Carbohydrate-rich diet increases critical thermal maximum in ants
Abstract
To understand species’ responses to climate change, we must better comprehend the factors shaping physiological critical thermal limits. One factor of potential importance is nutrient availability. Carbohydrates are an energy source that can directly affect an organism's physiological state. Ants are among the most omnipresent and ecologically relevant animal groups on Earth, and many ant species consume carbohydrate-based diets. Additionally, as ectotherms, ants are highly vulnerable to the effects of climate change. Here, we examined the relationship between foraging temperature, carbohydrate availability, and critical thermal maximum (CTmax) in ants (Hymenoptera: Formicidae). First, we conducted a laboratory experiment using 3–4 colonies of two species that forage at high temperatures (Camponotus blandus Smith and Dorymyrmex thoracicus Gallardo) and two species that forage at lower temperatures (Nylanderia fulva Mayr and Dolichoderus quadridenticulatus Roger). Each colony was divided into two experimental subcolonies, which were given diets containing different carbohydrate concentrations (5 vs. 20% sucrose solutions). We then measured CTmax. We also collected ants belonging to these species in the field and measured their CTmax. We found that CTmax was highest for the two species that forage at higher temperatures. For C. blandus and D. thoracicus, workers given 20% sucrose had higher CTmax than workers given 5% sucrose. No diet-mediated differences in CTmax were seen for N. fulva and D. quadridenticulatus workers. Additionally, the experimental ants in both treatment groups had higher CTmax than their field-collected conspecifics, except in the case of C. blandus. If carbohydrate-rich diets can boost heat tolerance in some species, it is possible that changes in resource availability could determine how climate change affects ants, especially species with carbohydrate-based diets. Furthermore, these impacts could ripple across the entire trophic network.
INTRODUCTION
Climate change can affect the geographical distribution of species, shaping biodiversity patterns over space and time (Parmesan, 2006; Bellard et al., 2012). To understand the responses of animals to climate change, we need to better comprehend their physiological traits (Arnan & Blüthgen, 2015; Roeder et al., 2021a). Of key importance is a species’ critical thermal maximum (hereafter, CTmax): the maximum temperature that induces muscle spasms and/or death under controlled laboratory conditions (Sinclair et al., 2016). An organism's CTmax reflects its physiological tolerance of high temperatures. The CTmax is particularly important in the lives of poikilothermic ectotherms (Sinclair et al., 2016; Bennet et al., 2021), whose body temperatures depend exclusively on environmental temperatures. Furthermore, CTmax can determine species distribution and abundance at regional and local scales (Deutsch et al., 2008; Kearney & Porter, 2009; Clusella-Trullas et al., 2011; Buckley et al., 2012; Arnan & Blüthgen, 2015; Sunday et al., 2019), and, ultimately, their responses to disturbance and climate change (Roeder et al., 2021a; Nascimento et al., 2022). Finally, because CTmax also affects species performance, activity, and behaviour, it may also impact the outcome of ant-plant mutualisms (Fitzpatrick et al., 2014; Tamashiro et al., 2019).
Whereas CTmax is thought to be under genetic control, it may also be influenced by diet (Bujan & Kaspari, 2017). The nutritional composition of an organism's diet can have physiological effects, where the availability of certain nutrients can yield physiological benefits (Feldhaar, 2014). Proteins are mainly important in growth and reproduction, whereas carbohydrates can be more directly deployed for immediate physiological needs such as for warming up (Dussutour & Simpson, 2009). Indeed, a carbohydrate-rich diet can allow for quick physiological changes, particularly in the CTmax of species that forage at high temperatures (Bujan & Kaspari, 2017). For instance, species that mainly forage during the hottest hours of the day could use the extra energy to increase CTmax, whereas species that mainly forage at lower temperatures would not need the extra carbohydrates (Diamond et al., 2012a; Roeder et al., 2021a). However, to date, research exploring these ideas is lacking.
