Evolutionary neuroecology of olfactory-mediated sexual communication and host specialization in Drosophila – a review
Abstract
In humans, finding a partner is quite a difficult task because there are many criteria that one needs to consider. However, in comparison to many animals, when choosing a partner, we easily discriminate between ourselves and members of other species through various communication systems. On the contrary, many fly species (Diptera) are morphologically similar and overlap in their geographical distributions and ecological habitats. Sexual interactions of most drosophilid flies occur on their hosts. Therefore, flies rely on olfactory sex pheromones, as well as on non-pheromonal chemicals such as host volatiles – which guide and restrict the search for conspecifics within limited locations – as honest signals for pre-mating reproductive isolation. A subtle divergence in the perception of these signals can lead to accumulated changes among populations of the same species, and ultimately to a reduction in gene flow and reproductive isolation. In recent years, we have seen an increased interest in how olfactory systems diverge to drive host adaptation and speciation. In this review, we discuss the evolutionary changes of the neural circuits that underlie mate recognition. We shed light onto sex pheromone communication systems, the construction of olfactory nervous systems, and the role of host specialization in reproductive isolation. Finally, leveraging the incipient speciation of Drosophila mojavensis Patterson populations, we highlight the underlying sensory mechanisms of the reproductive isolation barriers. In the end, we propose future research topics of the evolutionary neuroecology field of sexual communication.
INTRODUCTION
We live in a world full of odors – at least a trillion that our noses can distinguish (Bushdid et al., 2014) and that have significant impact on our life and emotions. Amazingly, our noses are also developed to smell feeling-related chemicals (fear, joy, sexual arousal) of other people, and therefore, we, like other creatures, ‘talk’ to each other through chemical signals (de Groot et al., 2012).
In the 1870s, 90 years before the olfactory intra-specific communication signals were directly explored and named, Jean-Henri Fabre wondered how a male moth found a hidden female behind wire-gauze while ignoring a visible female. Fabre (1916) suspected the presence of scents that attract conspecific partners and are not detected by the human nose. In 1959, Karlson & Lüscher (1959) proposed the new term ‘pheromone’ – derived from the Greek pherein, to transfer, and hormōn, to excite – for the chemicals that mediate communication between individuals of the same species, in which both the sender and the receiver of the signal gain benefit (Wyatt, 2017). Two months later, the German scientist Adolf Butenandt and his team succeeded in isolating and identifying the first sex pheromone – bombykol – which the female silk moth Bombyx mori L. releases to attract a male (Butenandt et al., 1959, 1961a,b). In these early days, half a million female moths were needed to extract and identify the sex pheromone (Butenandt et al., 1959). Currently, the development of analytical techniques, particularly gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectroscopy (LC-MS), has enabled us to identify numerous odorants emitted from a single insect as small as a vinegar fly, Drosophila melanogaster Meigen (Diptera: Drosophilidae) (Khallaf et al., 2021).
Moreover, in the light of modern technological innovations such as CRISPR-Cas9 genome editing, we have seen an increased interest in how olfactory systems diverge to drive speciation and adaptation to new ecological niches (Zhao & McBride, 2020). Therefore, the study of evolutionary changes of neural circuits that underlie species-specific behaviors in an animal’s ecological niche – a field referred to as evolutionary neuroecology – has become an attractive topic in the last 2 decades (de Bono & Bargmann, 1998; Lim et al., 2004; Newcomb & Katz, 2009; Bendesky et al., 2017; Prieto-Godino et al., 2017; Seeholzer et al., 2018; Ding et al., 2019; Hong et al., 2019; Zhao & McBride, 2020). By tracing the accumulated changes in the olfactory communication systems between closely related Drosophila spp., this review advances our understanding of how peripheral and central neural circuits are modulated over relatively short evolutionary time scales. The following sections provide a comprehensive overview on intra-specific olfactory communication via sex pheromones to attract a mating partner, the architecture of the insect olfactory system, and the transformation of the sex pheromone signal into an electrical signal to elicit sexual behaviors. Furthermore, we discuss how host shift, and the usually accompanying changes in olfactory preferences in flies, can contribute to sexual isolation and, hence, speciation.
