Associative learning in immature lacewings (Ceraeochrysa cubana)
Abstract
It is known that many social insects and arthropod predators and parasitoids can learn the association between a resource and volatile cues. Although there are various studies on the effect of experience in immature arthropods on behavior later in adult life, not much is known about the effects of such experiences on immature behavior. This was investigated here in the lacewing Ceraeochrysa cubana (Hagen) (Neuroptera: Chrysopidae). Whereas adults of this lacewing feed on plant-provided food and honeydew, larvae are voracious polyphagous predators of several insect pests, and therefore important for biological control. Hence, studying the foraging behavior and the effects of learning in immatures of this species is important. We exposed immatures to the volatile methyl salicylate (MeSA), which was either associated with food or with the absence of food. Subsequently, their response to this volatile was tested in an olfactometer. Immatures that had experienced the association of MeSA with food were attracted to it and immatures that were exposed to MeSA during food deprivation were repelled. Subsequently, predator immatures that had experienced the association between MeSA and food were released on a plant without food and were found to use this volatile in locating patches with food. In contrast, larvae without such experience were found equally on food patches with and without the volatile. We conclude that these immature predators are capable of learning the association between volatiles and food, or the absence of food, and use this during foraging.
Introduction
Learning has been defined as a change in behavior as a consequence of experience (Papaj & Prokopy, 1989). It can help an animal to adapt its behavior in response to changing environmental circumstances by exploiting correlations in their environment that are particular for a certain time and place (Dukas, 2008; Morand-Ferron, 2017). An animal′s ability to learn will depend on the amount of information it can respond to (Dukas, 2008). Learning has been demonstrated in many arthropods, in particular in social insects (Takeda, 1961), parasitoids (Thorpe & Jones, 1937; Vinson, 1976; Lewis & Tumlinson, 1988; Lewis & Takasu, 1990; Wäckers & Lewis, 1994; Giunti et al., 2015; Steven et al., 2019), and, to a lesser extent, in arthropod predators (Drukker et al., 2000a,b; de Boer & Dicke, 2004a).
Arthropods are known to use olfactory and visual cues to locate food and habitats, and they can learn to associate these cues with profitable habitats or food (Vet & Groenewold, 1990; Drukker et al., 2000a), and even with specific nutrients (Simpson & White, 1990; Gadd & Raubenheimer, 2000). Arthropod predators and parasitoids of herbivores often use volatile organic compounds produced by plants to locate their prey/host (Dicke & Sabelis, 1988; Lewis & Takasu, 1990; Sabelis et al., 1999b). This response to volatiles may be innate or acquired during an individual′s life. Acquiring volatile preferences may arise through (1) imprinting, defined as rapid learning during a sensitive period early in life – either with or without new experience of the individuals with the cues (Gould, 1993; Hall & Halliday, 1998); (2) sensitization, when the response to a stimulus increases as a result of exposure to that stimulus (Papaj & Prokopy, 1989; Hall & Halliday, 1998); and (3) associative learning, where a conditioned stimulus (i.e., volatile) and an unconditioned stimulus (i.e., food) are paired, and the response (positive or negative) to the conditioned stimulus depends on the unconditioned stimulus (Thorpe, 1956; Lewis & Tumlinson, 1988; Hall & Halliday, 1998). Associative learning as studied in this paper requires that the conditioned stimulus (a volatile) and the unconditioned stimulus (i.e., the presence or absence of food) be paired, resulting in context-dependent preference or aversion, depending on the unconditioned stimulus. This kind of learning was demonstrated for predators such as predatory mites (Drukker et al., 2000a) and heteropteran bugs (Drukker et al., 2000b).
