A 30-year systematic review reveals success in tephritid fruit fly biological control research
Abstract
Fruit flies (Diptera: Tephritidae) are among the main pests in horticulture, impacting field crops and export markets. The biological control of fruit flies has become an important method for environmentally friendly crop production. Although biological control is an age-old practice and the mass-rearing of some biological agents boomed in the 1990s, efforts in fruit fly control programs still face several challenges. We conducted a systematic review to investigate publications assessing the success or failure of using natural enemies against tephritid fruit flies. Our goal was to compile and summarize information from over 30 years of research on biocontrol efforts, including groups and species of control agents and fruit flies tested, methodological approaches applied, the country where the study was performed, and the scope adopted. We also analyzed effectiveness, efficiency, and success rates in biocontrol studies published from 1990 to 2021. Our review showed 2986 records, of which 225 publications matched the criteria. The most-studied biocontrol agents for the suppression of fruit flies were parasitoids, fungi, and nematodes. A few studies assessed bacteria, predators, and viruses. We found a total of 55 fruit fly species described as hosts of the biological agents. Natural/conservation biological control was the main scope studied; however, augmentative biological control had a higher rate of successful studies and potential success, followed by classical biological control. The effectiveness and efficiency parameters are discussed to provide support for researchers in future studies.
INTRODUCTION
True fruit flies (Diptera: Tephritidae) are important pests of agricultural commodities infesting a wide range of fruits and vegetables. Including more than 5000 species and 500 genera, this family is one of the largest among Diptera, with a worldwide distribution (Scolari et al., 2021). Despite that, 250 species (<6%) are of economic importance (FAO/IAEA, 2019; Rendón & Enkerlin, 2020). These insects are polyphagous pests that cause direct damage to fruits through female puncturing, larval feeding, and fruit dropping (Aluja, 1994). Several fruit flies also limit fresh fruit exports through quarantine restrictions (Ovruski & Schliserman, 2012).
Historically, fruit fly control was almost exclusively performed using insecticides or bait sprays (a mixture of insecticide and a food attractant) (Urbaneja et al., 2009; Flores et al., 2011). However, because restrictions on effective broad-spectrum and systemic insecticides have increased in many countries (Böckmann et al., 2014), research focused on the use of environmentally friendly approaches to reduce the use of chemical control methods. Currently, pest control research applied to fruit flies is dedicated to assessing biocontrol tactics, behavioral control using the sterile insect technique (SIT), post-harvest treatments, and bioinsecticides (Dias et al., 2018).
The biological control of fruit flies has become an important method for environmentally friendly crop production. Significant efforts were made in fruit fly biocontrol research from 1950 to 2020, following the examples of studies performed by Clausen (1956), Clausen et al. (1965), Wharton & Marsh (1978), Wharton & Gilstrap (1983), Wharton (1989), Sivinski (1996), Purcell (1998), Ovruski et al. (2000), Montoya et al. (2000, 2011), Stibick (2004), Bokonon-Ganta et al. (2007), Vargas et al. (2007, 2008, 2012), and Garcia et al. (2017, 2020).
Biological control consists in reducing pests by manipulating the populations of biocontrol agents, called BCAs (Hodek et al., 2012). The most common BCAs against insects comprise living organisms or natural enemies, such as parasitoids, predators, fungi, nematodes, bacteria, and viruses. According to Eilenberg et al. (2001), biological control involves the use of living organisms, whereas genes or gene fragments, metabolites from insect or weed pathogens, or competitors to plant pathogens, used without the organisms producing them, are not considered biological agents. Approaches adopted can be conservation, classical, and augmentative biological control. The first scope involves the action of natural enemies in the environment and employs techniques to provide resources to optimize the effect of these agents in the field (Naranjo et al., 2015). Classical biological control (CBC) refers to the intentional introduction of an exotic natural agent from a distant area for the permanent establishment and long-term pest control in another area that the pest has invaded (Eilenberg et al., 2001). Augmentative biological control (ABC) is, briefly, based on the release of commercially reared BCAs for pest control in crops (Van Driesche & Abell, 2008).
Successful biocontrol programs have been used to control the most important tephritid pests, such as Ceratitis capitata (Wiedemann) (Mediterranean fruit fly, medfly), Bactrocera spp., Anastrepha ludens (Loew), and Anastrepha suspensa (Loew) (Caribbean fruit fly, caribfly) (Sivinski et al., 1996; Ovruski et al., 2000; Montoya et al., 2007; Vargas et al., 2007; Garcia et al., 2020). Although biological control is an age-old practice and the mass-rearing of some BCAs boomed in the 1990s, efforts in fruit fly control programs still face several challenges (Enkerlin et al., 2017). The main limiting factors for the biological control of fruit flies are reported in the use of parasitoids. These studies emphasize legislative issues for exotic biological agents, parasitism levels in the field, and high costs of mass-rearing as the main difficulties (Ovruski & Schliserman, 2012). The small margin between control levels and fruit infestation is also listed as a challenge. Even knowing that parasitoids can reach high parasitism levels, fruit infestation rates higher than 5% are considered unacceptable in fruit production systems (Purcell, 1998).
Here, we investigate publications assessing the success or failure of BCAs against tephritid fruit flies using a systematic literature review. Specifically, we set out to answer the following questions: (1) which group of BCAs has been most studied? (2) Which fruit fly species have been most studied? (3) Which scope has been most adopted in biocontrol research (natural/conservation, classical, augmentative, or survey)? (4) What methods (laboratory, semi-field, or field) have been used? (5) How was success of control determined in biocontrol studies? And (6) when and where has the research taken place? By quantifying the number of studies investigating the research on biocontrol efforts and summarizing information from the past 30 years, we can support researchers in future studies.
MATERIALS AND METHODS
We performed a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA statement and Checklist; http://www.prisma-statement.org). Systematic reviews aim to identify and summarize the findings of all relevant individual studies, thereby making available evidence more accessible to researchers and decision-makers. The information discussed here is based on the largest number of studies, following the PRISMA guidelines. Full references of all publications found in our systematic research and the dataset are provided in Supporting Information 1. An overview of the search process is given in Figure 1.
