Influence of host larval weight of Anastrepha ludens on production parameters and quality attributes in the mass rearing of the parasitoid Diachasmimorpha longicaudata
Abstract
Larvae of Anastrepha ludens (Loew) (Diptera: Tephritidae) are non-native hosts of the parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). These hosts appear to be a very good resource for the development of D. longicaudata in a wide range of larval weights. Therefore, the present study aimed to evaluate whether the larval weight of A. ludens influences the emergence and mass-rearing parameters of D. longicaudata, including the parasitoid's adult attributes and host selection capacity. Three mean larval weights of 29, 25, and 21 mg were considered as high, medium, and low categories for the analysis. The tests included the effect of management of host larvae in the mass rearing of the parasitoids. In addition, evaluations of flight ability, survival, fecundity, and size were carried out in the adults that emerged from the larvae of the various weight categories. There was a direct relationship between host larval weight and parasitoid pupal weight, but there was no relationship with the emergence of adult parasitoids. However, a high percentage of females emerged from heavier larvae. Similarly, although adults that developed in the heaviest larval category were larger, adult quality parameters were not affected. In the evaluations of host selection, the parasitoids oviposited randomly in the larvae from the various weight categories; there was only selection if larvae of different weights were mixed. In addition, superparasitism did not differ between larval weight categories. In the evaluation of competition for hosts in cages under field conditions, larger female parasitoids that developed in the heaviest host larvae landed more frequently on infested fruit, but oviposition was similar between females of different sizes. In conclusion, the larval weight of A. ludens influenced the size of the host puparia and of adults of D. longicaudata, but the emergence and attributes of the developed adults were similar. Therefore, it can be concluded that the low larval weights of A. ludens evaluated in this study are within the range for proper development of D. longicaudata in mass rearing. This allows several options to produce hosts at a lower cost without negatively affecting the quality of parasitoid production.
INTRODUCTION
Host size is generally considered a factor that directly influences the performance, size, and evolution of adult parasitoids that develop inside the host (Elzinga et al., 2003). There is evidence that parasitoid development in larger hosts favors host regulation, survival, progeny, fecundity, and host-searching ability (Cohen et al., 2005; Reudler et al., 2007; Ueno, 2015). Larger hosts offer abundant food resources and facilitate adequate parasitoid feeding, with a high availability of energy for dispersal activities and a high amount of protein essential for fecundity (Firlej et al., 2006). The availability of larger hosts also allows a change in strategy from individual to gregarious oviposition by female parasitoids (West et al., 2001).
There is a guideline in parasitoid mass rearing that indicates that using larger hosts is a primary requirement to maintain the quality of the production (Ode & Heinz, 2002; Paine et al., 2004). Most of the quality standards of mass production are established based on the size or weight of the host (Beckage & Gelman, 2004). According to Charnov & Skinner (1985), there is a direct and beneficial relationship between host size and weight and the performance, competitiveness, or quality of the developed parasitoids. One advantage of using larger hosts is that a female-biased offspring sex ratio can be maintained (Ode & Heinz, 2002).
However, there are also cases where an increased host weight or size does not have a direct effect on the biological attributes of the parasitoids. This may be mainly because heavier hosts generate more defenses or may be less physiologically viable, which means that their management requires a more expensive investment for the development of parasitoids (Xu et al., 2008; Liu et al., 2011). This calls into question the direct relationship between parasitoid quality and host size (Häckermann et al., 2007).
In various countries of the Americas, the parasitoid Diachamimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) is mass-reared using larvae of the genus Anastrepha (Diptera: Tephritidae) as hosts. This is a case where an introduced Indomalayan parasitoid was used to control a native pest in the Neotropical region (Meirelles et al., 2016). At the beginning of the host-searching process, D. longicaudata responds favorably to semiochemicals emitted by the interaction between Anastrepha larvae and the fruits they infest (Greany et al., 1977; Carrasco et al., 2005). The viability of Anastrepha larvae as hosts for the oviposition and subsequent development of D. longicaudata is very broad (López et al., 1999; Ovruski et al., 2011); the development of D. longicaudata is successful in various Anastrepha species – for example, Anastrepha ludens (Loew), Anastrepha suspensa (Loew), Anastrepha obliqua (Macquart), and Anastrepha fraterculus (Wiedemann). Some advantages in the management of the host by D. longicaudata have been found; for example, the oviposition of eggs in larvae of A. suspensa is mixed with the presence of entomopoxviruses, which decrease the antagonistic activity of the host (Lawrence, 2005).
