Lack of lipid accumulation in two species of chalcidoid wasps with secondarily evolved phytophagy
Funding information
This work was supported by a grant to JE from The Netherlands Organization for Scientific Research (NWO, VICI grant no. 865.12.003). The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the preparation and writing of the manuscript, or in the decision to submit the article for publication.
Abstract
Stable co-evolutionary relationships between species may result in the loss of autonomous synthesis of essential nutrients when these can be obtained from the ecological partner. Parasitoid insects obtain most of their nutrients from their host, and many lineages do not increase their adult lipid reserves even when feeding on a surplus of sugars. Several clades within the parasitoid Hymenoptera have lost the parasitoid lifestyle and switched to either a predatory or a phytophagous lifestyle. Here, we test whether adult lipid accumulation is reactivated in two species that independently evolved a phytophagous lifestyle from parasitoid ancestors. The larvae of Megastigmus aculeatus (Swederus) (Hymenoptera: Chalcidoidea: Torymidae) and Bruchophagus platypterus (Walker) (Hymenoptera: Chalcidoidea: Eurytomidae) obligatorily feed on seeds of specific plants. Seeds of wild host plants were collected and the emerging wasps were tested for the lipid accumulation phenotype using whole-body diethyl ether extraction. Both species had significantly reduced lipid content after 1 week ad libitum access to sugars compared to the lipid content at emergence, indicating a lack or negligible level of lipogenesis. Therefore, the secondary switch to phytophagy was not associated with recovery of lipid accumulation in these species. We discuss these results in relation to the lipid content of the host seeds and the reversibility of the lipid accumulation phenotype. We infer that the close relationship among seed-feeding insects and their hosts allows the seed-feeders to rely on a diet with predictable lipid quantities which relaxes the need for autonomous lipid synthesis.
INTRODUCTION
In insects, lipid accumulation mostly results from a diet rich in carbohydrates. Lipid reserves are important as an energy source during periods of poor nutrition and play a key role in survival and reproduction. Lipid accumulation is a conserved metabolic function, but some species have evolved deviations from the general pattern due to their specific lifestyle. Parasitoid insects feed on other animals as larvae and complete their development on a single host (Godfray, 1994). In many parasitoid species, the vast majority of their adult lipid reserves is carried over from the larval stage. Although adult parasitoids are capable of utilizing dietary carbohydrates to meet immediate energy demands (Jervis et al., 2008), the conversion of such carbohydrates to long-term storage in the form of lipids is impaired even in the presence of excess carbohydrates (Ellers, 1996; Giron & Casas, 2003; Visser & Ellers, 2008; Visser et al., 2017).
Phylogenetic analysis showed that a lack of lipid accumulation has evolved repeatedly in insects, each time concurrent with the origin of a parasitoid lifestyle (Visser et al., 2010). Impaired lipogenesis has also been found in several host-associated organisms unrelated to insects: mycorrhizal fungi (Luginbuehl et al., 2017), apicomplexan parasites (Mazumdar & Striepen, 2007), and the dandruff-fungus, Malassezia globosa Midgley, E Guého & J Guillot (Xu et al., 2007). Whereas in most animals some unsaturated fatty acids are classified as essential nutrients, in such fatty acid auxotrophs the dietary uptake of any lipid has become essential (Berg et al., 2006; Liu et al., 2015). Like parasitoids, these organisms share a stable co-evolutionary relationship with an ecological partner from which they can obtain essential nutrients such as lipids (Ellers et al., 2012).