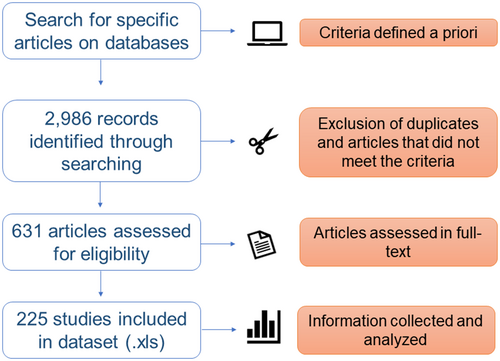
Search strategy
Three databases covering peer-reviewed literature were used to search for published studies that assessed BCAs in fruit fly species of the family Tephritidae. We accessed Web of Science Core Collection (from 1864 to 2021), Science Direct (from 1997 to 2021), and Scopus (from 1960 to 2021) to search for studies focused on fruit fly species and BCAs. We queried using the following search terms to capture relevant literature in all databases: ‘fruit fly’ and ‘biological control’, ‘fruit fly’ and ‘parasitoids’, ‘fruit fly’ and ‘predators’, ‘fruit fly’ and ‘bacteria’, ‘fruit fly’ and ‘fungi’, ‘fruit fly’ and ‘virus’, and ‘fruit fly’ and ‘nematodes’. The first search using the key terms generated 2986 records (last access date: 10 February 2021), and the results were imported into a library of Mendeley Reference Manager.
Screening and eligibility criteria
The screening strategy included selecting original research articles written in English, Spanish, and Portuguese. To ensure the consistency of the research included in our review, we chose to use only peer-reviewed journal articles that reported primary research. We did not include review articles, conference proceedings, editorial material, or book chapters in our search. Two criteria were established to include the study in our dataset: (1) studies including fruit fly species from the family Tephritidae, and (2) studies that used one or more BCAs of fruit flies in a pest control context. We decided not to include studies evaluating only commercially formulated products (e.g., bioinsecticides).
Data extraction
Our research resulted in 225 publications (see Supporting Information 1) that were used to obtain the complete reference of each article and extracted information on the following aspects: (1) groups and species of BCAs (parasitoids, predators, bacteria, fungi, viruses, and nematodes); (2) fruit fly species studied; (3) scope of the study (a survey or a natural/conservation, classical, or augmentative biocontrol study); (4) methodological approach used (laboratory settings, semi-field or field performance, or combined approaches when at least two approaches were used in the same study); (5) evidence of biological control based on the effectiveness (potential of the BCA to the conclusion of the study) and efficiency (maximum efficiency parameter obtained by BCAs); and (6) country where the study was performed. Studies of competition, interaction, or laboratory evaluations that did not specify a scope of biological control were included as surveys. Studies of diversity and new records of biological agents were included as natural biocontrol studies. We used the first author’s location for studies lacking information on where the research was conducted.
Data analysis
To analyze the studies providing evidence of successful biological control, we defined the following two criteria: (1) studies with field performance, and (2) studies meeting criterion 1 with evidence of fruit fly biological control >30%, based on at least one efficiency parameter. Studies with an efficiency parameter of 30–50% were included as potential biological control, and studies with one of these parameters >50% were included as successful biological control. The extracted data were subjected to descriptive analysis (Proc UNIVARIATE).
RESULTS
Studies assessing biocontrol agents
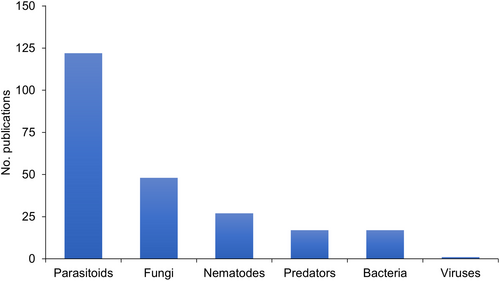
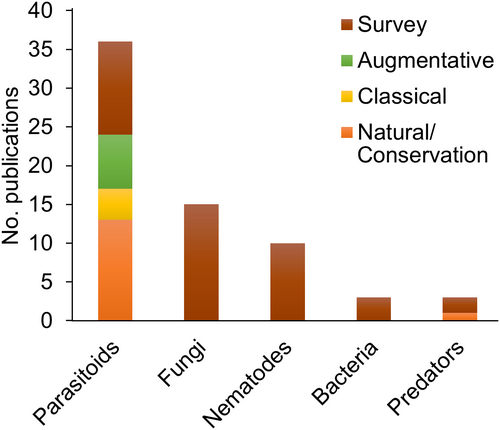
The most-studied groups of BCAs were parasitoids (54%), fungi (21%), and nematodes (12%) (Figure 2). Bacteria and predators were studied in 8% of the publications. Viruses were assessed in <1% of the studies. The predominant primary biological mechanism studied was parasitism, found in 66% of the studies (n = 149), followed by pathogenicity (n = 66; 29%), and predation (n = 17; 8%). In total, 148 species of BCAs were assessed in the studies. Among the 15 most-studied BCA species, eight were parasitoids (Table 1). The complete list is shown in Supporting Information 1.