The rearing of D. longicaudata at the Moscafrut Facility, in Metapa de Dominguez, Chiapas, in southeast Mexico, produces more than 50 million puparia per week with more than 50% emergence (from parasitized host larva to pupa) using A. ludens larvae as hosts (Cancino & Montoya, 2006). The parasitoids developed in A. ludens have been reported as efficient control agents of populations of Anastrepha spp. (Montoya et al., 2000).
The mean weight of third-instar A. ludens of 24.06 ± 2.02 mg at 9 days of larval development is considered the basic parameter indicative of maintenance of stability in the quality of parasitoid production (Cancino et al., 2006). However, it is unknown whether the weight of A. ludens larvae influences the various attributes of D. longicaudata as a control agent of fruit fly populations. Maybe Anastrepha larvae of lower weights are efficient hosts for the development of D. longicaudata, maintaining its important biological and behavioral attributes, as this parasitoid species originates from larvae of Asian Bactrocera spp., which are in most cases smaller than Anastrepha larvae (Vargas et al., 1993).
Considering the above, we proposed the following objectives: (1) to determine the effect of the host larval weight of A. ludens on the mass-rearing of D. longicaudata, (2) to study the influence of A. ludens larval weight on various attributes of D. longicaudata adults, (3) to assess the preference of D. longicaudata for host larvae of different weights and the over-oviposition (superparasitism) in different larval weights, and (4) to assess the competitiveness of adult parasitoids developed in larvae of different weights.
In general terms, 70% of the production cost of D. longicaudata corresponds to the costs of the diets for host larval development. Therefore, it is important to determine the effect of using lighter-weight larvae of A. ludens, which can be used as hosts in the mass rearing of D. longicaudata, as lighter-weight host larvae can be produced using low-cost options such as developing hosts at higher larval densities per kg of diet, as well as preparing larval diets with cheaper ingredients or eliminating or reducing proportions of high-cost ingredients (torula yeast, corn cob fractions, etc.). This would result in a substantial reduction in the cost of the mass production of D. longicaudata.
MATERIALS AND METHODS
Biological material
Anastrepha ludens larvae and D. longicaudata parasitoids from the respective mass rearing laboratories of the Moscafrut Facility (SADER-IICA) were used. The larvae are irradiated (45 Gy) before being exposed to the parasitoids to avoid the emergence of flies from unparasitized larvae. This parasitoid has been maintained in this facility for a period of close to 500 generations. Since approximately the 100th generation, the host larval weight of 24.06 ± 2.02 mg was established as the quality standard for mass production of D. longicaudata. The standard was defined based on an analysis of more than 50 lots of mass production, each of which was related to the respective quality parameters such as larval mortality, parasitoid pupal weight, and parasitoid adult emergence (standard operation procedures of quality control of parasitoids, Moscafrut Facility; Cancino et al., 2006).
The temperature during the development of the parasitoids from egg to pupa was 26 ± 2 °C. The laboratory tests were carried out at 24 ± 2 °C and 60–80% r.h.
Determination of various larval weights
The analysis of the larval weight of A. ludens as a host for the development of D. longicaudata was performed considering a range of 20–28 mg. The evaluations were carried out with three categories established with the criteria of low (20 mg), medium (24 mg), and high (28 mg) weight. The weight categories were obtained from a sample of 500 g of 9-day-old third-instar A. ludens. The larvae were mixed with cob powder (Mafornu, Ciudad Guzmán, Jalisco, Mexico) and passed through a seed separating machine (Lab-line Instruments, Melrose Park, IL, USA) where they were classified into three size groups. The weights of each group were determined using a Pioneer PA512C semi-analytical balance (Ohaus, Parsippany, NJ, USA) with samples of 100 larvae. Host larvae used in the standard rearing of D. longicaudata were used as a control.
Random sampling was performed for the evaluations of the effects of mass-rearing management process, although the means are reported according to weight category. For practical reasons, the evaluations of parasitoid attributes and larval weight selection were performed using only samples of each weight category.