Ants (Hymenoptera: Formicidae) are among the most ubiquitous, diverse, and abundant groups of ectothermic animals on Earth and represent about 15% of the total biomass attributable to terrestrial animals (Hölldobler & Wilson, 1990; Elizalde et al., 2020). This is reflected in their ecological importance, as ants mediate many ecological processes and provide core ecosystem services, including seed dispersal, bioturbation, and protection against herbivory, among many others (Del Toro et al., 2012; Elizalde et al., 2020). Ants are an excellent study system for exploring how carbohydrate availability could affect CTmax. First, they are very sensitive to environmental changes and rapidly respond to shifts in both environmental conditions and resource availability (Arnan et al., 2012; Andersen, 2019). Second, CTmax is highly variable among ant species, populations, and even nestmates (Villalta et al., 2020; Roeder et al., 2021b; Nascimento et al., 2022). Whereas many abiotic and biotic factors may explain this variability, much of it remains unexplained (Nascimento et al., 2022). Finally, carbohydrates are an important nutritional component in the diets of many ant species because greater carbohydrate availability boosts colony activity, worker survival, and food resource defence (Hölldobler & Wilson, 1990; Blüthgen & Fiedler, 2004; McGlynn & Parra, 2016). Carbohydrate-rich diets might also allow ants to continue foraging under extreme thermal conditions that would otherwise be intolerable. For instance, sucrose can be stored and quickly metabolised, thereby generating ATP (Suarez et al., 1996) and fuelling the synthesis of heat shock proteins, which help limit the effects of heat stress (King & MacRae, 2015). However, to date, research is lacking on how dietary carbohydrates can affect ant CTmax (and that of other ectotherms). Only one study has explored this question (Bujan & Kaspari, 2017). It found that CTmax increased by 5 °C in Azteca chartifex Forel worker ants fed carbohydrates. We need more research to understand whether this response is seen in other ants and whether there is interspecific variability in dietary modulation of CTmax. It is essential to examine this relationship given its major implications for how ants in particular, and organisms in general, respond to environmental changes. If higher levels of carbohydrates can boost critical thermal maxima, then dietary changes could help mediate the impacts of climate change and other disturbances in animal populations, at least in the short term.
This study experimentally examined the effects of carbohydrate availability on CTmax using four ant species that are naturally active under different thermal conditions: two that forage at high temperatures and two that forage at lower temperatures. We reared 3–4 colonies per species in the laboratory. Each colony was split into two subcolonies that were fed different carbohydrate concentrations, and worker CTmax was subsequently measured. We also collected workers of these species in the field to characterise their natural CTmax values. We tested two hypotheses: (1) ant species active at higher temperatures will have higher CTmax than ant species active at lower temperatures, and (2) ants given higher concentrations of carbohydrates will have an increased CTmax, with a more pronounced effect in ant species active at higher temperatures.
MATERIALS AND METHODS
Study area
This study was conducted in the city of Garanhuns (8°52′55″S, 36°30′07″W, 950 m above sea level) in Pernambuco, a rural region in northeastern Brazil. The climate is classified as tropical wet and dry (Köppen climate classification type As), with a dry summer and wet winter. Annual mean temperature is 21.7 °C, and annual mean precipitation is 660 mm. Humidity is high throughout the year (68–85%; Climate-Data, 2021). Despite the tropical climate, temperatures are mild due to the altitude. The study area is ideal for addressing our hypotheses because it has high temperatures during the day (mean maximum temperature: 28.5 °C in summer and 23.4 °C in winter) and relatively low temperatures at night (mean minimum temperature: 19.3 °C in summer and 17.5 °C in winter).
Study species
We worked with four ant species that commonly occur in the study area: two species that are active during the hottest periods of the day, Dorymyrmex thoracicus Gallardo (Dolichoderinae) and Camponotus blandus Smith (Formicinae), and two species that are active during the milder to colder periods of the day, Dolichoderus quadridenticulatus Roger (Dolichoderinae) and Nylanderia fulva Mayr (Formicinae). We have observed these activity patterns in the field: during the hottest hours (around noon), C. blandus and D. thoracicus were active, but D. quadridenticulatus and N. fulva were not (X Arnan, pers. obs.). We deliberately chose pairs of species within two subfamilies to control for phylogenetic history: one member of each pair was more heat tolerant, and the other was more heat intolerant.