| Biological agent species | Group | No. studies | Main fruit fly host | Total no. hosts |
|---|---|---|---|---|
| Diachasmimorpha longicaudata | Parasitoid | 46 | Ceratitis capitata | 19 |
| Doryctobracon areolatus | Parasitoid | 22 | Anastrepha fraterculus | 17 |
| Fopius arisanus | Parasitoid | 21 | Bactrocera dorsalis | 8 |
| Beauveria bassiana | Fungus | 21 | C. capitata | 13 |
| Metarhizium anisopliae | Fungus | 19 | C. capitata | 10 |
| Heterorhabditis bacteriophora | Nematode | 17 | A. suspensa | 10 |
| Utetes anastrephae | Parasitoid | 16 | A. fraterculus, A. obliqua, A. sororcula, and A. serpentina | 10 |
| Steinernema carpocapsae | Nematode | 14 | B. dorsalis and B. oleae | 10 |
| Ganaspis pelleranoi | Parasitoid | 10 | A. fraterculus | 12 |
| Metarhizium brunneum | Fungus | 9 | C. capitata, B. oleae, and Rhagoletis suavis | 6 |
| Psyttalia concolor | Parasitoid | 9 | B. oleae | 5 |
| Bacillus thuringiensis | Bacterium | 8 | A. fraterculus, A. ludens, and Zeugodacus cucurbitae | 9 |
| Steinernema feltiae | Nematode | 8 | C. capitata and A. suspensa | 6 |
| Diachasmimorpha tryoni | Parasitoid | 8 | C. capitata | 3 |
| Doryctobracon crawfordi | Parasitoid | 8 | A. ludens | 8 |
| Opius bellus | Parasitoid | 7 | A. fraterculus and C. capitata | 10 |
| Coptera haywardi | Parasitoid | 7 | A. ludens | 5 |
Parasitoids
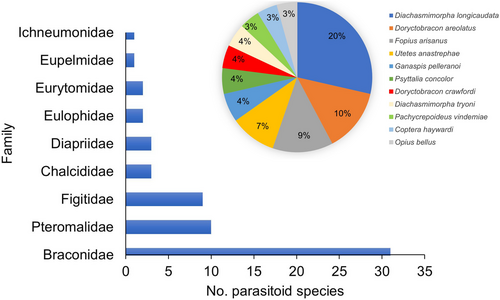
Among the 225 publications, 61 species of parasitoids were studied in a biological control context. The family Braconidae (Opiinae) was the main family studied (Figure 3). Pteromalidae was the second most-studied family, followed by the Figitidae (Eucoilinae). Diachasmimorpha longicaudata (Ashmead), Doryctobracon areolatus (Szépligeti), Fopius arisanus (Sonan), Utetes anastrephae (Viereck) (Hymenoptera: Braconidae, Opiinae), and Ganaspis pelleranoi (Brèthes) (Hymenoptera: Figitidae, Eucoilinae) were the five most-studied species.
Fungi
Twenty-one fungal species were assessed in the studies. Metarhizium anisopliae (Metschn.) Sorokin and Beauveria bassiana (Bals.-Criv.) Vuill. (Ascomycota: Hypocreales) were the most-studied fungi, found in 9 and 8% of the studies, respectively. Metarhizium brunneum (Petch) and Isaria fumosorosea Wize (Hypocreales) were studied in <5% of the publications. Other fungal genera studied were Aspergillus, Lecanicillium, Mucor, Penicillium, Purpureocillium, and Trichoderma.
Nematodes
Nineteen studies assessed nematodes as BCAs. Heterorhabditis bacteriophora Poinar (Rhabditida: Heterorhabditidae) and Steinernema carpocapsae (Weiser) (Rhabditida: Steinernematidae) were the most-studied nematode species for the control of fruit fly species (Table 1). Seven other Heterorhabditis species and nine Steinernema species were also studied.
Predators
Thirty-six predator species were assessed in fruit fly biocontrol studies. However, there were no predominant species in the number of publications. Predators studied included spiders, ants, and coleopterans. Species found in more than one publication were Argiope keyserlingi Karsch (Arachnida: Araneidae), Oecophylla longinoda (Fabricius) (Hymenoptera: Formicidae), Pseudophonus rufipes (De Geer) (Coleoptera: Carabidae), and Ocypus olens (Müller) (Coleoptera: Staphylinidae).
Bacteria and viruses
In total, 11 bacterial species were studied as BCAs of fruit flies. The most-studied bacterium was Bacillus thuringiensis (Berliner) (Bt). Other potential groups studied were Klebsiella, Candidatus, Lactobacillus, Pantoea, Providencia, Serratia, Streptomyces, Wolbachia, and Zygosaccharomyces. Viruses were evaluated in only one study. Three picornaviruses that were reported infecting medfly were Ceratitis capitata iflavirus 1, Ceratitis capitata iflavirus 2, and Ceratitis capitata noravirus.
Studies assessing fruit fly species
In total, 55 species were found in publications assessing BCAs. Table 2 shows the fruit fly species that were studied in more than one publication (complete list in Supporting Information 1). The most-studied species was C. capitata, followed by A. ludens, Bactrocera oleae (Rossi), Bactrocera dorsalis (Hendel), and Anastrepha fraterculus (Wiedemann). Studies related to Rhagoletis genus included Rhagoletis cerasi (L.), Rhagoletis pomonella (Walsh), Rhagoletis suavis (Loew), Rhagoletis fausta (Osten Sacken), Rhagoletis indifferens Curran, and Rhagoletis mendax Curran (Supporting Information 1).
| Fruit fly species1 | No. publications |
|---|---|
| Ceratitis capitata | 80 |
| Anastrepha ludens | 32 |
| Bactrocera oleae | 31 |
| Bactrocera dorsalis 2 | 27 |
| Anastrepha fraterculus | 25 |
| Anastrepha obliqua | 19 |
| Zeugodacus cucurbitae | 15 |
| Ceratitis cosyra | 9 |
| Anastrepha serpentina | 7 |
| Bactrocera zonata | 7 |
| Anastrepha spp. | 6 |
| Anastrepha suspensa | 6 |
| Anastrepha sororcula | 6 |
| Bactrocera tryoni | 6 |
| Ceratitis rosa | 4 |
| Anastrepha striata | 4 |
| Rhagoletis cerasi | 3 |
| Rhagoletis pomonella | 3 |
| Rhagoletis mendax | 3 |
| Anastrepha distincta | 3 |
| Anastrepha zenildae | 2 |
| Anastrepha leptozona | 2 |
| Anastrepha pickelli | 2 |
| Anastrepha antunesi | 2 |
| Rhagoletis suavis | 2 |
| Other fruit fly species | <2 |
Scope of the studies
Sixty-eight % of the studies assessing fruit fly biological control were included as a survey (n = 154), and 22% (n = 50) of the studies were included as natural/conservation biological control. Classical and augmentative biological control were assessed in 6% (n = 13) and 5% (n = 11) of the studies, respectively, where the predominant fruit fly species were C. capitata (n = 9), B. oleae (n = 6), A. fraterculus (n = 5), Anastrepha obliqua (Macquart) (n = 3), and A. ludens (n = 2). Most of the biological agents studied in classical and augmentative studies were D. longicaudata (n = 5), D. areolatus (n = 4), G. pelleranoi (n = 3), F. arisanus (n = 3), and Psyttalia concolor (Szepligeti) (n = 2).