Effect of mass-rearing management process on host larval weight
In order to know the effect of the standard mass-rearing management process on host larvae of different weights, host mortality at 72 h after exposure, pupal weight, parasitoid emergence, and offspring sex ratio were evaluated. The standard process involves massive separation (4–5 million larvae) of larvae from the diet by vibration. Approximately 15 kg of larvae mixed with diet are separated by placing them in hexagonal cylinders (160 cm long, 60 cm diameter) with 1-cm-diameter lateral perforations, which are covered with galvanized metal mesh (8 threads per 2.5 cm). The cylinders are placed horizontally in a metallic structure where they rotate and vibrate mechanically. Larvae without diet are received in trays (58 × 52 × 11 cm) and packed in fiberglass trays (38 × 27 × 11 cm) for irradiation (dose of 4.5 Gy, applied at a rate of 3.9 Gy/min, ration rate), and are subsequently distributed in trays at a ratio of 1:1 (vol/vol) with recycled diet (used for the larval development of A. ludens, sterilized and dehydrated). The larvae processed this way were called ‘with process’ (WP). These larvae were compared with larvae obtained with a control treatment where they were separated from the diet by washing small volumes of 0.5 L of larvae (ca. 16000 larvae) with a sieve, and they were called ‘without process’ (NP). The larvae of different weights obtained with the two procedures were exposed to parasitoids to determine the effect of the management process on parasitoid development. Samples of 100 larvae were taken randomly from each weight category. Each sample was weighed in a semi-analytic balance (Pioneer PA512C), placed in Petri dish-type parasitization units (Petri dish base of 10 cm in diameter and 1.5 cm in height, covered with tricot fabric secured with an elastic band), and exposed to D. longicaudata of 5–10 days of age at a female:male ratio of 30:15 in a Hawaii cage (27 × 27 × 27 cm wooden frame; Wong & Ramadan, 1992).
After exposure, the larvae were kept on a diet for 24 h, and then washed and placed in vermiculite for pupation inside cylindrical plastic containers (7.5 cm diameter, 7 cm high). Samples of 100 units were used to count the dead host larvae 72 h after exposure, and on day 14 they were separated from the pupation substrate and pupal weight (mean weight of pupae in the host puparia and empty puparia) was obtained with a semi-analytical balance (Pioneer PA512C). After adult emergence, the females and males were counted to obtain the emergence percentage and sex ratio.
Influence of host larval weight on parasitoid attributes
These tests were performed with adult parasitoids that emerged from larvae-pupae obtained from each larval weight category. The sample of adult parasitoids was identified by their respective category. Four attributes (quality parameters) were assessed: flight ability, survival, fecundity, and size.
Flight ability
One hundred 14-day-old pupae (1 day before emergence) developed in each larval weight category exposed to parasitoids were placed separately in black PVC tubes (10 cm diameter, 9.8 cm high). The internal walls of the tubes were previously covered with neutral talc to prevent the emerged adult parasitoids from walking out. Each tube was introduced in a Plexiglas cage (30 × 30 × 30 cm). The flying parasitoids that came out of the tube were removed daily, and on day 5, the number of flying parasitoids was obtained from the difference between the number of empty puparia and the number of walking adults contained inside the tube.
Survival
A sample of 30 females and 15 males emerged from each larval weight category was placed in 30 × 30 × 30 cm Plexiglas cages without water and food. Daily survival was measured by removing the dead parasitoids every day from the 1st day until reaching total mortality. The number of surviving parasitoids per day was used to calculate survival percentage and construct the corresponding survival curves.
Fecundity
Another sample of 30 females and 15 males emerged from each larval weight category was placed in 30 × 30 × 30 cm Plexiglas cages with water and food (honey). Females were kept for up to 6 days for sexual maturation. In order to determine female fecundity capacity, 100 host larvae (of standard weight) were exposed to the female parasitoids in a Petri dish-type parasitization unit (as described above) for 1 h per day for five consecutive days (6–10 days of age). The exposed larvae were kept in plastic containers (7.5 cm diameter, 7 cm high) with vermiculite until adult emergence. The number of emerged parasitoids was recorded and associated with the number of mother parasitoids to obtain the mean offspring number per female per day.
Size
The size of the female ovipositor and the size of the body, wings, antennae, and hind tibiae of the females and males emerged from each larval weight category was measured with a Zeiss Stemi 305C stereoscopic microscope (Carl Zeiss, Jena, Germany) coupled with a digital camera (Zeiss AxioCam Erc5s) and with image editor software (Zeiss Blue v.3.0). Ten adult parasitoids were taken randomly from dead individuals of each weight category during the different evaluations.
Parasitoid preference for host larval weight
- Choice with separate options. One hundred larvae from each of the three weight categories and 100 control larvae were exposed separately; each sample was placed separately in a Petri dish-type exposure unit. All larvae were exposed at the same time to 120 females and 60 males of D. longicaudata for 1 h, during which the females were allowed to select the best option.
- No choice. Samples of 100 larvae of each weight category and a control were exposed separately. Each sample was exposed to 30 females and 15 males of D. longicaudata that were confined in separate cages. Larval exposure was also for 1 h.
- Choice with options without separation. In this evaluation, four samples of 100 larvae without separating them by weight category were exposed to 120 female and 60 male parasitoids for 1 h in a Petri dish-type unit each. After exposure, the larvae were separated mechanically by weight (for which larval size was used as an indicator).
After parasitoid exposure, the larvae from each weight category were washed and kept at 26 °C and 60–80% r.h. for pupation. Parasitoid preference was determined by the emergence of adults.