Dorymyrmex thoracicus frequently occurs in dry disturbed environments in South America and usually nests in bare soil. It can be found in urban buildings and gardens, and it feeds on insects, seeds, and sugary substances (Cuezzo & Guerrero, 2012). Camponotus blandus is a polymorphic, generalist species that is often found in urban areas. It nests in the soil, decaying tree trunks, or termite mounds; it predominantly forages in trees during the day and preferentially harvests sugar- or animal-based resources (Orivel & Dejean, 2002). Dolichoderus quadridenticulatus is an omnivorous species with arboreal habits that is common in tropical forests. It nests on the ground, in the leaf litter, in tree trunks, or in the tree canopy and forages both on the ground and in the vegetation (Aguiar & Santos, 2017). Nylanderia fulva is a native species in South America but a major invasive species in the southern USA. Its nests are in the ground and contain multiple queens. The nests are interconnected, generating extraordinarily dense populations whose numbers greatly exceed the combined numbers of all the other ants in the community. They feed on small insects, small vertebrates, and aphid honeydew (Kumar et al., 2015).
Study design
Field sampling
Between December 2020 and November 2021, we collected 20 workers from four colonies of each study species using an entomological aspirator. The only exception was C. blandus, for which ants were collected from two colonies. The workers were placed in 50-ml Falcon tubes. Each tube contained a small cotton ball soaked in water, which kept the workers from drying out. The workers were immediately taken to the laboratory, and their CTmax values were measured within 4 h of collection to limit acclimation effects.
Carbohydrate supplementation experiment
Between April and July 2021, we collected 3–4 colonies of each species using an entomological aspirator. We gathered enough of each colony (i.e., 50–100 workers) to perform the experiments; no larvae, queens, or sexuals were sampled. Each colony was stored in a plastic container (ca. 15 × 20 × 15 cm) lined with a talcum powder and alcohol paste, which prevented the ants from escaping. Each colony had access to an artificial nest: a Falcon tube with water behind a cotton plug. The colonies were kept in the laboratory for 2 days (25–30 °C, 50–65% r.h., L12:D12). They were fed a Bhatkar-Whitcomb diet (i.e., a mixture of agar, whole egg, honey, vitamins, and minerals; Bhatkar & Whitcomb, 1970) and, occasionally, dead insects. After this acclimation period, each colony was equally divided into two experimental subcolonies. They were deprived of food for 24 h. Then, one subcolony was given a 5% sucrose solution, the other subcolony was given a 20% sucrose solution. The subcolonies had access to the solutions for 24 h. Although ants could theoretically compensate for lower concentrations of dietary nutrients by increasing the amount of food collected, this strategy does not appear to be used in colonies without larvae, such as ours (Dussutour & Simpson, 2009). Following this 24-h period, we sampled 10 workers from each subcolony to measure CTmax. We did not use an experimental control (i.e., starved colonies) because it would have represented unrealistic conditions. Instead, we compared the CTmax values of the field-collected ants and the ants from the experiment.
Thermal tolerance assays
Overall, we measured CTmax for 537 workers. To estimate CTmax, workers were placed in an Eppendorf tube plugged with cotton. The tube was positioned in a randomly chosen slot of a dry heat bath (8 × 6 Thermal-Lok Dry Heat Bath; USA Scientific, Orlando, FL, USA) prewarmed to 38 °C. We then increased the temperature at a rate of 1 °C every 3 min until 100% of the ants had lost locomotor function or had died; we noted the temperature.
After the assays, we measured Weber's length for all the workers using a stereomicroscope (2.5×) and graph paper. Weber's length is the diagonal length of the mesosoma in profile from the point at which the pronotum meets the cervical shield to the posterior basal angle of the metapleuron (Weber, 1938); it serves as a proxy for body size (Weiser & Kaspari, 2006; Boyle et al., 2021). It was used to control for the potential correlation between body size and physiological traits (Peters, 1983).