Lipid accumulation is defined as a significant increase in per capita lipid content during a given observation time (Teixeira et al., 2003; Schmid et al., 2005; Guo et al., 2008). The lipid accumulation phenotype can readily be assessed in adult insects with a simple assay in which lipid content is measured using diethyl ether extraction before and after ad libitum sugar-feeding. This assay has merits as it is relatively fast and cheap to measure whole-body lipid content, thereby providing a measure for whole-body lipid stores that are directly linked to organismal fitness (Ellers, 1996). However, applying this technique has two drawbacks. Firstly, it is impossible to measure the same individual before and after treatment due to the destructive nature of the methods. Secondly, in situations where lipid reserves are depleted, one cannot distinguish between a limited amount of metabolic de novo fatty acid biosynthesis and complete absence of lipid production (Ruther et al., 2021). Metabolic regulation normally co-regulates these processes so that they do not happen at the same time as conversion inefficacies would result in a waste of energy (Berg et al., 2006). It is therefore unlikely that during depletion of lipid stores a simultaneous lipid biosynthesis occurs. This drawback does not apply when one is interested in lipid accumulation, or a lack thereof, as is the case in this manuscript. Using this assay, significant lipid accumulation was observed for non-parasitic insects such as Drosophila melanogaster Meigen and Athalia rosae (L.) (Visser et al., 2010). However, most parasitoid species tested did not accumulate lipids as adults despite feeding on a surplus of sugars (Visser et al., 2010; Wang et al., 2020b).
A lack of lipid accumulation in parasitoids seems not to be caused by gene loss or degradation. All species studied to date have the full complement of genes required for the de novo fatty acid biosynthesis pathway (e.g., Werren et al., 2010; Kraaijeveld et al., 2019; Wang et al., 2020b) and expression of these genes has been documented (Visser et al., 2012; Lammers et al., 2019; Wang et al., 2020b). Rather, it seems that sugar-feeding and de novo fatty acid biosynthesis have been decoupled through up to now unknown gene regulatory changes (Lammers et al., 2019). A few generalist parasitoid species seem to be exceptions and accumulated lipids in the assays, which shows that expression of the lipid accumulation phenotype potentially depends on ecological factors such as host range (Visser et al., 2010, 2018). A tentative explanation would be that generalists may not have the requisite host-manipulating abilities (Visser et al., 2010).
To better understand the conditions that determine the expression of the lipid accumulation phenotype, it is necessary to investigate species in a wider spectrum of lifestyles. Within the Chalcidoidea (Hymenoptera), a superfamily mostly consisting of parasitoids, there are several clades that have independently lost the parasitoid lifestyle and secondarily evolved a predatory or a phytophagous lifestyle (Clausen, 1940; Peters et al., 2017). Because of the close association between the lack of lipid accumulation and the parasitoid lifestyle, and given the reversibility of the lack of lipid accumulation, we might expect such groups to have regained their lipogenic capacity.
So far only two non-parasitoid species of cynipid wasps have been tested for their lipogenic ability: the gall-inducer Diplolepis rosae (L.), which manipulates its host plant’s physiology to increase lipid levels in cells surrounding the developing larva, and its inquiline Periclistus brandtii (Ratzeburg) (Harper et al., 2004; Visser et al., 2010). The adults of both species showed no increase in lipid reserves following sugar-feeding (Visser et al., 2010, 2013), despite their non-parasitoid lifestyle.
In the present study, we tested the lipid accumulation phenotypes of two genera in the superfamily Chalcidoidea that independently evolved a phytophagous lifestyle from parasitoid ancestors (Heraty et al., 2011, 2013; Munro et al., 2011; Peters et al., 2017). These chalcidoid wasps are seed-feeders – also called ‘seed-predators’ by Janzen (1971) and subsequent authors –, i.e., larvae feed and develop on or in seeds, rendering the seed inviable. Seeds are typically rich in oils (Janzen, 1971), which could enable the seed-feeding larvae to obtain a surplus of lipids and store them for adult use. We report the lipid content of these species under various treatments and relate these results to published data on lipid content of seeds in the wasp’s larval diet. If the larval diet has sufficient lipids to sustain the insects for their entire adult life, we expect selection for autonomous lipid production to remain relaxed (Lahti et al., 2009) and to find no expression of the lipid accumulation phenotype.