Superparasitism in different larval weights
One hundred larvae of each weight category were exposed to 30 females and 15 males separately in a Hawaii cage for 1 h. The larvae were kept on a diet for 24 h, then placed on pupation substrate (vermiculite), and 2 days later, the scars per puparium (attempts or oviposition) were counted using a stereoscopic microscope (Zeiss Stemi 305C) in samples of five pupae, and the immature stages of the parasitoids were also counted by dissecting the pupae.
Competition for hosts between parasitoids of different sizes in field cages
Twenty female parasitoids (5 days old) were placed in a cage of 1 × 1 × 0.4 m. The females were divided into four groups with five females each corresponding to parasitoids developed in the three selected larval sizes and a control group. Parasitoid size was observed to be related to host weight in previous evaluations. The various parasitoid groups were identified inside the cage by a colored dot (yellow, red, blue, and green) on the dorsal part of the thorax, which was applied manually with vegetable paint (McCormick and Company, Naucalpan, Mexico) and a no. 0 camel hair paintbrush. Five guavas artificially infested with 20 larvae of A. ludens (9 days old, standard size) were previously hung from the ceiling of the cage with a wire hook. One guava was placed in the center of the cage and the other four were separated from the central fruit by 20 cm in the direction of the corners of the cage. To infest the guavas, a circular opening was made on the top of the fruits to remove the pulp with a spoon, which was then mixed with the larvae and introduced back into the fruit. The opening was covered again and sealed with elastic film (Parafilm M, Darmstadt, Germany). Ten min after introducing the parasitoids, observations were made every 10 min for 1 h to determine the total number of parasitoids of each size that landed or oviposited on/in the five fruits. Observations were made in the same way in another evaluation without competition, where the parasitoids were separated by size (developed in different larval sizes). In this case, five female parasitoids (5 days old) per size category were introduced separately in three 30 × 30 × 30 cm cages with a guava infested with 20 larvae. The observations without competition were made at the same time and a control evaluation was considered. The test was performed in an orchard with mango and zapote trees. Four repetitions were carried out at a mean 28.5 °C and 90% r.h.
Data analysis
The mean values of each larval weight category were analyzed with ANOVA and Tukey's multiple range test. The various production parameters (with and without the management process) and adult attributes between the larval weight categories were also analyzed with ANOVA and Tukey's multiple range test. A linear regression was used to evaluate the relationship between larval weight vs. adult parasitoid emergence, host larval weight vs. parasitoid pupal weight (transformed to an exponential variable), and host larval weight vs. female parasitoid emergence. The survival curves of the adults developed in the various larval weights were analyzed using a long-rank test. Mean emergence in the host preference choice and no-choice tests and the superparasitism data (no. scars and immature parasitoid stages) obtained in the various treatments were also analyzed with ANOVA and Tukey's multiple range test. The mean number of female parasitoids that landed and oviposited on/in the fruits in the field cages was analyzed using a bifactorial design (adults developed in the larvae of different weights with and without competition) of repeated measures with a binominal response using a generalized linear model. All analyses were performed in JMP v.16 (SAS Institute, Cary, NC, USA) and R v.4.0.2 (R Core Team, 2020).
RESULTS
Effect of mass-rearing management process on host larval weight
Larvae were divided into three size categories, with the following mean (± SE) weights (mg) (range in parentheses): high, 28.77 ± 0.29 (26.60–31.50); medium, 25.36 ± 0.30 (22.40–25.90); and low, 20.96 ± 0.29 (19.40–22.10), and the control samples taken from the standard mass rearing weighed 25.02 ± 0.31 (22.60–26.40) mg. Mean larval weight differed among the three larval size categories, both in larvae with (WP: F3,98 = 34.73) and without the management process (NP: F3,115 = 115.68, both P < 0.0001). Larval mortality 72 h after exposure to parasitoids was not different between the larval weight categories, both in larvae with and without process (WP: F3,38 = 55.03, P = 0.43; NP: F3,40 = 43.73, P = 0.75). However, parasitoid pupal weight differed between larval weights in the larvae with process (F3,16 = 16.78, P < 0.0001) – pupal weight was similar in medium-weight and control larvae, and heavier larvae led to heavier pupae. In parasitoids that developed in larvae without process, there were differences in pupal weight between low- and high-weight larvae (F3,21 = 5.07, P = 0.008). Still, parasitoid adult emergence was similar between the three larval weight categories either with or without process (WP: F3,115 = 0.28, P = 0.83; NP: F3,98 = 1.65, P = 0.18). In general, the mean sex ratio obtained indicated that more females emerged from heavier larvae (Table 1).