THE INS AND OUTS OF OLFACTORY COMMUNICATION
Since the first olfactory receptors have been described (Buck & Axel, 1991), more and more scientists started to investigate the evolution of chemical communication among species with respect to the chemical signal and the molecular and neural basis of the signal’s perception. However, olfaction is a challenging sense for several reasons. First, unlike with optical, thermosensory, hygrosensory, and mechanosensory stimuli that can be measured (such as wavelength, temperature, humidity, and pressure), olfactory stimuli do not follow a physical scale but present a nearly infinite number of possible combinations of molecules. Second, in contrast to vision and audition, where the stimulus travels with the huge velocity of light or sound, olfactory signals rely on the complex physical movement of molecules that take time to travel in air (or water). These molecules convey information not only about the sender, but also about the distance to the emitting source and the time elapsed since their release. Third, a relatively large portion of the genome is devoted to encoding the olfactory receptor genes. It is, therefore, hardly surprising that, aside from the ability to discriminate among millions of chemicals, the olfactory system can detect odorants that have never before naturally occurred on earth (i.e., that are synthesized for the first time by chemists) (Meunier & Rampin, 2017). The olfactory system is well suited for a prompt adaptation to ecological changes. Fourth, olfaction relies on large numbers of receptors, larger than for any of the other senses. For example, humans could differ significantly in how they perceive the same odor due to potential variation in ca. 400 functional olfactory genes, compared to limited differences in vision due to changes in the four opsin genes (Menashe et al., 2003; Wyatt, 2014). Fifth, the rapid evolution of olfactory sensory genes that have emerged independently in insects and vertebrates makes them a rich and challenging evolutionary subject to be investigated. Finally, in comparison to taste neural pathways that elicit attraction or aversion through labeled-line coding (Zhang et al., 2019), olfaction is to a large extent organized through high-level combinatorial processes (Malnic et al., 1999). This postulates that the olfactory system uses combinations of receptors to encode odor identities, which permit an enormous flexibility of signaling to evolve (Haverkamp et al., 2018).
Sex pheromone communication: a ‘curriculum vitae for mating’
Sex pheromones have a large impact on the life of an animal and represent a very detailed curriculum vitae to engage in copulation. They are the first and arguably the best-studied olfactory communication signals, from their production to detection and elicited behaviors (Dickson, 2008; Yu et al., 2010; Dahanukar & Ray, 2011). Sex pheromones are produced by members of almost all orders of animals, from worms (Jeong et al., 2005) and insects (Wyatt, 2017) to fish (Clarke et al., 1991), reptiles (Schoralkova et al., 2018), birds (Caro et al., 2015), and mammals (Goodwin et al., 1979). Animals use various mechanisms for releasing sex pheromones into the environment, e.g., they have been reported in the saliva of pigs, in urine and tears of mice, in vaginal fluids of hamsters, and in anal glands of insects (Brennan, 2010; Wyatt, 2017).
Although closely related species tend to produce similar sex pheromones, as a result of shared biosynthetic pathways that were present in common ancestors (Figure 1), they gain specificity by blending compounds in a species-specific concentration ratio. Aphids provide a fascinating example, as most species utilize the same four chemicals (nepetalactone and three isomers of nepetalactol), but each has its unique sex pheromonal blend due to a specific concentration ratio (Pickett et al., 1992; Hardie et al., 1994). Besides the various ratios, an enormous diversity of sex pheromones could be achieved by utilizing simple modifications (e.g., reduction and oxidation) to an existing sex pheromone (Lebreton et al., 2017; Borrero-Echeverry et al., 2021), which can explain the presence of different, but biosynthetically related (Figure 1), components in closely related species (Symonds & Elgar, 2008).
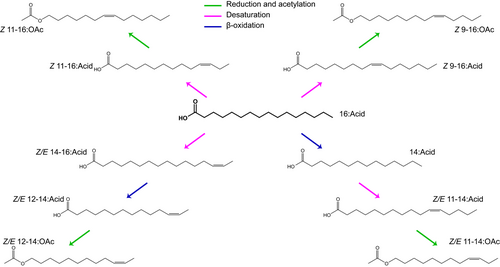
Contrary to the concept of species specificity, the same molecules could be used as a sex pheromone in different species, if: (1) they live in non-overlapping geographical regions, i.e., allopatric species (Wyatt, 2017), (2) the release time of sex pheromones is segregated to avoid heterospecific attraction (Ishikawa et al., 1999; Cardé & Haynes, 2004), or (3) the animals are unlikely to mate, as is the case for elephants and several moth species that utilize the same sex pheromone (Wyatt, 2014).