We focused on two unrelated genera of chalcidoid wasps that are present in The Netherlands and evolved phytophagy independently (Clausen, 1940; Munro et al., 2011): Megastigmus (Torymidae) and Bruchophagus (Eurytomidae). Species from both genera are considered pests as they attack seeds of plants with commercial value. Megastigmus is a genus of at least 126 species, of which 21 are found in the Western Palearctic (only 13 species are considered native). Its species’ biology is completely reviewed and revised by Roques & Skrzypczyńska (2003) and is briefly summarized here. The wasps have narrow host ranges; generally, a wasp species only attacks seeds of a single plant genus. Some species attack seeds in conifer cones (Pinaceae, Cupressaceae), whereas others oviposit in seeds of a few berry-bearing Rosaceae and some Anacardiaceae. Each individual develops inside an individual seed and winter diapause may extend up to several years. Eleven species of Megastigmus are known from The Netherlands (Gijswijt, 2003). As the host seeds are present once per year only, it is assumed that the wasps are univoltine. Five European species mostly reproduce by thelytokous parthenogenesis: Megastigmus aculeatus (Swederus), Megastigmus pictus (Förster), Megastigmus pinsapinis Hoffmeyer, Megastigmus pistaciae Walker, and Megastigmus suspectus Borries.
Bruchophagus is a genus of at least 32 species, most of which attack seeds of clover and related Fabaceae (McDaniel & Boe, 1991; Lotfalizadeh et al., 2007). Three species of Bruchophagus occur in The Netherlands (Gijswijt, 2003): Bruchophagus gibbus (Boheman) on Trifolium spp., Bruchophagus platypterus (Walker) on Lotus spp., and Bruchophagus roddi Gussakovskiy on Medicago spp. The biology of these species was studied by Batiste (1967) and is briefly summarized here. Females oviposit into seeds when the seedpod is in an intermediate stage of development. Only one egg is laid per seed and each individual larva feeds on a single seed only. Egg-to-adult development time is 29–36 days under greenhouse conditions, but larvae can undergo a facultative diapause extending development beyond winter. Host seeds are available for many consecutive months and consequently these Bruchophagus spp. reproduce for several generations each summer.
MATERIAL AND METHODS
Seed sampling
Seeds of a variety of plants were collected across The Netherlands when they were expected to be ripe, and hence were likely to contain fully-grown larvae or pupae of seed-feeding wasps of the target genera. Intact seed pods and berries were picked by hand from multiple plants and these were stored separately for each site and species in labelled plastic bags. In the laboratory, the seeds were put in a Petri dish in small cages (BugDorm model 4S2222; MegaView Science, Taichung, Taiwan), one for each site–species combination. Seeds were left in their original pods, cones, and berries, to not damage any. All seeds were incubated in a climate chamber at 20 °C, L16:D8 photoperiod, and 75% r.h. Food was not provided in the cages to ensure all wasps remained unfed prior to the treatments.
- Rowan (Sorbus aucuparia L.) berries on the island Schiermonnikoog (53.483°N, 6.183°E), on 16 October 2016. Megastigmus brevicaudis Ratzeburg is the only species from this genus known to attack Sorbus seeds.
- Rose (Rosa spp.) hips in Zuid-Kennemerland National Park (52.405°N, 4.590°E), on 15 February 2017. The species developing on seeds of this host plant are M. aculeatus, Megastigmus nigrovariegatus Ashmead, and Megastigmus rosae Boucek.
- Norway spruce [Picea abies (L.) H Karst.] cones from a forest near Lunteren (52.083°N, 5.642°E), on 22 October 2016. Megastigmus strobilobius Ratzeburg and possibly Megastigmus atedius Walker attack this tree species.
- Douglas fir [Pseudotsuga menziesii (Mirb.) Franco] cones from Lunteren, the same date and location as above. Megastigmus spermotrophus Wachtl is the only Megastigmus species known to feed on this tree.
- Birds-foot trefoil (Lotus corniculatus L.) seed pods from a roadside in Amsterdam (52.335°N, 4.800°E), on 26 July 2017. Bruchophagus platypterus is a common wasp in this plant’s seed pods.
Cages were checked for emerging wasps every 2 days. When wasps started to emerge, the cages were checked in the morning at around 09:00 hours, removing any wasps that emerged before that time. Then at around 13:00 hours, wasps that had emerged were certainly at most 4 h old and only these wasps were randomly allocated to treatments (see below). Two sets of collected seeds, rose hips and birds-foot trefoil seed pods, yielded a large number of seed-feeding wasps that were used in the experiments. All other insects that emerged from the seeds were stored separately. No seed-feeding wasps emerged from the other tested substrates (rowan berries, spruce and fir cones), and 34 male and 29 female parasitoids from the genus Bracon emerged from the rowan berries.