| Larval weight category | Larval weight (mg) | Larval mortality (%) | Pupal weight (mg) | Emergence (%) | Sex ratio (♀/♂) | ||||
|---|---|---|---|---|---|---|---|---|---|
| WP | NP | WP | NP | WP | NP | WP | NP | ||
| Low | 20.96 ± 0.29c | 2.52 ± 0.59a | 1.23 ± 0.30a | 10.36 ± 0.58c | 10.36 ± 0.70b | 51.32 ± 0.87a | 54.23 ± 2.07a | 2.20 | 1.14 |
| Medium | 25.36 ± 0.30b | 1.62 ± 0.37a | 0.94 ± 0.26a | 13.62 ± 0.58b | 12.28 ± 0.70ab | 54.52 ± 1.08a | 56.52 ± 1.59a | 3.24 | 1.83 |
| High | 28.77 ± 0.29a | 2.15 ± 0.54a | 0.82 ± 0.31a | 16.18 ± 0.58a | 14.02 ± 0.64a | 52.35 ± 1.25a | 53.12 ± 1.66a | 5.26 | 2.12 |
| Control | 25.02 ± 0.31b | 2.25 ± 0.64a | 1.09 ± 0.36a | 13.52 ± 0.58b | 11.73 ± 0.70ab | 53.20 ± 0.84a | 55.63 ± 1.86a | 4.49 | 2.15 |
- Means within a column followed by different letters are significantly different (Tukey's test: P < 0.05).
There was no relationship between host larval weight and parasitoid emergence (F3,36 = 3.88, P = 0.06; r2 = 0.09). Host larval weight and parasitoid pupal weight had a direct relationship (F3,138 = 240.79, P < 0.001; r2 = 0.63). Also parasitoid pupal weight and number of emerged females had a direct relationship (F1,140 = 55.20, P < 0.001; r2 = 0.29; Figures 1 and 2).
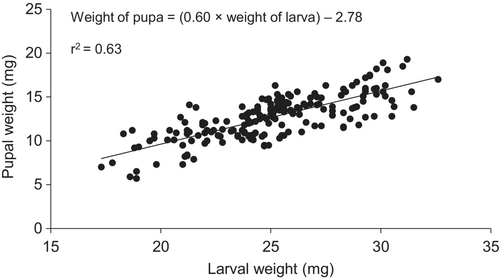
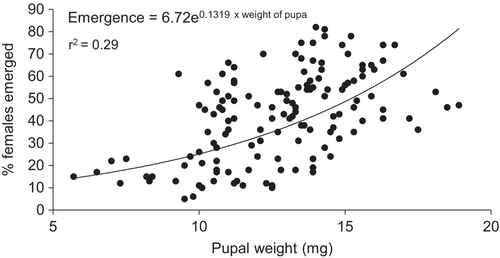
Influence of host larval weight on parasitoid attributes
Flight ability did not differ among host larval weight categories (F3,41 = 0.49, P = 0.68). Similarly, larval weight did not influence female fecundity (F3,124 = 0.55, P = 0.64; Table 2). There was also no effect of host larval weight on the survival of females that emerged from pupae developed in the three larval weight categories or the control treatment (χ2 = 0.24, d.f. = 3, P = 0.97; Figure 3).
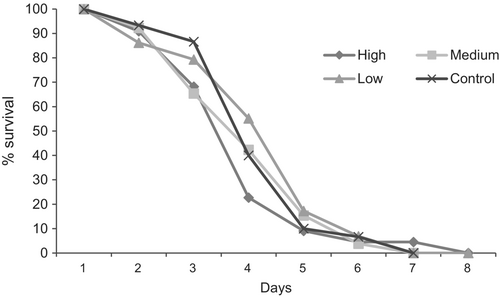
Parasitoid size variables did differ among the larval weight categories, both in females (body: F3,56 = 67.3; tibia: F3,56 = 82.21; ovipositor: F3,56 = 51.69) and in males (body: F3,56 = 29.37; antenna: F3,56 = 13.58; wing: F3,56 = 15.75; tibia: F3,56 = 19.58, all P < 0.001). In most cases, the parasitoids that developed in heavier larvae exhibited larger structures. Parasitoids with smaller structures were those that developed in low-weight larvae. In females, structure size was similar between females that developed in heavier vs. standard-weight larvae used in mass rearing, whereas in males, structure size was similar between males that developed in medium- vs. standard-weight larvae (Table 3).
| Larval weight category | Flight ability (%) | Fecundity (offspring/♀/day) |
|---|---|---|
| High | 75.24 ± 5.92a | 2.93 ± 0.32a |
| Medium | 75.68 ± 6.27a | 2.46 ± 0.30a |
| Low | 78.68 ± 5.61a | 2.60 ± 0.36a |
| Control | 83.61 ± 2.76a | 2.92 ± 0.30a |
- Means within a column followed by the same letter are not significantly different (Tukey's test: P > 0.05).