Methodological approach per year of publication
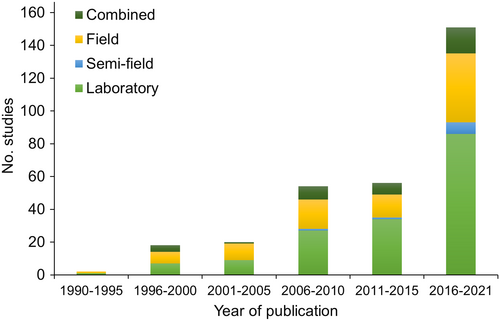
Years of publications that returned hits ranged from 1990 to 2021. Of >30 years of research assessing biological control of fruit flies, 73% of the studies were performed using laboratory settings, 41% were carried out in field conditions, and 4% in semi-field conditions (e.g., greenhouses). Seventeen % of the studies were performed using combined approaches. Most of the studies were conducted between 2015 and 2021 for all methodological approaches (Figure 4); 2019 had the largest number of studies, followed by 2018.
Evidence from biocontrol assays
Effectiveness
We analyzed two parameters from the publications to obtain knowledge about successful fruit fly biocontrol studies worldwide. The first parameter analyzed was the effectiveness of the BCAs assessed in the studies. This parameter was defined based on the recommendation of the BCA studied according to the study's conclusion. Eighty-three % (n = 186) of the studies recommended at least one BCA evaluated, and 4% (n = 9) did not recommend the BCA under the conditions tested. Thirteen % (n = 30) of the studies did not determine the effectiveness.
Efficiency
The second parameter evaluated was the efficiency of the BCAs used in the studies based on the rate obtained for the maximum efficiency parameter. Among 225 publications, 69% of the studies (n = 156) showed at least one efficiency parameter (Table 3). Twenty % of the studies (n = 44) showed at least one efficiency parameter between 90-100%, including parasitism of larvae and pupae, pupal predation, larval, pupal, and adult mortality, conidia germination, pupae or adults showing mycosis, and fruit fly suppression after releases in the field. Forty-eight % of the studies (n = 108) showed >50% efficiency. In general, the parameters evaluated more frequently in the studies were larval mortality (n = 34), adult mortality (n = 28), and larval parasitism (n = 29). In 17 studies, the stage of the parasitized fruit fly was not specified. The full list showing the parameters evaluated in the studies is shown in Supporting Information 1.
| Efficiency (%) | No. studies | EP | LP | PP | P | PR | LE | PE | AE | LM | PM | AM | LI | CG | MY | RD | SR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0≤E≤10 | 6 | ☑ | ☑ | ☑ | ☑ | ||||||||||||
| 10<E≤20 | 7 | ☑ | ☑ | ||||||||||||||
| 20<E≤30 | 10 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ||||||||||
| 30<E≤40 | 12 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ||||||||||
| 40<E≤50 | 11 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | |||||||||
| 50<E≤60 | 15 | ☑ | ☑ | ☑ | ☑ | ☑ | |||||||||||
| 60<E≤70 | 16 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | |||||||||
| 70<E≤80 | 15 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | |||||||||
| 80<E≤90 | 18 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ||||||||
| 90<E≤100 | 44 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ |
- No. studies: number of studies showing at least one parameter of efficiency; EP: egg parasitism, LP: larval parasitism, PP: pupal parasitism; PR: parasitoids recovered after releases; LE: larval predation; PE: pupal predation; AE: adult predation; LM: larval mortality; PM: pupal mortality; AM: adult mortality; LI: larval infection by nematodes; CG: conidia germination; MY: pupae or adults showing mycosis; RD: reduced fruit fly damage in the field; SR: fruit fly suppression after releases in the field.
Success rate
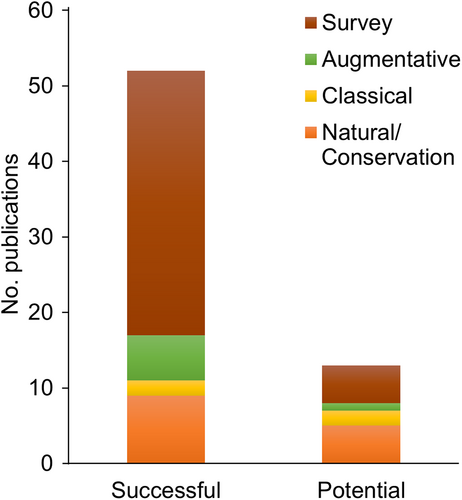
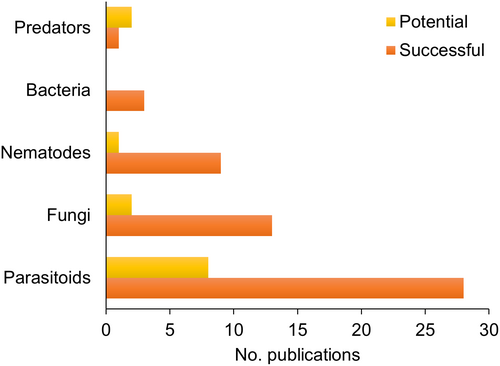
To evaluate the success or potential of the BCAs, we established two criteria based on the efficiency parameters. Forty-three studies met at least one criterion. Most studies with evidence of success were included as a survey, followed by natural/conservation, augmentative, and classical biological control (Figure 5). The predominant studies showing the potential for biological control used a natural/conservation scope. Studies discussing natural/conservation biological control were performed using parasitoids or predators (Figure 5). Successful classical and augmentative biocontrol studies assessed only parasitoids as BCAs (Figure 6). Fungi, nematodes, and bacteria were included only in surveys that met the criteria for evidence of success. The publications classified as successful biocontrol studies assessed parasitoids, followed by fungi, nematodes, bacteria, and predators (Figure 7).