In total, 140 female Megastigmus wasps emerged between 27 March and 5 April 2017 (40–49 days after seed collection) from the collected rose hips, whereas not a single male emerged. All wasps belonging to the genus Megastigmus (Figure 1) were identified to species level using Roques & Skrzypczyńska (2003). Identification was confirmed by Dr. S. Ulenberg at Naturalis Biodiversity Centre (Leiden, The Netherlands). Voucher specimens are stored at the Department of Ecological Science at the Vrije Universiteit Amsterdam, The Netherlands, under no. ML.V001.016-017.

Bruchophagus platypterus (Figure 2) is hitherto only known from L. corniculatus and no other Bruchophagus have been found on this host (McDaniel & Boe, 1991). A total of 326 female B. platypterus emerged between 31 July and 7 August 2017 (5–12 days after seed collection) as well as a similar number of males. Hence, females were presumably mated. Voucher specimens of B. platypterus are stored under no. ML.V001.031-032.

Feeding treatments
Newly emerged female wasps were randomly assigned to one of three treatments: ‘emergence’, ‘fed1wk’, and ‘water1wk’. In total, 38, 40, and 36 female M. aculeatus were allocated to these three treatments, respectively. One specimen in the water1wk treatment was later identified as M. rosae and was therefore excluded from further analysis; all other emerged wasps were identified as female M. aculeatus. In total, 115, 112, and 99 female B. platypterus were allocated to the emergence, fed1wk, and water1wk treatments, respectively. Wasps in the emergence treatment were sacrificed on the day of emergence by placing them in a −20 °C freezer. Wasps allocated to a feeding treatment (fed1wk or water1wk) were kept in groups of maximum 18 females in a plastic tube (2.35 cm diameter, 7.5 cm high) with a sponge stopper and were provided with ad libitum access to either water (water1wk) or a fresh 20%-sucrose solution (wt/vol, fed1wk), which was applied to the stopper of the tubes. The sucrose solution has a sugar concentration similar to natural sources of carbohydrates encountered by free-living insects, such as nectar (Wykes, 1953) and honeydew (Hendrix et al., 1992; Fischer et al., 2005). Every 2nd day the water and sucrose solutions were renewed. All wasps were observed to start drinking soon after food had been offered. After 1 week, survival of these wasps was scored. Dead wasps were removed from the tubes and the survivors were killed by freezing.
Lipid content measurements
For measurements of dry weight and fat-free dry weight, we followed the protocol of David et al. (1975) and Ellers (1996), with modifications. A random subset of 24 female wasps from the emergence treatment, and 24 survivors from the fed1wk treatment, were selected for each species for lipid content measurements. These wasps were individually verified to have intact bodies under a microscope at up to 60× magnification (WILD M8; Leica, Wetzlar, Germany). Hind tibia length was measured as a proxy for body size independent of body mass using an SC180 camera and CELLSENS ENTRY v.1.18 software (Olympus, Tokyo, Japan). Hind tibia length was also measured for 24 wasps of the water1wk treatment to compare body size distributions among treatments. Wasps selected for lipid content measurement were placed in labelled glass vials (Lenz Laborglas, Wertheim, Germany), freeze-dried for 2 days and subsequently weighed on a UMT2 microbalance [readability (d) = 0.1 µg; Mettler Toledo, Greifensee, Switzerland]. Next, 4 ml di-ethyl ether (≥99.7%; VWR International, Darmstadt, Germany) was added to each vial in order to dissolve all lipids from the wasps’ bodies. After 2 days the wasps were removed from the ether and dipped in fresh ether to wash off any residue. All wasps were freeze-dried again and weighed on the same microbalance. Afterwards, all wasps were checked for integrity again. Wasps that lost body parts in the measurement process would have been removed from the analysis, but this was not required in any of these data sets.