| Larval weight category | Body | Antenna | Wing | Hind tibia | Ovipositor | |
|---|---|---|---|---|---|---|
| Females | High | 5.13 ± 0.07a | 7.04 ± 0.09a | 4.54 ± 0.03a | 1.86 ± 0.02a | 5.63 ± 0.07a |
| Medium | 4.60 ± 0.07b | 5.48 ± 0.25b | 4.17 ± 0.08b | 1.27 ± 0.02b | 5.23 ± 0.09b | |
| Low | 3.97 ± 0.06c | 4.59 ± 0.27c | 3.73 ± 0.05c | 1.37 ± 0.05b | 4.29 ± 0.09c | |
| Control | 5.32 ± 0.08a | 6.95 ± 1.29a | 4.72 ± 0.06a | 1.88 ± 0.02a | 5.76 ± 0.10a | |
| Males | High | 5.57 ± 0.10a | 8.25 ± 0.20a | 4.87 ± 0.05a | 1.92 ± 0.02a | |
| Medium | 4.65 ± 0.27b | 7.07 ± 0.45b | 4.10 ± 0.23b | 1.46 ± 0.12bc | ||
| Low | 3.50 ± 0.07c | 5.67 ± 0.16c | 3.57 ± 0.06c | 1.22 ± 0.04c | ||
| Control | 5.03 ± 0.12ab | 7.01 ± 0.23b | 4.31 ± 0.09b | 1.65 ± 0.02b | ||
- Means within a column and within a sex followed by different letters are significantly different (Tukey's test: P < 0.05).
| Larval weight category | Choice 1 | No choice | Choice 2 |
|---|---|---|---|
| High | 67.75 ± 6.14a | 60.00 ± 7.39a | 32.60 ± 6.78b |
| Medium | 68.00 ± 5.01a | 64.00 ± 8.26a | 58.16 ± 6.19ab |
| Low | 69.20 ± 5.49a | 61.50 ± 8.26a | 47.16 ± 6.19ab |
| Control | 70.46 ± 5.49a | 63.00 ± 8.26a | 60.16 ± 6.19a |
- Means within a column followed by different letters are significantly different (Tukey's test: P < 0.05).
Parasitoid preference for host larvae of different weights
There were no differences in adult emergence among larval weight categories in either the choice or the no-choice tests (choice: F3,16 = 0.04; no-choice: F3,13 = 0.05, both P = 0.98). A difference was only observed if the larvae of different weights were not separated when exposed to the females and were separated by category after exposure (F3,19 = 3.73, P = 0.02) – the heaviest larvae were selected less frequently (Table 4).
Superparasitism in larvae of different weights
The number of scars and the number of immature stages did not differ among larval weights (scars: F3,186 = 0.10, P = 0.95; immature stages: F3,186 = 0.09, P = 0.96). Both numbers (scars: 3.3–3.4 range; immature stages: 1.5–1.6) were higher in heavier larvae; however, no relationship indicating a decrease with lower larval weight was found.
Competition for hosts between parasitoids of different sizes in field cages
Females developed in heavier larvae were observed to land more frequently on the fruits (χ2 = 8.74, d.f. = 3, P = 0.03), and the landing frequency of these females was higher without competition with other females (χ2 = 3.89, d.f. = 1, P = 0.04) and showed no differences over time (χ2 = 4.64, d.f. = 5, P = 0.46) (Figure 4A). However, oviposition frequency did not show differences between groups of parasitoids developed in larvae of different weights (χ2 = 2.37, d.f. = 3, P = 0.49). There was also no interaction with competition (χ2 = 2.34, d.f. = 1, P = 0.12) and mean ovipositing frequency did not show differences over time (χ2 = 7.40, d.f. = 5, P = 0.19) (Figure 4B).
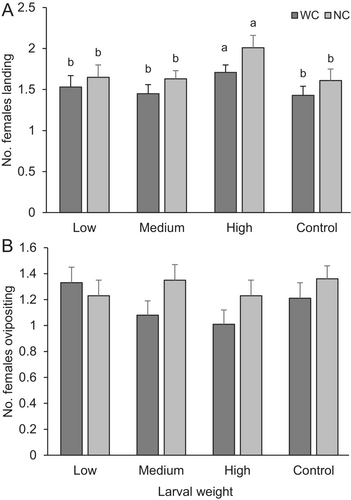
DISCUSSION
There was no relationship between the emergence percentage of D. longicaudata and the weight of the A. ludens larval host. However, a positive relationship was observed between host larval weight and parasitoid pupal weight; larger adult parasitoids emerged from heavier host larvae, and more of these parasitoids were female. Nevertheless, this did not affect the various attributes of adult parasitoids considered as quality parameters for mass rearing.