Animals often synthesize sex pheromones in a single form of two stereoisomers, i.e., enantiomers. Enantiomers are two compounds with the same molecular formula and order of atoms, but with different spatial orientations of the atoms, similar to the right and left human hands. For example, the mammalian pheromone 3,4-dehydro-exo-brevicomin has two chiral centers (RR and SS enantiomers); only the RR enantiomer is produced and detected by male mice (Novotny et al., 1995). Moreover, the biologically ‘wrong’ enantiomer sometimes acts as an inhibitor of the physiological activity of the ‘right’ component. It is, therefore, not surprising that racemic mixtures of pheromones (1:1 mixture of both enantiomers) often result in poor behavioral responses (Leal et al., 1998). An interesting example is provided by two Japanese beetles species, Popillia japonica Newman and Anomala osakana Sawada, that produce different enantiomers of γ-lactone. The two species can accurately distinguish between the two signals, because one of the enantiomers is an attractant whereas the other acts as an inhibitor, and vice versa for the other species (Leal et al., 1998).
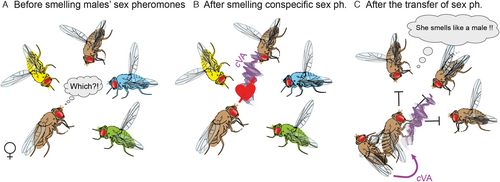
Sex pheromones induce hard-wired and innate behaviors with immediate (i.e., releaser effects, such as courtship rituals) and long-lasting (i.e., primer effects, such as phase changes in locusts) consequences. However, the interpretation of the meaning of sex pheromones could vary among individuals due to context, age, sex, or other factors including internal or mating state (Wyatt, 2014). For example, the urinal male-specific pheromone of mice induces aggression in males, attracts females, induces estrus in mature females, and accelerates maturation of young females (Novotny, 2003). Similarly, in the vinegar fly, 11-cis-vaccenyl acetate (cVA), a male-specific sex pheromone that transfers to females during copulation, triggers many behavioral outputs (Figure 2). The compound induces sexual receptivity in virgin females (Bartelt et al., 1985; Ha & Smith, 2006; Kurtovic et al., 2007), but not in mated females (Lebreton et al., 2014; Das et al., 2017). On the other hand, it suppresses courtship and elicits aggression in males (Jallon et al., 1981; Ejima et al., 2007; Wang & Anderson, 2010). Furthermore, cVA works as an oviposition cue (Dumenil et al., 2016) and aggregation pheromone for both sexes (Bartelt et al., 1985), and induces long-range attraction behavior that could be modulated by starvation (Lebreton et al., 2015).
Sex pheromone processing: turning the chemical codes into electrical signals
Insects detect odors with their antennae – specifically the third antennal segment (the funiculus) – and maxillary palps (Figure 3). Olfaction starts when odor molecules diffuse into the pores of hair-like structures called sensilla, which cover the surfaces of these olfactory organs (Hansson, 1999; Menini, 2010). Each sensillum houses a different number of olfactory sensory neurons (OSNs). Once an odor binds to the cognate chemoreceptor, which is located on the surface of the ciliary membranes of OSNs, the responsive OSN transforms this chemical information into electric signals that are conveyed further to the primary olfactory neuropil (Figure 3). In insects, this neuropil is located in a region called the antennal lobe (AL), which is analogous to the olfactory lobe in crustaceans or the olfactory bulb in vertebrates (Ache & Young, 2005; Homberg et al., 1989). The OSNs send their axons to the AL (Figure 3) to innervate discrete spherical subunits of this neuropil − the olfactory glomeruli.
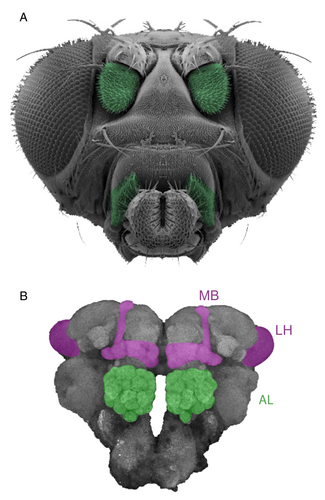
The number of glomeruli varies widely across animal species and correlates to the number of odorant receptors (ORs), e.g., 62 ORs and 52 glomeruli in D. melanogaster, 170 ORS and 165 glomeruli in honeybees, around 380 ORs and 400 glomeruli in ants, and ca. 1000 ORs and 1800 glomeruli in the olfactory bulb of mice (Galizia et al., 1999a,b; Schachtner et al., 2005; Maresh et al., 2008; Zube et al., 2008; Krieger et al., 2010; Braubach et al., 2012; Grabe et al., 2016). In both invertebrates and vertebrates, each glomerulus is innervated by a group of OSNs that express the same chemoreceptor(s) (i.e., OSNs with identical odor response profiles) (Mombaerts et al., 1996; Gao et al., 2000; Vosshall et al., 2000). The glomeruli are connected by a network of local interneurons (LNs), which represent an early processing step for shaping the efferent olfactory information into the higher brain centers [reviewed in Grabe & Sachse (2018) and Yang et al. (2019)]. The LNs’ arborization patterns diversify their anatomical structure, with some LNs innervating only a few glomeruli (so-called patchy LNs) and others targeting many, if not all, glomeruli (so-called panglomerular LNs) (Chou et al., 2010; Seki et al., 2010; Liou et al., 2018; Mohamed et al., 2019). Two types of LNs are distinguished by the impact of the signal and use of neurotransmitters: inhibitory and excitatory neurons (Shang et al., 2007; Silbering et al., 2008).
