CO2 produced by each fly), was subtracted from the other seven recordings of chambers with flies.
Starvation resistance
Fifteen replicates for each Sex*Strain combination were conducted 3 days after emergence. Five virgin adults per replicate were placed in each vial (replicate) containing agar 1.5% (Foodchem Inc., USA) and antifungal solution (methylparaben 1.8 g/L, Nipagin, Sigma-Aldrich, USA), which provided moisture but no food.
Fecundity
Three days after emergence, 40 sexually mature virgin females and 80 sexually mature virgin males from each strain were placed together in a 200 cm3 chamber with standard medium. Two replicates were set for each strain. Ten fertilized females of each strain were then individually placed in 45-mL plastic vials containing 1.5% agar and commercial yeast as substrate and allowed to oviposit for 24 h. Afterwards, vials were renewed, and the process was repeated for 8 consecutive days in D. buzzatii and 4 days in D. koepferae, as there were no eggs after the fourth day in the latter. The eggs were then counted in the Petri dishes, and fecundity was estimated as the mean number of eggs laid per female per day for each species separately.
Longevity
Ten 45-ml vials with 5 three-day-old virgin adults per replicate for each Sex × Strain combination were set. Each vial contained 10 mL of 1.5% agar, antifungal solution (Nipagin 1.8 g/L), and commercial yeast as substrate. Individuals were transferred to new vials every 2 days until the end of the experiment. Dead individuals were counted at 9, 14, and 19 h (light cycle). Longevity of each individual was estimated as the time (in hours) elapsed from the start of the experiment to the checkpoint when its death was recorded.
Statistical analyses
To determine differences between species, strains, and sexes, we applied GLMs, utilizing the gls and glmmTMB packages (v 1.1.9) in R (v. 4.0.0) (Brooks et al., 2017; Lindstrom & Bates, 1990). Three-way factorial GLMs with Species, Strain, and Sex as fixed factors and a Gaussian distribution were employed for metabolic rate, longevity, and starvation resistance, whereas a two-way GLM with Species and Strain as a fixed factors and a Gaussian distribution was used for fecundity. The emmeans package (v. 1.10.7) was used for post hoc contrasts, and p-values were corrected using the Holm–Bonferroni method (Lenth et al., 2019).
RESULTS
Mean phenotypic values (and corresponding confidence intervals) for each trait measured in each strain and species are reported in Table S1. Table 1 shows the principal results regarding the GLM analyses for each trait assessed.
| Trait | Source of variation | Chi square | df | p-value |
|---|---|---|---|---|
| Metabolic rate | Species | 0.2182 | 1 | 0.6400 |
| Strain | 1.1163 | 1 | 0.2900 | |
| Sex | 5.0137 | 1 | 0.0200 | |
| Species × Strain | 0.3409 | 1 | 0.5500 | |
| Species × Sex | 0.0026 | 1 | 0.9500 | |
| Strain × Sex | 0.5441 | 1 | 0.4600 | |
| Species × Strain × Sex | 4.8274 | 1 | 0.0200 | |
| Starvation resistance | Species | 595.244 | 1 | <0.001 |
| Strain | 77.052 | 1 | <0.001 | |
| Sex | 18.4115 | 1 | <0.001 | |
| Species × Strain | 228.7137 | 1 | <0.001 | |
| Species × Sex | 1.1404 | 1 | 0.2855 | |
| Strain × Sex | 1.5261 | 1 | 0.2167 | |
| Species × Strain × Sex | 3.8291 | 1 | 0.0503 | |
| Fecundity | Species | 100.192 | 1 | <0.001 |
| Strain | 16.377 | 1 | <0.001 | |
| Species × Strain | 18.489 | 1 | <0.001 | |
| Longevity | Species | 5675.5788 | 1 | <0.001 |
| Strain | 0.0386 | 1 | 0.8442 | |
| Sex | 13.0228 | 1 | <0.001 | |
| Species × Strain | 4.8663 | 1 | 0.0277 | |
| Species × Sex | 61.5914 | 1 | <0.001 | |
| Strain × Sex | 15.5591 | 1 | <0.001 | |
| Species × Strain × Sex | 0.1103 | 1 | 0.7398 |
- Note: Bold: p-values < 0.05. Abbreviation: df, degrees of freedom.