Immatures of the lacewing Ceraeochrysa cubana (Hagen) (Neuroptera: Chrysopidae) are important natural enemies in Neotropical America, with a potential for biological control (López-Arroyo et al., 1999; Albuquerque et al. 2001). The adults feed on plant-provided food and honeydew, and larvae prey on arthropod eggs and soft-bodied insects such as aphids and mites (New, 1975). Whereas lacewing adults are known to respond to a variety of volatiles, such as aphid sex pheromones (Boo et al., 2003), aggregation pheromones of conspecifics (Zhang et al., 2004), and herbivore-induced plant volatiles (James & Grasswitz, 2005; Jones et al., 2011), nothing is known about the response of larvae, the most important predatory stage. It has been suggested that dispersion of immatures is random (Hajek, 2004). Here, this is questioned by studying the effect of experience of immature C. cubana with the volatile methyl salicylate (MeSA), one of many components of herbivore-induced plant volatile mixtures (Dicke et al., 1990; de Boer & Dicke, 2004b; Arimura et al., 2009). The volatiles were associated with the presence or absence of food to determine whether lacewing larvae were able to learn this association. Because changes in response to such volatiles would only be useful if immatures leave their natal patch in search for new patches with prey, we also investigated whether the immatures left a plant without food and used volatiles to search for new prey patches.
Materials and methods
Rearing methods
The culture of C. cubana was established using insects from a stock colony of the Empresa de Pesquisa Agropecuária de Minas Gerais (EPAMIG Sudeste, Viçosa, Minas Gerais, Brazil). Immatures were fed with eggs of the Mediterranean flour moth, Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) and adults received a yeast–honey diet (1:1, wt:wt) (Batista et al., 2017). Rearings were maintained at controlled temperature (25 ± 2 °C), relative humidity (75 ± 5%) and photoperiod (L12:D12).
Volatile source
Volatile dispensers were made of Parafilm, which was cut into strips of 5.2 cm2, rolled up, and tightly flattened in layers of ca. 5 mm. Each roll was cut into five pieces of 7 mm long (Janssen et al., 2014). One group of dispensers was immersed in 99% liquid synthetic methyl salicylate (MeSA) (Sigma-Aldrich, Shanghai, China) in a closed Petri dish and the other was kept in a clean Petri dish. After 24 h, the dispensers were taken from the Petri dishes and placed on a tissue paper to dry. Dispensers with and without MeSA were used as volatile sources in olfactometer tests and in training trials. The dispensers release the volatiles for at least 24 h (Janssen et al., 2014). We chose MeSA because this compound has been used in studies of learning (Drukker et al., 2000b; de Boer & Dicke, 2004a) and it is present in volatile blends released by plants upon herbivore attack.
Y-tube olfactometer tests
An olfactometer (Sabelis & van de Baan 1983; Janssen, 1999) was used to test preference for or aversion to MeSA compared to ambient air. The olfactometer consisted of a Y-shaped glass tube (27 cm long, 3.5 cm diameter), with a black Y-shaped metal wire in the middle to guide the predator, with the base of the tube connected to a pump that causes an airflow from the arms of the tube to the base (Janssen, 1999). Each arm was connected to a glass container (50 × 36 × 43 cm), either containing three dispensers with the volatile (as above) or three clean dispensers. The airflow in each arm of the olfactometer was calibrated to 0.50 m s−1 (VelociCalc Air Velocity Meter 9545-A; TSI, Shoreview, MN, USA), so that the air from the containers formed two separate fields in the base of the Y-tube (Sabelis & van de Baan 1983). One immature predator, 10 days old (since hatching), was released at the downwind end of the Y-tube and was allowed to walk upwind along the base of the Y-tube to choose either the arm connected to the container with the volatile or to the container with a clean dispenser. A trial ended when the predator reached the end of one of the arms of the Y-tube or after 5 min, after which it was removed, and the next predator was introduced. Each replicate consisted of 20 predators that had made a choice, hence, the total number of individuals tested per replicate varied according to the numbers of predators not responding within 5 min. After each five animals that made a choice, the Y-tube was cleaned with alcohol and the containers were connected to the opposite arm of the olfactometer to correct for unforeseen asymmetries in the set-up and the experiment was continued with the same volatile dispensers. The olfactometer, the volatile containers, and the connecting tubes were cleaned with detergent and water in between replicates. Unless stated otherwise, predators were starved for 24 h prior to testing. Four replicate experiments were carried out for the innate response and three replicate experiments for the learning experiment, each on a different day with a different group of predators and different volatile dispensers.