Data analysis
Survival was compared between the two feeding treatments to validate the wasps’ feeding abilities and to verify their use of sucrose for somatic maintenance. Survival was scored as a binary variable: whether the individual was dead or alive after 1 week. Differences in survival of wasps between the water1wk and fed1wk treatments were analysed using generalized linear models with an overdispersion-corrected binomial distribution. Measurements of tibia length were compared among the treatments to verify whether bias due to body size is sufficiently precluded by randomization. Differences in mean body size were analysed using ANOVA, and the distributions of the data points were compared using two-sample Kolmogorov-Smirnov tests. The lipid content of each wasp was calculated as the dry weight before minus the dry weight after ether extraction. We initially planned to use ANCOVA with tibia length as covariate to test for differences in lipid content between the treatments; however, such an approach was precluded for both species tested due to violation of the assumption of homogeneity of variance and heteroscedasticity of the residuals of the tests. Instead, for each wasp, the lipid content was recalculated as the percentage lipid out of total body weight (wt/wt). These percentages lipid content were compared between treatments using Mann-Whitney U tests. Additionally, we investigated whether the feeding tube that housed the wasps had an effect on the observed lipid content using linear mixed models (of the formula: lipids ~ treatment , random = ~1|tube) with the function ‘lme’ from the R package ‘nlme’. Lipid accumulation would be inferred if mean lipid levels were significantly higher after 1 week of feeding than at emergence. All analyses were run in R v.4.0.3 (R Core Team, 2015). The data from this study are available in Dryad under reference no. wwpzgmsk0 (http://doi.org/10.5061/dryad.wwpzgmsk0).
RESULTS
Megastigmus aculeatus
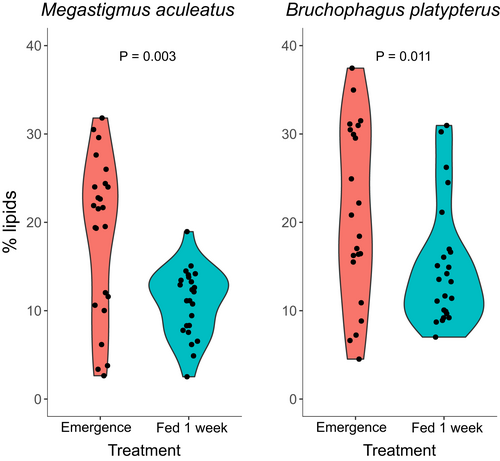
Survival of M. aculeatus was higher in the wasps that received a sucrose solution than in the wasps that were fed only water for 1 week (89 vs. 18%; GLM: χ2 = 48.85, d.f. = 1, P<0.001). Body size was highly variable among individuals of this species: hind tibia length ranged from 469.1 to 961.1 µm, and dry weight from 91.4 to 988.0 µg. The mean body size (i.e., tibia length) was similar between wasps at emergence and after feeding for 1 week on a sucrose solution (ANOVA: F1,46 = 0.54, P = 0.47), and the distribution of the data was similar as well (Kolmogorov-Smirnov test: D = 0.17, P = 0.9). Wasps had a higher lipid content at emergence than after feeding for 1 week on a sucrose solution (mean ± SE = 18.6 ± 1.8 vs. 10.9 ± 0.8%; Mann-Whitney U test: W = 431, P = 0.003; n = 24; Figure 3). Lipid content was also significantly different when including ‘feeding tube’ as a random effect in a linear mixed-effects model (treatment: F1,11 = 9.76, P = 0.0097).
Bruchophagus platypterus
Survival of B. platypterus was higher in the wasps that received a sucrose solution than in the wasps that were fed only water for 1 week (71 vs. 0%, respectively; GLM: χ2 = 313.3, d.f. = 1, P<0.001). Body size varied, with hind tibia length ranging from 401.6 to 556.6 µm, and dry weight from 106.4 to 307.1 µg. The mean body size (i.e., tibia length) was similar between wasps at emergence and after feeding for 1 week on a sucrose solution (ANOVA: F1,46 = 0.91, P = 0.35), and the distribution of the data was similar as well (Kolmogorov–Smirnov test: D = 0.17, P = 0.9). Wasps had a higher lipid content at emergence (%) than after feeding for 1 week on a sucrose solution (mean ± SE = 23.0 ± 2.4 vs. 15.0 ± 1.4%; Mann-Whitney U test: W = 411, P = 0.011; n = 24; Figure 3). However, lipid content was also significant when including ‘feeding tube’ as a random effect in a linear mixed-effects model (treatment: F1,14 = 6.62, P = 0.022).