Metabolic rate
GLM analysis detected a significant interaction between strains, sexes, and species in the metabolic rate (Figure 1A, Table 1). According to post hoc contrasts, no differences were observed between combinations of species, strain, and sex factors (Table S2).
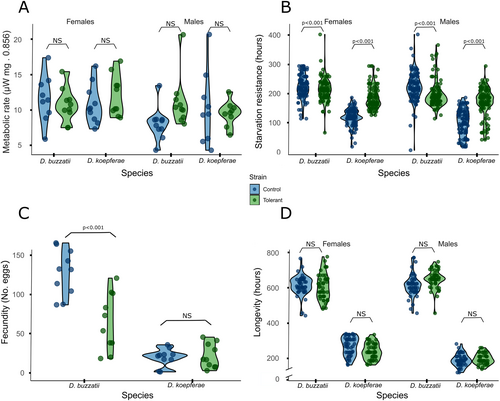
Starvation resistance
Differences in starvation resistance between sexes, strains, species, and the interaction between strains and species were significant (GLM, Table 1, Figure 1B). Therefore, contrasts between combinations of strain and species were performed while, separately, contrasts between sexes were also analyzed. Control outlived the tolerant strain in D. buzzatii. In contrast, the tolerant strain exhibited a remarkably higher survival in starvation conditions than the control in D. koepferae. Interspecific differences were observed since D. buzzatii outlived its sibling species. Finally, females outlived males (Table S2).
Fecundity
Differences in fecundity between strain, species, and the interaction between both variables were significant (GLM Table 1, Figure 1C). According to post hoc contrasts, D. buzzatii exhibited a higher fecundity than its sibling species; the fecundity in the control strain in D. buzzatii was higher than that in the tolerant strain, while no differences between strains were observed in D. koepferae (Table S2).
Longevity
Differences in longevity between sexes and between species were significant. Further interactions between strain and species, strain and sex, and sex and species were also significant (GLM, Figure 1D, Table 1). Therefore, contrasts between combinations of both factors of each of the three significant interactions were performed. No differences between control and tolerant strains were observed either in D. buzzatii and D. koepferae.
Interspecific differences were observed between species where D. buzzatii outlived its sibling species (Table S2).
Significant differences between strains were observed in each sex. In this sense, control females outlived tolerant females, while tolerant males exhibited higher longevity than control males. In the case of D. buzzatii, males outlived females, while in D. koepferae, females exhibited higher longevity than males.
DISCUSSION
Correlated evolution constitutes a critical factor in the evolution of lineages, involving much of their phenotypic variation (Mauro & Ghalambor, 2020; Price & Langen, 1992). In this study, we investigated correlated evolution in life history and physiological traits associated with increased tolerance to phytochemicals in artificially selected strains of two Drosophila species with differing degrees of cactus host specialization.
D. buzzatii, a generalist species, demonstrated the capacity to exploit various cacti genera as hosts (Hasson et al., 1992; Soto et al., 2012). It exhibited higher fecundity, longevity, and starvation resistance compared with its sibling species. However, when adapted to high concentrations of T. terscheckii phytochemicals, D. buzzatii showed reduced fecundity and starvation resistance, suggesting a general fitness cost due to increased energy allocation for detoxification.
In contrast, D. koepferae, as a specialist species, has a more restricted distribution intimately linked to the limited species of cacti it exploits, likely due to a reduced capacity to occupy vacant ecological niches. D. koepferae showed no such trade-offs and even demonstrated enhanced survival under starvation conditions, indicating a more efficient adaptive strategy with minimal fitness costs.
These interspecific differences in fecundity, longevity, and starvation resistance align with previous studies (Sambucetti et al., 2005; Soto et al., 2012). However, the patterns of trade-offs and costs in fitness-related traits have diverged since the speciation event, reflecting the unique adaptations to phytochemicals in each species. Despite these ecological adaptations, metabolic rates remained similar between the two species.
According to the disposable soma theory, organisms maximize fitness by energy allocation to different metabolic functions (Kirkwood & Austad, 2000; Kirkwood & Cremer, 1982; Kirkwood & Holliday, 1979). Adaptation to high concentrations of T. terscheckii phytochemicals in D. buzzatii led to a reduction in fecundity and starvation resistance, while metabolic rate and longevity remained unaffected. This suggests a general fitness cost due to increased energy expenditure on detoxification, compromising other fitness components to maximize larval survival in the selective regime. Consequently, the production of eggs in females and the maintenance of the adult soma might be compromised to achieve a higher detoxification capacity.