After processing by LNs, afferent olfactory information is conveyed from the AL to higher brain centers via second-order neurons, so-called olfactory projection neurons (PNs, analogous to mitral/tufted cells in vertebrates). The PNs send their dendrites to the AL glomeruli and their axons to higher-order brain centers. The PNs reveal a clear spatial segregation with respect to their response, to either pheromones or food odors (Jefferis et al., 2007), attractive amines or aversive acids (Min et al., 2013), and opposing hedonic valences and odor intensity (Strutz et al., 2014). The PNs convey information through the inner, middle, and outer antennocerebral tract (iAct, mAct, and oAct) (Stocker et al., 1990) to the mushroom body (MB) and the lateral horn (LH) (Figure 3) (Vosshall & Stocker, 2007). The mushroom body is analogous to the piriform cortex in mammals (Su et al., 2009) and is thought to be a center for olfactory learning and memory (Davis, 1993; Heisenberg, 2003; Fiala, 2007; Waddell, 2013), whereas the lateral horn shares many similarities with the mammalian amygdala (Miyamichi et al., 2011; Sosulski et al., 2011) and is presumed to mediate innate behavior (Heimbeck et al., 2001). However, recent studies have documented many roles of the MB in innate behaviors (Bracker et al., 2013; Lin et al., 2014; Lewis et al., 2015) and of the LH in modulation of the learning processing of the MB (Dolan et al., 2019). The LH output neurons project their axonal terminals to many other brain regions (Tanaka et al., 2004; Frechter et al., 2019) and then to descending neurons that will ultimately activate the motor system, to enable the animal to perform odor-guided behaviors.
Drosophila as a model to study the evolution of sex pheromone communication
Since 1903, when William Ernest Castle started to use D. melanogaster as a research organism in the laboratory, five Nobel prizes in physiology or medicine have been awarded to nine scientists for their vinegar fly-based discoveries: (1) In 1933, to Thomas Hunt Morgan for his pioneering discovery that the ‘chromosome plays a key role in heredity’ (Morgan, 1910). (2) In 1946, to Hermann Joseph Muller for the discovery that the ‘mutation rate of genes increases by x-ray radiation’ (Muller, 1930). (3) In 1995, to Edward B Lewis, Christiane Nüsslein-Volhard, and Eric F Wieschaus for understanding the genetic control of embryonic development (Lewis, 1978; Nüsslein-Volhard & Wieschaus, 1980). (4) In 2011, to Jules A Hoffmann for discoveries concerning the activation of innate immunity (Lemaitre et al., 1996). And (5) in 2017, to Jeffrey C Hall, Michael Rosbash, and Michael W Young for uncovering the molecular mechanisms that control the circadian rhythm (Bargiello et al., 1984; Zehring et al., 1984; Siwicki et al., 1988; Hardin et al., 1990; Liu et al., 1992; Vosshall et al., 1994; Price et al., 1998).
All of these discoveries were possible due to the ease of manipulating fly genomes by unique and sophisticated genetic tools, which are capable of activating or silencing the gene(s) of interest (Senturk & Bellen, 2018). Besides our ever-increasing bounty of knowledge and tools, and the ability to extrapolate to related species, their wealth of ecological diversity has made Drosophila spp. an excellent model to elucidate the genetic and neural correlates of their evolution. The genus Drosophila includes more than 1500 described species that live in quite diverse environments (Markow & O'Grady, 2005; Jezovit et al., 2017). Drosophilids’ various ecological niches and species-specific behaviors and neural circuits, as well as the available genome sequences and experimental tools, have made insect sexual communication a valuable model to study evolutionary neuroecology.