Statistical analysis
To test our hypotheses, we performed linear mixed models (LMMs) with normal error distribution. In all the models, body size (Weber's length) was a covariate, and colony identity was a random factor (i.e., to control for within-colony effects). To test hypothesis 1, we compared CTmax among species using the field-collected ants; CTmax was the response variable, and species the explanatory variable. To test hypothesis 2, we compared CTmax among the experimental groups; CTmax was the response variable, and the explanatory variables were species, sucrose concentration, and their interaction. Then we compared CTmax between the field-collected ants and the ants from the experiment. We used two models in which CTmax was the response variable; the explanatory variables were species, data group (field vs. experimental), and their interaction. One model was performed for each treatment group (5 vs. 20% solution). It was impossible to conduct all these comparisons within a single model because of the nature of the data. Notably, the field-collected ants belonged to different colonies than the experimental ants, which came from the same batch of colonies. Whenever species emerged as a significant variable in any of the models, we conducted a Tukey honestly significant difference (HSD) test (95% confidence level) to compare results between species. Model residuals were visually inspected to make sure that assumptions of normality and homoscedasticity had been met. All the statistical analyses were carried out in R v. 4.1.1 (R Core Team, 2021).
RESULTS
There were significant differences in CTmax among species for the field-collected ants (LMM: χ2 = 575.9, d.f. = 3, P < 0.0001). The two more heat-tolerant species had higher CTmax values (significantly higher in C. blandus than in D. thoracicus: mean ± SE = 48.9 ± 0.1 vs. 47.5 ± 0.2 °C) than did the two more heat-intolerant species (significantly higher in D. quadridenticulatus than in N. fulva: 45.7 ± 0.1 vs. 43.4 ± 0.1 °C) (Figure 1). In the experimental results, there was an interaction between sucrose concentration and species (LMM: χ2 = 8.9, d.f. = 3, P = 0.031). Thus, C. blandus and D. thoracicus workers that had been given a higher-sucrose diet displayed higher CTmax values (0.3 and 0.5 °C higher than in the lower-sucrose group); however, N. fulva and D. quadridenticulatus were unaffected by sucrose concentration (Figure 1).
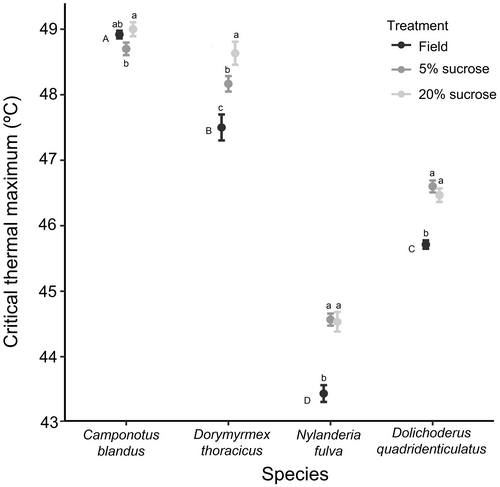
When we compared the field-collected and experimental ants, we observed an interaction between data group and species (LMM; 5% sucrose: χ2 = 6.6, P = 0.0002; 20% sucrose: χ2 = 11.7, P = 0.0090, both d.f. = 3). CTmax was higher for all the experimental ants than for the field-collected ants in three of the four study species, notably D. thoracicus (5% sucrose: 0.7 °C higher; 20% sucrose: 1.2 °C higher), D. quadridenticulatus (0.9 °C higher), and N. fulva (1.2 °C higher) (Figure 1). Body size was not significant in any of the models (P > 0.5).
DISCUSSION
We demonstrated that the activity patterns of four common ant species in a semiarid tropical region were associated with CTmax values. More interestingly, we found that ants given higher concentrations of carbohydrates (i.e., a 20% sucrose solution) had slightly greater CTmax than ants given lower concentrations of carbohydrates. However, this difference was only seen in the more heat-tolerant species. Furthermore, in three of the study species, the experimental ants (those that received carbohydrates) had greater CTmax than the field-collected ants. Such was the case for one of the highly heat-tolerant species and the two more heat-intolerant species. Our findings have important implications for understanding the adaptations that ectotherm species already possess and could use to deal with climate change.