Experience
To test the innate response, four groups of 30 individual third-instar predators (10 days old) were taken from the rearing units. The insects were held for 24 h in individual plastic tubes (3 cm diameter, 7.5 cm deep) without food, with a hole in the lid covered with fine mesh for ventilation. Subsequently, their response to MeSA or ambient air was tested. Each group was tested on a different day with a new set of volatile dispensers.
To offer experience to predators, they were individually held in plastic tubes as above and all plastic tubes were placed inside a plastic box (35 × 24 × 20 cm) with two openings. One opening (3 cm diameter) was connected to a pump that produced an airflow from the room into the box at 0.45 m s−1 at the entry the box. The other side of the box had an air outlet. Three volatile dispensers with MeSA were put in Petri dishes below the input of the airflow (Figure 1). Before the experiments, we verified whether the volatile would enter the tubes by putting cotton wool inside the tubes used in the training procedure, placing three volatile dispensers inside the box and pumping air through the box. After 24 h, the volatile could be perceived from dispensers as well as from the cotton wool that had been inside the tubes, the latter confirming that volatiles were carried into the tubes in our set-up.
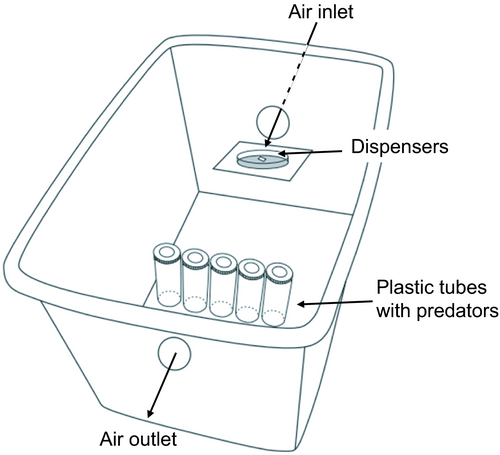
The training started with first-instar predators (24 h since hatching), that were taken from the rearing unit and randomly assigned to two groups (Table 1). During the first 96 h, the immatures of all groups received eggs of E. kuehniella in their tubes as food while perceiving the volatiles. Dispensers were replaced with new ones every 24 h. Subsequently, they were held in a new plastic tube without food and volatiles for 24 h. During the next 4 days, immatures were switched daily from tubes with food in the presence of volatiles to tubes without food and without volatiles. A control group received the same treatments with or without food, but was never exposed to the volatiles (Table 1). The day after this training period, the individuals of the two groups were transferred to new clean tubes and tested for their response to MeSA.
| Experience | Duration of each step of experience (h) | |||||
|---|---|---|---|---|---|---|
| 96 | 24 | 24 | 24 | 24 | 24 | |
| Food + MeSA | Food +a | No food − | Food + | No food − | Food + | No food − |
| Control | Food − | No food − | Food − | No food − | Food − | No food − |
| Food – MeSA | Food − | No food + | Food − | No food + | Food − | No food + |
| Control | Food − | No food − | Food − | No food − | Food − | No food − |
- Experience was started with immatures of 24 h since hatching. They were tested 1 day after the last experience.
- a +/− refers to the presence/absence of MeSA.
To assess whether associative learning was involved (operant conditioning; Hall & Halliday, 1998), it was necessary to show a switch in response when MeSA was associated with a negative stimulus. The same training procedure as described above was given to two other groups, but one group was exposed to MeSA when they were without food, and the control group was not exposed to MeSA (Table 1). These predators received the last experience with the volatile 1 day later as the predators trained with the association of volatiles with the food (Table 1). Papaj et al. (1994) showed that the effect of unrewarding experiences in a parasitoid was shorter than that of rewarding experiences; hence, the training schedule used here ensured the detection of the effect of unrewarding experiences. The response of these groups to the volatile was assessed as above. The response of the predators was assessed on different days with new control groups. The response of all predators was assessed in the olfactometer test within 3 h.