Sex pheromone signaling in D. melanogaster is well understood, from sensory signals and their cognate receptors to neurons and their connectivity (Auer & Benton, 2016). Therefore, sex pheromone signaling in drosophilid flies represents an ideal system to address the evolutionary basis of various sexual traits. Moreover, the small number of pheromone-sensing ORs compared to other ORs tuned to general odors (Couto et al., 2005) makes a comprehensive evolutionary study feasible. Out of the 52 OSN classes in D. melanogaster (Grabe et al., 2016), only four express OSNs tuned to pheromones, which are localized in a particular sensillum type (trichoid sensillum) (Couto et al., 2005). The OSNs housed in trichoid sensilla are activated exclusively by olfactory sex pheromones and consist of two classes (at1 and at4). Whereas at1 sensilla are located on the ventro-distal region of the funiculus and house one OSN, at4 sensilla are present on the dorso-distal region and house three OSNs (Clyne et al., 1997; van der Goes van Naters & Carlson, 2007; Datta et al., 2008; Dweck et al., 2015; Lin & Potter, 2015). The pheromone-tuned OSNs and their corresponding pheromones are Or67d and Or65a/b/c, which are both tuned to cVA, Or47b, tuned to methyl laurate, and Or88a, tuned to methyl laurate, methyl myristate, and methyl palmitate (Kurtovic et al., 2007; van der Goes van Naters & Carlson, 2007; Dweck et al., 2015).
ROLE OF HOST VOLATILES AND SEX PHEROMONES IN SPECIATION
Like other animals, drosophilids rely on chemical cues to locate and choose an appropriate mating partner (Johansson & Jones, 2007; Smadja & Butlin, 2009; Wyatt, 2010; Thomas & Simmons, 2011). These cues might vary among individuals of the same species to facilitate choosing an appropriate conspecific, i.e., at the right age and reproductive status and the most genetically compatible (Andersson & Iwasa, 1996; Andersson & Simmons, 2006). Olfactory sex pheromones, as well as non-pheromonal chemicals such as host volatiles – which guide and restrict the search for conspecifics within limited locations – are pre-mating isolation and speciation signals (Smadja & Butlin, 2009). Any change in these pre-mating signals and their associated preferences (Ritchie, 2007) may, hence, lead to reproductive isolation and ecological speciation (Mackay et al., 2005; Arbuthnott, 2009). It is still not well understood how sender and receiver of sexual communication signals coevolve, as novel signals may not be preferred and/or recognized by conspecifics, and would thus fall out of the population. The same would be expected for novel preferences.
Ecological speciation is the process by which adaptation to various ecological environments leads to the formation of reproductive barriers between populations of the same species that will later split to form different species (Schluter, 2000; Coyne & Orr, 2004; Gross & Rieseberg, 2005; Rundle & Nosil, 2005; Funk et al., 2006). For example, populations that adapt to a specific environment (e.g., desert vs. forest habitats) may evolve genetic differences affecting the way that individuals communicate or behave. Therefore, speciation occurs as a by-product of accumulated ecologically related variation that results in individuals from different populations that avoid mating with each other (Mayr, 1947, 1963; Rundle & Nosil, 2005; Vines & Schluter, 2006). Ecological speciation can occur under either geographic arrangement, allopatry (where populations are geographically separated), or sympatry (where populations speciate in the absence of geographical barriers) (Rundle & Nosil, 2005). However, sympatric speciation requires a pronounced divergent selection to overcome the homogenizing effects of gene flow. On the other hand, the unimpeded divergence by gene flow due to geographic barriers may lead to more distinct pre- and post-zygotic mechanisms between allopatric populations (Turelli et al., 2001).
An important line of inquiry to help understand isolation forces is to investigate the genetic and neural changes that underlie preferences among closely related species, especially those that diverged across short evolutionary time scales. The following sub-sections provide an overview of the impact of host volatiles and sex pheromones on reproductive isolation among drosophilids.
Reproductive isolation through host specialization
Colonization of a new host could directly lead to divergence in the olfactory communication systems through many ways (Figure 4). First, host shift has severe impact on the biology of animals, e.g., body size, shape, and color, as well as population density (Nosil et al., 2003; Messina, 2004). These changes, therefore, may impact communication signals, mate-searching strategies, and sexual preferences (Etges, 1998). For example, searching for mates in less dense environments could lead to the adoption of long-range sexual signals. Second, new hosts could provide new ways of signal transmission and perception, resulting in differential sexual signaling (Funk et al., 2002), a process called sensory drive (Endler & Basolo, 1998). Sensory drive concerns how sexual traits and preferences coevolve in response to new environments. Lastly, host volatiles may be perceived and preferred as background or as components of the sex pheromone blend. For example, exposure to a particular host plant while mating has a strong impact on the subsequent reproductive preferences of male moths (Proffit et al., 2015; Zakir et al., 2017).