Our first hypothesis – ant species active at higher temperatures would have higher CTmax than ant species active at lower temperatures – was supported by our findings. Arnan & Blüthgen (2015) demonstrated that CTmax was a good predictor of the activity niches of 17 Mediterranean ant species. Diamond et al. (2012a) used species-specific CTmax to predict the responses of ant communities to experimentally induced forest-floor warming in eastern North America. They found that CTmax predicted the responses of 19 ant species to warming in areas where environmental conditions were relatively close to ant CTmax. In our study area, D. thoracicus and C. blandus are active during daily peaks in temperature, and they might be foraging close to their CTmax values. Our results support the idea that CTmax could be a good predictor of ant activity in semiarid tropical regions. Other studies found that CTmax was more strongly associated with microclimatic than macroclimatic gradients (Baudier et al., 2018; Nascimento et al., 2022). This result could explain why CTmax is tightly linked to local ant activity.
Our second hypothesis – higher concentrations of carbohydrates would boost CTmax in ants, especially in species that are active at higher temperatures – was also supported by our results. To date, research exploring this relationship in ants, and other ectotherms in general, is extremely rare. A single laboratory study has shown that CTmax climbed by 5 °C when A. chartifex workers were given a supply of carbohydrates (Bujan & Kaspari, 2017). In our study, the CTmax of D. thoracicus and C. blandus, the two most heat-tolerant species, increased by only a few 10ths of a degree. These differences do not appear to be explained by phylogenetic history, as the genera Dorymyrmex and Azteca belong to the subfamily Dolichoderinae (Moreau & Bell, 2013). The ants’ different lifestyles could play a role – Azteca are largely arboreal nesters and foragers, whereas Dorymyrmex are ground nesters and forage both on the ground and in the vegetation. Arboreal nesters are more exposed to ambient temperatures and, consequently, tend to have higher CTmax (Kaspari et al., 2015). It might be that such species acquire greater thermotolerance by consuming a carbohydrate-rich diet (i.e., extrafloral nectar and aphid honeydew; Hölldobler & Wilson, 1990). However, we cannot rule out an effect of methodological differences. Whereas all our experimental colonies received a carbohydrate-based diet (albeit of different concentrations), the control colonies in Bujan & Kaspari (2017) received only water and were starved, which may have amplified the difference in CTmax. Regardless, our findings suggest that diet composition can affect ant CTmax.
Our experiment showed that CTmax increased in the most heat-tolerant ants. Ant species that forage at very high temperatures might quickly metabolise carbohydrates to fuel the synthesis of heat shock proteins (King & MacRae, 2015), which would allow them to remain active during hotter periods of the day or warmer seasons (Bujan & Kaspari, 2017). However, when we compared the CTmax values for the field-collected vs. the experimental workers, we found that carbohydrates could play a more generalised role in boosting thermal tolerance. Indeed, having greater access to carbohydrates could increase CTmax in more heat-tolerant and heat-intolerant species alike. This may be explained by a relationship between CTmax and foraging temperature. Whereas most ant species forage at temperatures far from their physiological limits (i.e., they are thermally risk adverse), some do collect rich food resources at temperatures close to their CTmax (Cerdá et al., 1998; Roeder et al., 2022). It is possible that the two more heat-intolerant study species have CTmax values that are similar to the temperatures at which they most commonly forage, which means that an increase in CTmax could allow them to forage over longer windows of time (Cerdá et al., 1998).