Countries
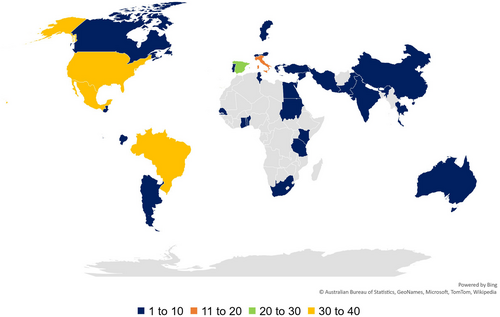
Research studies assessing fruit fly biological control were conducted in 34 countries (Figure 8). Most of the studies were performed in Mexico and the USA (16%), followed by Brazil (13%), Spain (10%), and Italy (6%). Thirty-four % of the studies were conducted in North America, followed by Europe (20%), South America (18%), Africa (13%), Asia (12%), and Oceania (3%).
DISCUSSION
Biological control is a safe and sustainable pest management technology that takes advantage of natural enemies present in the agroecosystems (Wang et al., 2019). The adoption of natural enemies to control fruit flies has received increasing attention in worldwide research, and here, we demonstrated this by quantifying the studies using biological agents.
Parasitoids are the most-studied fruit fly biocontrol agents
Parasitism was studied in more than half of the publications, followed by pathogenicity, whereas predation was found only in a few studies. Biological control using parasitoids offers the advantage of well-chosen agents with high host specificity. The compatibility with the conditions where they are released can maintain self-perpetuating populations from generation to generation, providing reasonable control (Vreysen & Robinson, 2011). Therefore, parasitoids are often considered a better option than predators, as parasitoids are target-specific, thus reducing the risk of attacking non-target species (Cossentine, 2000; Zamek et al., 2012).
The remarkable advances in rearing techniques and artificial diets for rearing hosts over the past 30 years have allowed parasitoid wasps to be used as biological agents in fruit fly programs (Cancino & Montoya, 2008). The Mexican National Campaign against Fruit Flies (NCFF) is an example of a successful case of fruit fly control using parasitoids (Montoya et al., 2007). This program was created in 1992 to establish areawide integrated pest management (IPM) control measures against four Anastrepha species, with augmentative biological control as one of the main strategies (Gutiérrez-Ruelas et al., 2016). Parasitoids also have been used successfully in augmentative release programs in Hawaii (USA), Guatemala, and Israel (Ovruski et al., 2000; Argov & Gazit, 2008).
Studies on superparasitism, parasitoid learning, competition, and interactions between parasitoids and other BCAs, such as fungi, predators, and nematodes (Montoya et al., 2012; Tormos et al., 2012; Aluja et al., 2013; Van Nieuwenhove et al., 2016; Masry et al., 2018; Murillo et al., 2019a,b; Paranhos et al., 2021), have also contributed to increasing the number of successful studies of biological control as shown in our results. These types of studies are increasing in number as the success of a biological technique is strongly associated with the ability of released parasitoids to disperse, survive, and find hosts (Paranhos et al., 2007). Thus, parasitoids that exhibit a capacity for rapid host-location have a higher probability of successfully controlling target pests (Monsia et al., 2019). However, host fruit size appears to be a significant limitation associated with this type of natural enemy, as the host larvae can move deep within the fruit, out of reach of the female parasitoid’s ovipositor (Montoya et al., 2016).
Important role for Diachasmimorpha longicaudata and Fopius arisanus and new attention to Neotropical parasitoids
The most-studied agent, D. longicaudata, is a solitary larval parasitoid originally from southeast Asia and successfully introduced into Hawaii in 1948. At present, this wasp is disseminated worldwide and is studied to control fruit flies of the genera Ceratitis, Anastrepha, and Bactrocera (Ovruski et al., 2000; Schliserman et al., 2003). Diachasmimorpha longicaudata has been mainly studied against C. capitata, A. ludens, A. fraterculus, and A. obliqua. This parasitoid had the highest number of host fruit fly species, demonstrating the research interest in this species worldwide.
Currently, D. longicaudata is mass-reared in several facilities worldwide, and it is applied in augmentative releases to complement other control methods such as SIT (Paranhos et al., 2007; Suárez et al., 2019). In our review, we found studies of competition between D. longicaudata and Neotropical and exotic parasitoids, such as Doryctobracon crawfordi (Viereck) (Miranda et al., 2015) and F. arisanus (Liang et al., 2018), and studies of the combined use of two parasitoids, such as Aganaspis daci (Weld) (de Pedro et al., 2019), Coptera haywardi (Ogloblin) (Montoya et al., 2019), D. crawfordi, U. anastrephae, and Opius hirtus (Fischer) (Murillo et al., 2019a,b). Some studies have focused on using this wasp with entomopathogenic nematodes (EPNs) to control fruit flies (Heve et al., 2018a,b). Heve et al. (2018b) studied the effects of three nematodes (H. bacteriophora, Steinernema feltiae, and Heterorhabditis indica) on D. longicaudata for the control of the caribfly. The number of emerged parasitoids in each nematode species treatment was similar to the control, suggesting that none of the EPNs affected the emergence of D. longicaudata.