Similarly, in D. melanogaster, food odors (vinegar) boost the perception of the male sex pheromone – cVA – in virgin females, but not in males nor in mated females (Das et al., 2017). Although vinegar does not directly activate glomerulus DA1 – a specific glomerulus that responds to cVA (Kurtovic et al., 2007) – vinegar odors potentiated the cVA response on the PNs projecting to this glomerulus through electrical synapses between excitatory LNs and PNs in the antennal lobe. In line with these findings, other food odors (phenyl acetic acid and phenyl acetaldehyde) induce courtship in male flies through the ionotropic receptor (IR84a) (Grosjean et al., 2011). Vinegar is a complex blend that consists of many odorants, of which acetic acid is the most abundant compound (Becher et al., 2010). Although acetic acid alone is sufficient to elicit attraction in flies, it failed to potentiate the cVA response in combination with cVA, indicating the vinegar blend is necessary to mediate this synergism. Notably, the synergistic pattern, which was observed on the electrophysiological level, has been translated into increased female receptivity (Das et al., 2017). Interestingly, males showed neither synergistic effects nor behavioral responses in the presence of vinegar and cVA (Das et al., 2017). In behavioral assays, fed virgin Drosophila females were more attracted to the blend of cVA and vinegar than to vinegar alone, whereas males were not (Lebreton et al., 2014). Obviously, female flies benefit from not only focusing on male odors when looking for a mating partner, but also keeping the odors of potential food sources in mind.
Drosophila spp. live in a wide range of habitats across all climatic conditions, from deserts and caves to mountains and forests (Markow & O'Grady, 2005; Jezovit et al., 2017). In these environments, they feed and breed on diverse hosts such as decaying fruits, slime fluxes, mushrooms, and flowers, as well as frog spawn. On the one hand, many drosophilids share the same habitat (Markow & O'Grady, 2005; Jezovit et al., 2017); to reduce competition, they might allocate more energy to a distinct sensory modality (Keesey et al., 2019). On the other hand, many drosophilids specialize on a single host resource, such as Drosophila mojavensis Patterson on cacti, and Drosophila erecta Tsacas & Lachaise on Pandanus fruit. Another notable drosophilid that has received particular attention with respect to its chemoreception is Drosophila sechellia Tsacas & Bachli, which is endemic to the Seychelles archipelago and has evolved extreme specialism for noni fruits (Morinda citrifolia L.), which are toxic for most (if not all) other drosophilids, including its sibling species Drosophila simulans Sturtevant and D. melanogaster (Legal et al., 1992).
The extreme specialism that D. sechellia has evolved compared to its close cousins did not only result in an increased metabolic tolerance of noni fruit toxins (R'Kha et al., 1991; Jones, 1998), but also in changes in courtship and mate choice (Cobb et al., 1989; Coyne, 1992), oviposition (Moreteau et al., 1994; Amlou et al., 1998), and pupariation site selection (Erezyilmaz & Stern, 2013). Recently, these shifts were shown to be correlated with changes at the periphery of the fly’s olfactory system, i.e., whereas the odorant receptor OR22b is lost, the flies exhibit an increased number of neurons expressing OR22a (Auer et al., 2020). However, the many behavioral shifts raise the possibility that the peripheral changes of olfactory coding are accompanied by novel central projection patterns. Indeed, circuit tracing experiments revealed the presence of increased sensory pooling onto interneurons and novel branches of PNs (Auer et al., 2020). Consistent with these findings, Or22a displays substantial intra- and interspecific nucleotide and copy number variation in many drosophilids (Guo & Kim, 2007; Nozawa & Nei, 2007; Aguade, 2009; Goldman-Huertas et al., 2015). In addition, the number of Or22a neurons has also expanded in D. erecta, a specialist on Pandanus fruit (Linz et al., 2013). Obviously, OR22a is an important receptor when it comes to host specificity. However, despite the importance of the Or22a pathway, several other olfactory channels play a role in noni attraction. These include Or85c/b neurons, which have conserved physiological properties among drosophilids but increased in number in D. sechellia, and Ir75b neurons, which have changed in both function and number while preserving central projections (Prieto-Godino et al., 2017). Such observations indicate that neural pathways may adapt in distinct ways, possibly reflecting diverse selection pressures and roles that enable animals to specialize to a unique host, and thus limit encountering heterospecific flies.