Although we know nothing about the carbohydrates that had been consumed by field-collected ants, we did see that the latter had lower CTmax than the experimental ants (except in the case of C. blandus). This finding fits with what Bujan & Kaspari (2017) observed. Consequently, it seems reasonable to hypothesize that this increase results from having greater access to a carbohydrate-rich diet. The question then arises: why did field-collected ants generally display lower CTmax? There are several possible explanations. First, our study was conducted in an urban area, where carbohydrates might be readily available (Carpenter & Savage, 2021) but of lower quality than those provided in the experiment, which were in liquid form (Penick et al., 2015; Stahlschmidt & Johnson, 2018). Indeed, past field research has demonstrated that ants prefer liquid carbohydrates (Cannon & Fell, 2002). Second, we offered an unlimited supply of carbohydrates in the experiment, and the colonies could thus modulate their resource consumption levels (Dussutour & Simpson, 2009). Third, ant colonies that are in their reproductive phase usually favour proteins over carbohydrates so that they can better nourish their larvae (Dussutour & Simpson, 2009). Consequently, if colonies in the field were in their reproductive stage at the time of sampling, they could have been consuming very low levels of carbohydrates. It is possible that there was no difference in CTmax for field-collected vs. experimental C. blandus workers because the former came from colonies that were not in their reproductive stage.
These hypotheses aside, our results help clarify the factors that could explain variability in thermal limits, both in ectothermic organisms in general and in ants in particular. We discovered that access to carbohydrates can affect the thermal maxima of workers from the same colony. It is not a stretch to imagine that such differences could scale up to the colony and species level. Differences in resource availability along environmental gradients could help account for the marked variability in ant CTmax that has hitherto remained unexplained (Diamond et al., 2012b; Diamond & Chick, 2018). Our results also have broader implications for biodiversity conservation. To better understand how disturbance, such as climate change, could affect ambient temperatures, it is important to explore the impacts of food resource availability. The latter may have major consequences for the development, persistence, and distribution of organisms. For example, ants mainly obtain carbohydrates from aphid honeydew and plant extrafloral nectaries (Hölldobler & Wilson, 1990; Del Toro et al., 2012). Honeydew production is greater in forested areas (Dixon, 1975), thus ant communities in such locations may be better equipped to navigate the potential negative effects of climate change (IPCC, 2018) because they will be able to boost their CTmax values. At the same time, nectar production might drop as disturbance intensity and drought increase in seasonally dry tropical forests, which will face higher and higher temperatures (Magrin et al., 2014). Consequently, the persistence of many ant species could be at serious risk: if ambient temperatures rise sharply and nectar levels decline, then ants could be unable to access the dietary carbohydrates they need to boost CTmax. That said, patterns of nectar production along environmental gradients remain unclear and might be context-dependent (Arnan et al., 2022; Câmara et al., 2022). Finally, we believe that our results can also be extrapolated to other ectotherm organisms, mainly invertebrates. Although we found no studies directly analyzing the effects of a carbohydrate-enriched diet on thermal tolerance in ectotherms other than ants, there is evidence showing that carbohydrate consumption by these organisms increases at higher temperatures (e.g., Jandt et al., 2010; Rho & Lee, 2017). This might be associated with an improved physiological condition of ectotherms to cope with these higher temperatures.
In conclusion, research in this area remains limited. Clearly, more work is needed to see how generalised these findings might be among the great variety of ectothermic organisms that exist on Earth. Moreover, we must explore the effects of nutrients other than carbohydrates at a range of concentrations, both in ants in particular and in ectotherms in general.
AUTHOR CONTRIBUTIONS
Allana Freires: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – original draft (equal). Carlos Souza Ferreira: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – original draft (equal). Geraldo Nascimento: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Xavier Arnan: Conceptualization (lead); funding acquisition (lead); investigation (equal); project administration (lead); supervision (lead); writing – review and editing (lead).
ACKNOWLEDGEMENTS
We thank J. Pearce-Duvet for editing the manuscript's English. This study received financial support from the Universidade de Pernambuco (191_APQ 2020, Process 427). XA thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for his productivity grant (PQ-2, Process 307385/2020-5). ASAF and CASF thank CNPq (PIBIC-CNPq, process 5832) and the Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE) (BIC-0842-2.05/20) for their science initiation grants, respectively. GN thanks FACEPE (IBPG-0649-2.05/20) for his research grant.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