Superparasitism has been studied in D. longicaudata as an adaptive parasitism strategy under specific conditions (González et al., 2007; Montoya et al., 2011). This is a strategy in which a female wasp lays eggs on a previously parasitized host, and, in the past, it was attributed to the inability of females to discriminate between parasitized and non-parasitized hosts (Lawrence, 1988). This strategy may be one factor that explains the success of D. longicaudata as a BCA of fruit flies (Altafani et al., 2013). In A. ludens, some studies have shown that moderate levels of superparasitism result in a female-biased sex ratio and that both mass-reared and wild females superparasitize their hosts without detrimental effects on life-history traits (González et al., 2007). Similar results were found for this host and the parasitoid Diachasmimorpha tryoni (Cameron) (Ayala et al., 2014) and C. capitata and A. fraterculus superparasitized by D. longicaudata (Altafani et al., 2013).
Another important parasitoid studied was F. arisanus, indigenous to the Asian continent; this wasp is considered one of the primary parasitoids of Bactrocera species and C. capitata (Vargas et al., 2001). This parasitoid has been studied against B. dorsalis, Bactrocera carambolae (Drew & Hancock), B. oleae, C. capitata, Ceratitis cosyra (Walker), and Anastrepha spp. (Zenil et al., 2004). Given its success in Hawaii, F. arisanus was used in classical biocontrol programs in French Polynesia to control B. dorsalis spread (Vargas et al., 2007, 2016; Leblanc et al., 2013). This parasitoid was introduced in five French Polynesian islands from Hawaii in 2002. As a result of these efforts, the mean parasitism of fruit flies in 2009 reached from 70 to 95% (Leblanc et al., 2013). The establishment of F. arisanus in French Polynesia became the most successful example of classical biological control of fruit flies in the Pacific area outside Hawaii (Vargas et al., 2007, 2016). This experience supported the use of F. arisanus as a prime biocontrol candidate to suppress B. dorsalis in African countries and B. carambolae in South America (Vargas et al., 2016).
An opiine braconid native to the Neotropical region, D. areolatus was also one of the most-studied parasitoid BCAs. This parasitoid is a specialized, solitary, koinobiont endoparasitoid of larvae attacking inside host fruits (Aluja et al., 2013). Most studies with this wasp have focused on the fruit fly species A. fraterculus, followed by A. obliqua, C. capitata, and A. sororcula, from the Neotropics, except the medfly. The studies using D. areolatus aimed to assess the competition between native and exotic parasitoids, the host instars parasitized, and their population dynamics.
The potential of native parasitoids for the biological control of fruit flies is being explored in Brazil. For example, parasitoids of the genera Doryctobracon and Ganaspis can be found in orchards, together parasitizing up to 40% of flies (Garcia & Ricalde, 2013). In our review, other native parasitoids included among the first 15 studied BCA species were C. haywardi, U. anastrephae, G. pelleranoi, D. crawfordi, and Opius bellus Gahan, studied in Anastrepha species.
Biological control studies with fungi and nematodes show increasing success
Entomopathogenic fungi were the second most-studied BCAs. Beauveria bassiana and Metharizium anisopliae were the two most-studied fungi for the control of C. capitata, A. ludens, and A. obliqua. In general, entomopathogenic fungi caused high mortality of pupae and adults, but not of larvae. Most studies using fungi against fruit flies assessed the pathogenicity or virulence. Other studies aimed to evaluate the horizontal transmission capacity of these two fungi species. This ability involves the capacity of infected individuals to transmit the infection to untreated individuals during mating and physical contact (Toledo et al., 2007; Sookar et al., 2014). Horizontal transmission in fruit flies treated with fungi could serve as an efficient vector in cases where the biological agent has many host insect species. Toledo et al. (2007) performed assays using A. ludens females infected by B. bassiana and showed a transmission rate of 80–84% through mating and 15–22% through mating attempts. Montoya et al. (2020) showed that the simultaneous use of disseminator devices of B. bassiana in a zone where SIT was applied by releasing A. ludens sterile males enhances horizontal transmission to wild populations. Other promising results were also obtained using sterile males infected by M. anisopliae to control areas infested with C. capitata (Quesada-Moraga et al., 2008), Bactrocera zonata (Saunders), and Zeugodacus (Bactrocera) cucurbitae (Coquillett) (Sookar et al., 2014). Studies have been conducted on the control of Rhagoletis species with different entomopathogenic fungi and were well-reviewed by Daniels & Grunder (2012). Here, we found current articles covering the control of R. suavis, R. pomonella, R. indifferens, and R. mendax (Nisar et al., 2019; Behle, 2020; Renkema et al., 2020; Usman et al., 2020; Yee, 2020).
The two most-studied EPNs were H. bacteriophora and S. carpocaspae. These BCAs were evaluated against three species of Anastrepha, four species of Bactrocera, and one species each of Rhagoletis, Dacus, and Ceratitis. The last two fruit flies were studied only with H. bacteriophora. Barbosa-Negrisoli et al. (2009) showed that H. bacteriophora was more virulent to A. fraterculus larvae than pupae, with an efficient host search inside infested peaches, whereas Toledo et al. (2014) showed that humidity plays an important role in the capacity of H. bacteriophora to infect A. ludens larvae. However, most studies showed significant results in the control of fruit fly pupae, as some tephritid fruit flies spend several months in the soil at the pupal stage. Therefore, laboratory assays were conducted to assess the susceptibility of B. oleae pupae to two EPN species. Steinernema carpocapsae caused pupal mortality of 62.5% and infected 21.9% of the emerging adults (Torrini et al., 2017). A study performed by Rashad et al. (2015) showed that Steinernema species were more effective than H. bacteriophora against larvae and pupae of B. zonata. However, H. bacteriophora was more effective in the soil, inducing higher mortality in B. zonata pupae.