Reproductive isolation through mate selection
Sexual traits involved in communication between the sexes often provide an instant contribution to reproductive isolation (Blair, 1955; Bridle & Ritchie, 2001). Upon search for a mate, females rely on honest signals – including visual, acoustic, and chemical signals – to find high-quality males (Bateman, 1948; Grafen, 1990). Therefore, sexual selection has led to the evolution of sexual signals in males and the cognate sensory systems in females for many animal species. Compared to other sensory information, olfactory sex pheromones provide the first cue to recognize a conspecific individual and can include much valuable information, such as its sex, age, body size, reproductive status, and health condition (Martin et al., 2007; Nielsen, 2017). These olfactory cues not only provide information on the sender, but may also have negative or positive effects on the receiver. For example, in mice, male scent induces estrus in females, whereas female scent suppresses ovarian activity in other females (Whitten, 1959, 1966; Nielsen, 2017). Once they are attracted by sex pheromones, males and females engage in species-specific courtship rituals, by which both partners ensure their reproductive success.
Sex pheromones and courtship behaviors of drosophilid flies differ quantitatively and qualitatively (Spieth, 1952; Symonds & Wertheim, 2005) and therefore represent an attractive model to identify the genetic basis of phenotypic evolution. These species-specific behaviors represent a pre-mating isolation barrier, especially between sympatric species (Spieth, 1952). When meeting at the host, flies identify conspecific mating partners through integration of various sensory modalities including vision, audition, olfaction, and gustation (Markow & O'Grady, 2005), which leads to either increased or inhibited courtship behaviors. Fruitful insights have been gained regarding the evolution of sensory modalities that underlie the interspecific variations in drosophilid courtship behavior (Manoli et al., 2005; Ding et al., 2016; Tanaka et al., 2017; Seeholzer et al., 2018; Ahmed et al., 2019). Sexual behaviors in drosophilids consist of a series of actions and reactions that vary qualitatively and quantitively between species (Spieth, 1952) and result in species and sexual discrimination. At first, males orient to and follow the females. Subsequently, males tap the females’ bodies with their forelegs, followed by wing spreading and fanning for vibrational song production. Males follow the females by extending their proboscis to lick the females’ genitalia and then attempt copulation. Obviously, the male has to invest to convince the rather choosy female. Interestingly, female choosiness varies even within species. When population size is small, i.e., when potential mates are rare, females that are too choosy may not find any suitable males, whereas females that are less choosy might still reproduce, although with less satisfying males (de Jong & Sabelis, 1991; Martínez-Ruiz & Knell, 2017).
One remarkable drosophilid that splits into four geographically isolated and ecologically distinct populations, and represents a model of incipient speciation and host adaptation, is D. mojavensis (Etges, 2019). The northern subspecies D. m. wrigleyi and D. m. mojavensis utilize the prickly pear cacti in Santa Catalina Island (CA, USA) and the red barrel cactus in the Mojave Desert (CA, NV, AZ, and UT, USA), respectively. The southern subspecies D. m. sonorensis and D. m. baja breed and feed on the organ pipe cactus in the mainland Sonoran Desert (Mexico and AZ and CA, USA) and the agria cactus in Baja California (Mexico), respectively (Ruiz et al., 1990). Phylogenetic analyses revealed that the two northern and the two southern subspecies cluster with each other (Allan & Matzkin, 2019). These closely-related subspecies exhibit many differences, including in their morphological features (Pfeiler et al., 2009), neurophysiological responses (Crowley-Gall et al., 2016; Date et al., 2013; Nemeth et al., 2018), genomic (Matzkin, 2014) and transcriptomic (Matzkin & Markow, 2013) characteristics, and behavioral traits (Newby & Etges, 1998). Experimental reciprocal crosses between these allopatric subspecies revealed various pre- and post-zygotic isolation barriers (Zouros & Dentremont, 1980; Krebs & Markow, 1989; Knowles & Markow, 2001).
Leveraging the short evolutionary time scale between the closely related D. mojavensis populations, we elucidated the evolution of the reproductive isolation barriers among these populations and the underlying sensory mechanisms (Khallaf et al., 2020). We identified four male-specific acetates of which three were exclusively produced by the northern subspecies. This dramatic change in chemical profile was surprising given the short divergence time, ca. 0.25 million years, between D. mojavensis subspecies (Matzkin, 2014; Etges, 2019). The four populations were feeding on similar artificial media, suggesting that the differential male-specific compounds are not a consequence of nutrition but are rather genetically determined. When checking for the function of these novel compounds, we realized that R-(Z)-10-heptadecen-2-yl acetate (R-HDEA) induces female receptivity, whereas (Z,Z)-19,22-octacosadien-1-yl acetate (OCDA) reduces male courtship. Together, these two substances fulfil the same roles that are described for cVA in D. melanogaster. Our results support the findings of Chin et al. (2014), who showed that D. m. wrigleyi males avoid courting females perfumed with ejaculatory bulb extracts of mature males (which, according to our analyses, contain OCDA). By contrast, similar extracts from immature males (which do not produce OCDA) did not elicit this behavior (Chin et al., 2014). We provided direct evidence that, in the northern subspecies, the dedicated R-HDEA-sensing neurons and auditory cues collaborate to mediate mate recognition. However, the southern subspecies rely on auditory cues only (Etges et al., 2006) and rather ignore R-HDEA for mate recognition. As all D. mojavensis flies can detect R-HDEA (which is only present in the northern subspecies), the striking change of R-HDEA-induced behaviors among D. mojavensis subspecies implies the existence of differences in the central processing pathways (Khallaf et al., 2020). These findings are reminiscent of sexually dimorphic behavioral responses to cVA in D. melanogaster (Ruta et al., 2010; Kohl et al., 2015), where both males and females detect cVA, but interpret this in different ways.