The disposable soma theory predicts a trade-off between survival and reproductive functions, with each evolutionary strategy being specific to species and populations (Kirkwood & Austad, 2000; Kirkwood & Cremer, 1982; Kirkwood & Holliday, 1979). This theory is empirically supported in several species, including Drosophila (Kirkwood & Austad, 2000; Patridge & Mangel, 1999; Rose, 1991, 1999). Previous studies described a trade-off between survival and reproductive output in D. buzzatii, showing differences between natural populations (Kreiman et al., 2023; Norry et al., 2006; Sambucetti et al., 2005; Scannapieco et al., 2009). Our results showed that while longevity was not affected in D. buzzatii, fecundity output was reduced, indicating a cost in reproductive output but maintaining adult survival. However, under starvation conditions, survival was also reduced, suggesting that adaptation to T. terscheckii phytochemicals led to general fitness costs in adult traits such as fecundity and survival under starvation conditions. It is important to note that fecundity was measured in mated females, while longevity and starvation resistance were measured in virgin females and males, which may affect the results due to physiological and hormonal differences. Also, as genetic correlations between traits may explain the observed correlated evolution in fitness-related traits during experimental evolution, further experiments dissecting the genetic basis of detoxification are required.
The inferred ancestral host for the common ancestor of the Drosophila repleta group, which includes D. buzzatii, is the Opuntia genus, with host shifts to columnar cacti occurring multiple times in evolutionary history (Oliveira et al., 2012). Our results suggest that experimental evolution simulating a forced host shift led to rapid correlated evolution in key traits related to adult fitness. This supports the idea that hosts are a primary environmental factor driving the evolution of phytophagous insects. Most phytophagous insects specialize in a few plant families, making it difficult to exploit less frequently used hosts (Bernays & Graham, 1988; Futuyma & Moreno, 1988; Jaenike, 1990). This was evident in our experiments, where D. buzzatii achieved higher larval survival in the mild S1 regime compared to the more challenging S2 regime (Padró et al., 2018). The adaptation to S1 likely incurred energetic costs affecting several fitness-related traits, suggesting that adapting to new hosts in the wild may drive correlated evolution in crucial organism functions.
Conversely, D. koepferae populations maximized larval survival in the more demanding S2 regime (Padró et al., 2018). No reproductive or survival costs were observed, and under starvation conditions, tolerant strains exhibited increased survival. Additionally, the metabolic rate was unaffected, indicating no energy cost associated with adaptation to the selective regime. This efficient adaptation without significant fitness costs may be due to D. koepferae's specialization to T. terscheckii, which contains similar phytochemical concentrations to the selective regime. These findings are consistent with D. buzzatii's inability to ecologically displace D. koepferae in regions where columnar cacti are the main hosts (Fanara et al., 1999; Soto et al., 2014).
In summary, our findings suggest that the energetic costs in D. buzzatii provide the best explanation for the observed correlated evolution. As a generalist cactus feeder, D. buzzatii incurred costs in fitness-related traits absent in the specialized D. koepferae. These results highlight the role of host specialization in shaping evolutionary trade-offs and underscore the complexity of adaptive responses to phytochemical stress. The exploitation of a marginally used host can impose costs on crucial fitness-related traits, such as adult lifespan and reproductive output. Moreover, ignoring variation in tolerance to phytotoxins would overlook the diversity of responses across species. This variation is essential for understanding how different species manage to exploit toxic plants. Neglecting this aspect would result in a simplified view of insect–plant interactions, failing to account for the nuanced ways in which insects evolve strategies to detoxify or avoid harmful substances.
AUTHOR CONTRIBUTIONS
Santiago Bouzas: Data curation; formal analysis; investigation; methodology; writing – original draft; writing – review and editing. Valeria P. Carreira: Conceptualization; data curation; formal analysis; funding acquisition; investigation; supervision; writing – review and editing. Pablo E. Schilman: Formal analysis; investigation; methodology; resources; writing – review and editing. Lucas Kreiman: Formal analysis; investigation; writing – review and editing. Ignacio M. Soto: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
This work was supported by the National Agency for Scientific and Technological Promotion and with funds granted to I.M.S. (PICT 2021-0426), V.P.C. (PICT-2018-00753) and P.E.S. (PICT 2018-02810). V.P.C., P.E.S., and I.M.S. are fellow members of CONICET.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