The nematode H. bacteriophora was recently studied for the suppression of A. suspensa pupae, to develop a feasible and cost-effective method for IPM in guava fields (Heve et al., 2018a). Survival of the caribfly significantly decreased with an increasing infective juveniles (IJs) rate from 0 to 100 IJs cm−2. Optimum suppression (>60%) was achieved at 100 IJs cm−2. A profitability analysis showed that H. bacteriophora could be included in A. suspensa management tactics to produce guavas. Furthermore, the authors showed that the costs of using EPNs in caribfly IPM are minimized if the nematodes are applied by spot treatment of fruit.
Bacteria, predators, and viruses
Among the 11 bacterial species studied for the control of fruit flies worldwide, Bt is the most-studied entomopathogenic bacterium. This agent was studied to control nine fruit fly species, but mainly A. fraterculus and A. ludens. Martins et al. (2018) evaluated three strains of Bt against A. fraterculus larvae and found 60% of mortality at 2 × 109 CFU ml−1. Larvae that ingested spore/crystal suspensions exhibited significant larval and pupal deformations, leading to a 50% decrease in the completion of the biological cycle. In addition, Bt strains applied at the highest concentration combined with a food attractant in formulations of toxic baits resulted in adult mortality higher than 85%. This study provides a basis for developing Bt-based insecticides for the control of A. fraterculus.
Studies including predators with direct evidence of biological control are scarce. However, some studies showed an important role of predation in the conservation biological control of fruit flies. Albertini et al. (2017) performed a 1-year field study and found 35 species of carabids, nine of which were the most active, with scattered activity throughout the year. The authors identified species with useful traits for the predation of B. oleae pupae, such as high dispersal power, small to large body size, and main activity in late summer and autumn.
Some of the studies evaluated the interaction of predators with SIT. Rathnayake et al. (2019) assessed the effect of spiders and green mantises in combination with SIT to control Bactrocera tryoni (Froggatt) in Australia. These authors found a 40% reduction when both predators and irradiated males were present compared to the control and a 24% reduction compared to the presence of only irradiated males. Two studies evaluating the interaction between spiders and SIT in Mexico showed that A. ludens wild males had a better survival ability than mass-reared males (Rao et al., 2014; Dor & Liedo, 2019). These studies conclude that mass-rearing conditions negatively affect the ability of A. ludens males to escape from spiders. These studies are interesting because they show the differences in the responses exhibited by irradiated fruit fly species. Several quality parameters are applied to evaluate irradiated flies. When sterile males are released in the field, they need to avoid predators until they reach sexual maturity and survive long enough to mate with wild females (González-Lopez et al., 2016). González-Lopez et al. (2016) recommended using defensive wing display behavior as a quality parameter and proposed evaluating fly activity. A pattern shown by a fruit fly species or strain can be helpful for IPM strategies using SIT.
A unique study using viruses in a biocontrol context was performed by Llopis-Giménez et al. (2017). This study described three new picornaviruses infecting wild C. capitata collected in Hawaii and strains used in SIT programs worldwide (C. capitata V8, V8G, V8A, and wt). The viruses were present in most of the laboratory colonies and at lower frequencies in field populations. High viral titers of one virus (C. capitata norovirus – CcaNV) were associated with reduced irradiated male survival. The authors suggest that CcaNV may impair the fitness of sterile flies produced by SIT programs; however, no apparent effect was observed.
Scopes adopted in fruit fly biocontrol research
Most studies were performed as surveys; however, natural/conservation biological control was the most frequent among the scopes evaluated. These studies were conducted to identify the parasitoid fauna in areas infested by fruit flies and determine field parasitism levels and population dynamics. Natural/conservation biocontrol studies have a fundamental role in complementing classical and augmentative scopes that face inherent ecological and behavioral challenges, such as enemy dispersal, pest refugia, and deleterious predator/parasitoid guilds (Sivinski, 2013; Montoya et al., 2016).
Some studies used a conservation approach to maintain the presence of natural enemies in the field. Conservation biological control can be achieved by reducing pesticides, using selective pesticides, and carefully timing pesticide applications (El-Wakeil et al., 2017). The study performed by McQuate et al. (2005) is an example of a successful study using conservation biological control. A spinosad-based bait spray was applied to adjacent coffee plants before persimmon fruits became susceptible to oviposition by C. capitata. The bait spray suppressed the population of C. capitata and led to a reduced infestation of both coffee cherries and persimmon fruits. Moreover, parasitism rates of F. arisanus on C. capitata in coffee cherries were not significantly different between unsprayed and sprayed plots even after 11 weekly sprays. These results suggest that mass trapping, combined with spinosad-based bait sprays, are control components compatible with biological control and can be an IPM system against C. capitata.
Our review found that the rate of successful studies concerning classical biological control of fruit flies has increased by 15% since the 1980s. The factors affecting the success of classical biocontrol studies were described by Hokkanen & Sailer (1985). Overall, this increase reflects the impact of the increased knowledge on classical biological control. Successful cases of classical biological control include the introduction of F. arisanus in French Polynesia and Senegal. After a failure eradication of B. dorsalis in French Polynesia, F. arisanus and D. longicaudata were introduced from Hawaii to replicate the biocontrol success achieved 50 years earlier (Leblanc et al., 2013). The success of F. arisanus and D. longicaudata releases in Hawaii and French Polynesia was well discussed by Vargas et al. (2007, 2012) and Leblanc et al. (2013). During 2013 and 2014, F. arisanus was reared and shipped to Senegal inside puparia of C. capitata, where it led to an increase of parasitism rates of fruit flies in mango fruits to 25% (Vargas et al., 2016). This approach also served as a base for introducing F. arisanus in Brazil to control carambola fruit fly, B. carambolae (Vargas et al., 2016; Paranhos et al., 2021). Another example of classical biological control includes Psyttalia fletcheri (Silvestri) to control Z. cucurbitae in Réunion Island and Hawaii (Quilici et al., 2004; Vargas et al., 2004).