Most drosophilids are gregarious in mating, except the Hawaiian species, whose males display lekking behavior, i.e., they select and defend a unique territory on their host (Spieth, 1952; Carson et al., 1970). Similar to Hawaiian species, we demonstrated that D. m. wrigleyi males advertise their presence by pulsating a droplet of anal secretions, which contains the volatile sex pheromone, R-HDEA, in close proximity to females to enhance their receptivity (Khallaf et al., 2020). Such a trait is supportive to the observed sexual behaviors of D. mojavensis in nature, where males conquer undamaged areas next to feeding sites on cactus and attract females to this spot (O'Grady & Markow, 2012). Similarly, other members of the Drosophila genus show species-specific courtship behaviors (Spieth, 1952), which might include nuptial gift donation (Steele, 1986), partners’ song duet (O'Grady & Markow, 2012; LaRue et al., 2015), or territorial dating (O'Grady & Markow, 2012).
Divergence of the chemosensory genes could be accompanied by a rewiring of the central circuitry (Ding et al., 2016; Seeholzer et al., 2018). Indeed, neurons of D. mojavensis expressing the pheromone receptor Or65a do not innervate to the same glomerulus (DL3) as in D. melanogaster. Furthermore, despite expressing homologues of Or65a, the corresponding pheromone circuits of D. mojavensis and D. melanogaster govern different behaviors: the Dmoj-Or65a circuit detects R-HDEA and mediates female receptivity and conspecific recognition, whereas the homologous circuitry in D. melanogaster detects cVA and suppresses courtship and aggression behaviors in males (Ejima et al., 2007; Liu et al., 2011), as well as attraction towards cVA in mated females (Lebreton et al., 2014). These differences also suggest that the target domains in the higher brain centers of both circuits have changed. Notably, the processing pathway of a comparable pheromonal circuitry, between D. melanogaster and D. simulans (Seeholzer et al., 2018), has changed during less than one-tenth of the divergence time between D. melanogaster and D. mojavensis (ca. 40 million years ago). Thus, divergent functions of a homologous neural circuitry during a longer divergence time could be presumed.
FUTURE PERSPECTIVES
- What is the interplay between sexual and natural selection? Do these forces act in concert to result in speciation events?
- How do ecological conditions influence the evolution of sex pheromones and their perceptions?
- Do host volatiles influence the perception of sex pheromones in a species-specific manner? In other words, are sex pheromones of conspecifics perceived differently in a background of host volatiles?
- Does host specialization relieve flies of investment in sexual selection barriers (i.e., sex pheromones) as they may encounter only conspecific flies?
- Do genetic and neural variations act together to reinforce sexual isolation barriers, especially in sympatric species that require prominent divergences?
- How many and what types of genes need to mutate for flies to specialize on a new host?
With more than 1500 species, the genus Drosophila offers a fantastic resource to target these questions. Many of these species are nowadays available from Drosophila stock centers, and the establishment of molecular tools, e.g., CRISPR Cas9, allow us to investigate various aspects, e.g., the neuronal circuits of non-D. melanogaster drosophilids. However, what is lacking for many species is detailed knowledge about their ecology and distribution areas. Future drosophilists should, therefore, consider to visit more often their study flies in the field.
Acknowledgments
We thank Prof. Dr. Bill Hansson and members of the Department of Evolutionary Neuroethology, Max Planck Institute for Chemical Ecology, Jena, Germany, for discussions. This research was supported through funding by the Max Planck Society.
Author Contributions
Mohammed A. Khallaf: Conceptualization (lead); Visualization (lead); Writing – original draft (lead); Writing – review & editing (equal). Markus Knaden: Conceptualization (supporting); Supervision (lead); Writing – review & editing (equal).