DISCUSSION
Both species of seed-feeding wasps did not accumulate lipids by de novo lipogenesis despite having ad libitum access to sugars: M. aculeatus and B. platypterus lost a considerable part of their fat reserves in the 1st week after emergence (mean lipid content dropped by 7.7 and 8.0%, respectively). The reductions in lipid stores are within the range of those found in previous studies using the same assay (e.g., Visser et al., 2010). Both species readily fed on a sucrose solution and used these sugars for somatic maintenance, as demonstrated by the reduced survival when only water was offered. Although the wasps had opportunity for lipid accumulation, it was not observed. Therefore, these measurements provide clear evidence that the secondary switch to phytophagy in these species of seed-feeding wasps was not associated with expression of the lipid accumulation phenotype in our assay.
Seed-feeders as a guild are here represented by two species, with measurements on one population each. Future work could assess the generality of lack of lipid accumulation in seed-feeding wasps. Additionally, in the laboratory, wasps could be offered seeds of different size and quality to assess the influence of larval nutrition on adult lipid accumulation phenotype (Batiste, 1967).
Both wasp species tested in this study are nested in a clade of parasitoid Hymenoptera in which lack of lipid accumulation under sugar-feeding is inferred to be the ancestral state (Visser et al., 2010). The possibility of reactivating the lipid accumulation phenotype seemed probable a priori, as it occurred several times independently within this parasitoid clade, including one case in the superfamily Chalcidoidea (Visser et al., 2010). This begs the question why these two chalcidoid seed-feeders did not regain lipid accumulation upon the adoption of a phytophagous lifestyle. Also, in other clades that have switched from parasitism to phytophagy, such as in the gall-inducing wasps, no reversal to adult lipid accumulation was observed (Visser et al., 2010). Parasitoids have evolved the capacity to manipulate host metabolism to their own advantage using venoms, a trait that has been hypothesized to enable impaired lipid accumulation as it allows the parasitoid to obtain enough lipids to sustain it for a lifetime (Visser & Ellers, 2008; Wang et al., 2020a). Gall wasps also use host-manipulation to create a nutritious environment (Harper et al., 2004) and seed-feeders are arguably in similar close contact with their host, potentially enabling manipulation of host energy allocation. However, to our knowledge, there is no evidence for host manipulation by seed-feeding wasps; in fact, there are many examples in which plants abort seeds that are fed upon before they are fully mature (Janzen, 1971; Seifert et al., 2000). Only an anecdotal report by Batiste (1967, p. 429) states that seeds infested by Bruchophagus kolobovae Fedoseeva [a synonym of B. platypterus (Noyes, 2019)] “usually appear larger than uninfested seeds.” However, size bias in infested seeds could also result from oviposition preference of the female parasitoids. In summary, there is no indication that host-manipulation plays a role in these two seed-feeding wasps.
A possible explanation for why these seed-feeders did not regain lipid accumulation may lie in the lipid content of the larval diet. If the seeds that M. aculeatus and B. platypterus feed on as larvae contain sufficient quantities of lipids, adult lipid accumulation is not selected for. In order to test this hypothesis, we calculated seed lipid contents based on literature data. The total quantity of lipids per rose hip seed has to be estimated indirectly for M. aculeatus larvae because no seed weights have been published (File S1). Based on this calculation we estimate that a single rose hip seed contains 0.83–8.6 mg fat. The lipid content of the M. aculeatus specimens measured here is far lower: the maximum lipid content observed was 0.19 mg, which was only between 2 and 23% of the upper and lower bound of the estimated available lipids. We therefore conclude that the larval diet of M. aculeatus contains copious amounts of lipids.