The differences in larval weight were not determinant in the selection of a host and the development of the parasitoids. This is evident by the similar percentages of adult parasitoid emergence among the various larval weight classes. Even though larger parasitoids that developed in heavier larvae were more successful at landing on infested fruits in competitive conditions, this was not the case with oviposition. According to Lawrence (1981), larger adults of D. longicaudata can displace smaller ones in a direct interaction under laboratory conditions. The results obtained in the field evaluations in the present study indicate that, indeed, larger parasitoids (developed in heavier larvae) show higher dominance with respect to landing on infested fruit, possibly displacing the smaller females. Nevertheless, oviposition was similar between the different groups. Larger females may have the advantage of occupying the space more quickly and having a better opportunity to select their host (Gao et al., 2016). This may encourage small females to optimize their resources by shortening the perching period on the fruit and directly engaging in oviposition. Even though we did not evaluate this in the present study, it could explain the similarity in oviposition activity.
In this context, firstly, due to the wide weight range of A. ludens as a host for D. longicaudata, it may be considered a very complete food resource for parasitoid development and to obtain parasitoids with high-level attributes. Diachasmimorpha longicaudata is an introduced parasitoid that originally evolved in Bactrocera larvae, an Asian genus with typically smaller larvae compared to Anastrepha species (Chang, 2009). Since its introduction to the Americas, D. longicaudata has been an exotic parasitoid species that has established itself very quickly using Anastrepha spp. as hosts (Ovruski et al., 2000). One advantage of Anastrepha larvae as hosts may be that they provide more than the necessary quality and quantity in terms of nutritional requirements. Another advantage may be the susceptibility of Anastrepha larvae to the entomopoxvirus that D. longicaudata females introduce in the hosts during oviposition, as they can suppress the host's immunological antagonism. This was observed in a study by Lawrence (2005), where the entomopoxvirus reduced the immunological reactions of A. suspensa larvae. This strategy may not be effective in Bactrocera spp. larvae, as a decrease in the development of D. longicaudata has been observed due to the presence of immunological reactions (Mohamed et al., 2008). This may be the product of a more specific antagonistic reaction resulting from parasitoid–host coevolution (Kraaijeveld et al., 1998). In the case of Anastrepha larvae, little information is available on their capacity of immunological reaction to parasitoids. An exception is A. obliqua (Silva et al., 2002; Gómez-Alonso et al., 2022), which has the ability to suppress the development of immature stages of native parasitoids – Doryctobracon crawfordi (Viereck) or Opius hirtus (Fischer) –, also possibly the result of a coevolutionary relationship (Poncio et al., 2015; Gómez-Alonso et al., 2022). However, the development of D. longicaudata in A. obliqua larvae is viable, although at a lower level of emergence, based on results reported both in the laboratory and in the field (Sivinski et al., 1997; Isiordia-Aquino et al., 2017).
Another indication that Anastrepha larvae are high-quality hosts for D. longicaudata is the production of a female-biased offspring sex ratio. This has been observed in D. longicaudata developed in larvae of A. ludens, A. fraterculus, and A. suspensa (Ashley & Chambers, 1979; Montoya et al., 2011; Van Nieuwenhove et al., 2012). More females in the progeny of this parasitoid is perhaps the most important result of the use of Anastrepha larvae in the rearing of D. longicaudata. A female-biased offspring sex ratio directly favors higher population increase rates (Dyson & Hurst, 2004; Weerawansha et al., 2022), which is fundamental in the use of augmentative releases to maintain substantial effects in the suppression of pest populations (Heimpel & Lundgren, 2000; Ode & Heinz, 2002). The use of Anastrepha larvae (i.e., A. ludens, A. fraterculus) as hosts allows the emergence of adults with a higher proportion of females in a consistent mean ratio of two females to one male, which does not occur with the use of larvae of other species such as Ceratitis capitata (Wiedemann), where the sex ratio is almost one to one (Ovruski et al., 2011; Altafini et al., 2021). Offspring sex ratio is the result of various factors that influence female oviposition (Henter, 2004; Mohamad et al., 2015). Host size is considered an essential factor because females that parasitize larger hosts are usually larger, in addition to the basic protein requirement for fecundity (Ueno, 1999, 2015). Another factor that influences a female-biased progeny in D. longicaudata is directly related to superparasitism (Montoya et al., 2011).