Although natural/conservation biological control was the main studied scope, augmentative biological control had a higher rate of successful studies and potential success, followed by classical biological control. An important note is that a successful study is not the same concept as a successful case of biological control, but it can be a foundation for fruit fly control programs based on biological agents. All augmentative studies with successful evidence were performed using parasitoids. These publications involved mass releases to control fruit fly species, including P. concolor against B. oleae in Turkey (Hepdurgun et al. 2009) and California, USA (Yokoyama et al., 2008), D. longicaudata against A. suspensa (Sivinski et al., 1996) in Florida, USA, against A. ludens and A. obliqua in Mexico (Montoya et al., 2000, 2017), against C. capitata in Guatemala (Cancino et al., 2019), against C. capitata in Argentina (Sánchez et al., 2016), and against C. capitata and A. fraterculus in Brazil (Camargos et al., 2018).
What determines a successful biocontrol project for fruit flies?
Among the studies that showed at least once evidence of success for an evaluated BCA, rates higher than 90% of efficiency were more frequent. Our review found evidence supporting the effect of natural enemies on the reduction of fruit fly populations, as demonstrated by the parameters assessed for each BCA group. In general, studies assessing parasitoids propose as efficiency parameters larval parasitism rate and fruit fly population suppression after releases but find that large host fruit may represent a physical refuge for the pest, where female parasitoids are not able to reach the larval hosts (Sivinski et al., 2001; Montoya et al., 2016). In the case of predators, the studies we found evaluated the abundance of predators and pupal predation rates, even using gut content (i.e., mitochondrial DNA) of carabid predators. Studies evaluating the effect of fungi provided percentages of mortality of fruit fly pupae and adults showing mycosis, conidia germination, and adult mortality. Mortality of larvae and adults was mainly used for evaluating the effect of nematodes on fruit flies. These parameters were also considered by Stiling & Cornelissen (2005) to determine successful BCAs of various pests.
Unfortunately, when a successful case of biological control is analyzed, the study generally does not consider the economic value of the BCA. We found only one study showing a cost-effective BCA used to control fruit flies. This study was carried out by Heve et al. (2018a) and is an example of a successful study. These authors showed that repayment of costs by spot treatments of the EPN H. bacteriophora appears achievable in Florida. The low rate of studies showing cost-effective BCAs is due to the difficulty of evaluating treatment costs in the field for horticultural crops instead of major crops such as soybean, cotton, and maize. In several cases, biological control would probably be cost-effective if compared with chemical insecticides or bioinsecticides. Thus, cost-benefit analyses must include the environmental benefits associated with using BCAs in IPM plans.
Global distribution of research on BCAs against tephritid fruit flies
Most studies on fruit fly BCAs were performed in Mexico and the USA, followed by Brazil and Spain. Apart from the Medfly Regional Program Guatemala-Mexico-USA, created by a cooperative agreement to combat the medfly in the early 1980s, Mexico initiated a national campaign against native fruit flies in 1992 (Orozco et al., 2017), which resulted in significant efforts in biocontrol research. The medfly program and the national campaign against fruit flies use an areawide approach, combining surveillance tactics such as trapping and fruit sampling, bait sprays, SIT, augmentative parasitoid releases, and regulatory policies (Enkerlin et al., 2017; Orozco et al., 2017). In this case, biocontrol tools are compatible with an areawide control program as management is focused on the pests (Montoya et al., 2007).
Future directions
Several challenges around the research and application of biological control against fruit fly pests remain pending. In the case of augmentative biological control, efforts must be focused on improving the quality and costs of mass-production and releases of natural enemies as these issues are constantly in the eye of program managers. Efforts related to conservation biological control must also continue because fruit orchards offer some agro-ecological conditions that can promote higher effects of predator communities and other natural enemies present under these conditions. Finally, studies of how the pest-host fruit-larva-natural enemy system works should be performed to understand the scope of these strategies better.
As pest control is still emerging from an era of dependence on chemical pesticides, the importance of biological control has increased because it is safe for humans and environmentally friendly (Waage, 2001). However, despite these ecologically oriented characteristics, the concerns of environmental agencies and conservationists about their effects on non-target species persist (Wajnberg et al., 2001; Kindlmann et al., 2011), mainly those associated with introduced BCAs that pose potential risks of becoming established in the new habitat (Greathead, 1995). Therefore, a proper risk-benefit analysis of the introduction of natural enemies must be accomplished for a more accurate and predictive ecological theory (Simberloff & Stilling, 1996; van Lenteren et al., 2006).
CONCLUSIONS
Despite the increase in research on BCAs, biocontrol tactics represent a small portion of the control methods used to suppress fruit flies worldwide (Dias et al., 2018). This occurs for several reasons: low cost-effectiveness, low availability, level of impact on fruit fly pest populations, and little assistance from the public sector. The high number of studies performed in Brazil and Spain, for example, reveals the critical information available on natural enemies, especially parasitoids. The development of applied biocontrol programs, including mass production and augmentative releases, could be successful, especially when combined with other control methods under areawide concepts (Wong et al., 1992; Paranhos et al., 2019).
The advantages of fruit fly-free countries offer improvements in agriculture production and economy, such as increased fruit production and exports (Retamales & Sepúlveda, 2011). Public policies to maintain a country or a region free of fruit flies or provide surveillance strategies are already positively proven in Mexico and New Zealand (Montoya et al., 2007; Dhami et al., 2016). Biological agents applied within an IPM framework as part of a government’s commitment and improving public policies, in general, may promote the efficiency of biocontrol programs to protect the environment, biodiversity, and human health.
Author Contribution
Naymã Dias: Conceptualization (equal); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (lead); Writing – original draft (lead); Writing – review & editing (equal). Pablo Montoya: Conceptualization (equal); Investigation (equal); Methodology (equal); Supervision (equal); Writing – review & editing (equal). Dori Edson Nava: Conceptualization (equal); Investigation (equal); Supervision (equal); Writing – review & editing (equal).