Also for B. platypterus the lipid availability in the larval diet is based on estimations as there are no published data on the absolute lipid content of L. corniculatus seeds, despite the availability of multiple studies on relative fatty acid composition of these seed oils (Bakoglu et al., 2009; Kocak et al., 2011). Therefore, we used data on seed lipid content of related species (File S1), resulting in an estimated 0.001–0.059 mg fat in a single Lotus seed when based on lipid content of closely related species, or up to 0.323 mg when based on the more distantly related soy bean [Glycine max (L.) Merr.]. As the specimens of freshly emerged B. platypterus in our data set contained 0.0056–0.147 mg fat, it seems that this species could be rather limited in larval dietary lipids, in which case a regain of lipid accumulation would be expected. However, substantiating this inference would require direct measurements of the lipid content of L. corniculatus seeds. Such direct measurements of seed lipid content would also indicate the variation in lipid content among seeds. An additional source of nutrition for the developing larvae of the seed-feeding wasps could be seed starch that the developing wasps may or may not be able to convert to (essential) lipids. We therefore recommend to quantify seed starch in future work, besides measuring seed lipid content, and to assess to what extent these nutrients are incorporated into insect tissue.
Our results show that a lack of lipid accumulation in insects can be maintained after secondary evolution of phytophagy, i.e., loss of the parasitic lifestyle. We propose that the amount of available lipids in the diet of larvae, combined with reliable access to carbohydrates during the adult stage, is enough to facilitate enduring absence of lipid accumulation in these insects. These two nutritional conditions are shared by parasitoids and seed-feeders alike: for both guilds their larval diet contains surplus lipids and the adults can likely find ample sugars in flower-rich meadows where they have access to floral nectar, honeydew, extrafloral nectaries, and other sources (Segoli & Rosenheim, 2013).
Stable ecological interactions between seed-feeders and host plants may allow the seed-feeders to rely on a predictable amount of lipids in their diet so that the need for autonomous lipid synthesis remains relaxed (Ellers et al., 2012). We anticipate that any non-lipogenic insects in parasitoid clades that have access to abundant exogenous lipids in their larval stage and sufficient access to carbohydrates as adults are likely to maintain reliance on host lipids. This prediction could be extended to clades of insects with lifestyles that enable access to surplus lipids other than parasitoids or seed-feeders. If correct, this would imply that more guilds of insects without adult lipid accumulation are yet to be discovered. Candidate groups are insects with predatory larvae and sugar-feeding adults like Empis (Diptera: Empididae), Episyrphus (Diptera: Syrphidae), Maculinea (Lepidoptera: Lycaenidae), and insects whose nectar-feeding adults stock nests with paralyzed arthropods for oviposition, as found in spider wasps (Hymenoptera: Pompilidae) and many hymenopteran families of what was formerly known as the Crabronidae (Sann et al., 2018). Indeed, all age classes of adult Hemipepsis ustulata Dahlbom (Pompilidae) were found to have the same lipid content (Kemp & Alcock, 2003; Visser & Ellers, 2008). We conclude that lack of adult lipid accumulation may be more widespread than presently known.
ACKNOWLEDGEMENTS
We thank Dr. Sandrine Ulenberg (Naturalis Biodiversity Centre) for confirming the identity of Megastigmus aculeatus. Ole Bidstrup kindly provided the photo of Megastigmus aculeatus. This work was supported by a grant from the Netherlands Organization for Scientific Research (NWO, VICI grant no. 865.12.003).
AUTHOR CONTRIBUTIONS
Mark Lammers: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (equal); Project administration (lead); Software (lead); Visualization (lead); Writing – original draft (equal); Writing – review & editing (equal). Ken Kraaijeveld: Supervision (equal); Validation (equal); Writing – review & editing (equal). Jacintha Ellers: Funding acquisition (lead); Resources (lead); Supervision (equal); Validation (equal); Writing – review & editing (equal).
DECLARATIONS
The authors declare no conflict of interest. There are no disputes over the ownership of the data presented in the paper and all contributions have been attributed appropriately, via co-authorship or acknowledgement.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Dryad at http://doi.org/10.5061/dryad.wwpzgmsk0, reference no. wwpzgmsk0.