During the establishment of colonies of fruit fly parasitoids for mass rearing using Anastrepha eggs or larvae of A. ludens, A. obliqua, or Anastrepha serpentina (Wiedemann) as hosts, low emergence and development problems occurred (Zenil et al., 2004). For example, in the establishment of the egg-larval parasitoid Fopius arisanus (Sonan) and the larval parasitoid Diachamimorpha tryoni (Cameron) using A. ludens as a host, low emergence or poor development (structures such as deformed antennae or abdomen) has been observed (Cancino et al., 2009). It is unknown whether this non-viability is due to a deficiency or lack of adequacy in the diet or the antagonistic reactions of the host. However, D. longicaudata is more versatile, as it has been reported to rapidly adapt when new colonies of Anastrepha larvae or other Tephritidae species got established (Bautista & Harris, 1997; Viscarret et al., 2006; Ovruski et al., 2011).
According to Greany et al. (1977), D. longicaudata has structures that indicate the status of the host larva, such as size or physiological condition. This would imply that D. longicaudata has the capacity to discriminate hosts by developmental stage and optimize its sexual reproduction in suitable hosts. According to our results, D. longicaudata oviposited indiscriminately in larvae of different weights, which may indicate that D. longicaudata can develop in a wide weight range of Anastrepha host larvae. Even if the selection of larvae was more random, females were observed to prefer larvae of lower weight to oviposit, discriminating against higher weights. Consistent with these results, D. longicaudata has been reported to have the ability to develop in various instars of A. fraterculus and C. capitata (Rohr et al., 2019). Another indicator is the level of superparasitism observed, which was similar in the three larval weight classes. Superparasitism is very common in D. longicaudata when A. ludens larvae are used as hosts in a highly competitive and dynamic environment such as mass rearing (Montoya et al., 2011). This indicates that D. longicaudata does not increase oviposition when there are more resources, such as larvae with higher weight. Similarly, Altafini et al. (2013) did not observe differences in superparasitism by D. longicaudata between larvae of A. fraterculus and C. capitata, which differ in size.
It can thus be concluded that A. ludens larval weight does not have a direct influence on the emergence (independent of the management process) and attributes related to the production and quality of D. longicaudata. Larval mortality at 72 h after parasitization did not increase when larvae of lower weight were used, which indicates that this key mass-rearing parameter was not related to host larval weight. In addition, the parasitoids that emerged from larvae of the various weight categories did not show differences in survival and flight capacity, which are basic parameters that, together with adult emergence, define the quality of D. longicaudata in mass rearing. These results indicate that it is possible to reduce the costs in the production of A. ludens larvae as hosts. Immediate alternatives to achieve this are production at higher densities, reduction of high-cost ingredients (e.g., torula yeast, corn cob fractions, etc.), or the use of higher densities in oviposition units. However, it is important to clarify the aspects that must be taken care of to apply these changes in the mass rearing of both hosts and parasitoids. Firstly, it is important to maintain the high-quality parameters in the mother colonies of both hosts and parasitoids. Secondly, the management of mass production can have greater repercussions on low-weight larvae, and thus the necessary precautions must be taken with low-weight and undeveloped larvae because there is practical evidence of rapid changes in parameters such as oviposition, superparasitism, or sex ratio in a highly competitive and dynamic environment (López et al., 2009; Montoya et al., 2011).
An important question arises from this study: what is the optimal requirement of D. longicaudata to develop and nourish itself adequately using A. ludens larvae as hosts? The answer to this would ensure the production of parasitoids at reduced costs, with cheaper hosts and with great possibilities of improving parasitoid quality.
AUTHOR CONTRIBUTIONS
Jorge Cancino: Conceptualization (lead); data curation (lead); formal analysis (equal); investigation (lead); methodology (supporting); project administration (supporting); supervision (lead); writing – original draft (lead); writing – review and editing (lead). Amanda Ayala: Data curation (supporting); formal analysis (supporting); software (supporting); writing – original draft (equal); writing – review and editing (equal). Patricia López: Conceptualization (supporting); investigation (supporting); methodology (supporting); project administration (lead); supervision (supporting). Flor de María Moreno: Investigation (supporting); methodology (supporting); resources (supporting); supervision (supporting). Eduardo Solis: Funding acquisition (supporting); investigation (supporting); methodology (supporting); resources (supporting); supervision (supporting). Dina Orozco-Dávila: Conceptualization (supporting); funding acquisition (lead); project administration (supporting); resources (lead).
ACKNOWLEDGMENTS
We are grateful to the Technical Staff of the Mass Rearing Laboratory of Fruit Flies and Mass Rearing Laboratory of Parasitoids, which are both part of the Moscafrut Plant. The technical support from the staff of the Biological Control Department was essential to properly perform the evaluations.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