Per group of immatures that received a treatment, the preference for MeSA was assessed with a log-linear model for contingency tables with Generalized Linear Models (GLM) using a Poisson error distribution (log link) (Crawley, 2013) with volatile, side, replicate, and their interactions as factors. The minimal adequate model was obtained by removing non-significant interactions and factors with deletion tests using the ‘anova’ command in R. Subsequently, the responses were compared among groups with a GLM with experience of the predators as factor and a binomial error distribution. The analyses were performed with the statistical software R v.2.15.1 (R Development Core Team, 2017).
Release-recapture experiment
A release-recapture experiment was done outdoors to assess whether immature lacewings would move from a plant and use volatiles to localize new patches with food. A cage, consisting of a wooden frame (1.60 × 1.60 × 1.70 m) covered with fine mesh and placed outdoors, was surrounded by trees on one side, a building on the opposite side, and grass at the other two sides. A tray inside the cage was filled with soil and three black plastic discs (14 cm diameter) with volatile dispensers with MeSA, interspersed with three plastic discs with dispensers without volatile, were placed in a hexagon (diagonal = 1 m) on top of the soil. Eggs of E. kuehniella were added to all discs as food to arrest arriving predators. Discs with dispensers with and without MeSA occupied alternating positions to avoid any unforeseen directionality in predator dispersion (Janssen, 1999). In two replicates, the three plastic discs with dispensers with MeSA were put in positions 1, 3, and 5, and in the other two replicates the discs with MeSA were placed in positions 2, 4, and 6.
About 200 immature predators (24 h old) were taken from the laboratory colony. Each immature was trained as explained above, with MeSA paired with the presence of eggs of E. kuehniella as food source, and a control group that received the same treatment, but without the volatile. After the training period, the predators were carefully placed on a cabbage plant (Brassica oleracea L. var. capitata, eight-leaf stage) in the middle of the hexagon and were allowed to disperse from the plant to the discs. We released the predators on a cabbage plant to simulate a natural situation in which they would find themselves on a plant without food, from which they would need to disperse to find other prey patches. Starting 1 h after release, all discs were sampled once per hour, during a total of 6 h and again 24 h after release. At each check, all predators found on the plastic discs were removed. The temperature inside the cage was between 25 and 30 °C. For logistic reasons, the trained group and the control group were released on different days with new volatile dispensers, the groups with experience were released on September 24 and November 2, 17, and 19; the control groups on October 1 and 15 and November 5 and 14, 2014.
Per predator group (experienced with the volatile or control), the preference for MeSA was tested by comparing the total numbers of immatures that were recaptured during the experiments on the discs with or without volatile with a log-linear model for contingency tables (GLM; Crawley, 2013) with replicate and volatile as factors and a binomial error distribution. The difference in preference between the two treatment groups was compared by testing the proportion of predators recaptured on the discs with MeSA with a GLM using a binomial error distribution (logit link) with experience as factor. We also compared the proportions of released immatures that were recaptured on the discs between treatment groups.
Results
Associative learning
Naïve immature predators did not show attraction or repellence to MeSA: 55% chose the volatile (GLM: χ2 = 0.80, d.f. = 1, P = 0.37; Figure 2). The individuals that had experience with the association between MeSA and the absence of food did not show attraction or repellence to MeSA (χ2 = 2.41, d.f. = 1, P = 0.12). The control of this group showed a slight but significant preference for MeSA (χ2 = 4.31, d.f. = 1, P = 0.038). The response of these two groups differed significantly (experience*volatile: χ2 = 6.60, d.f. = 1, P = 0.010; Figure 2).
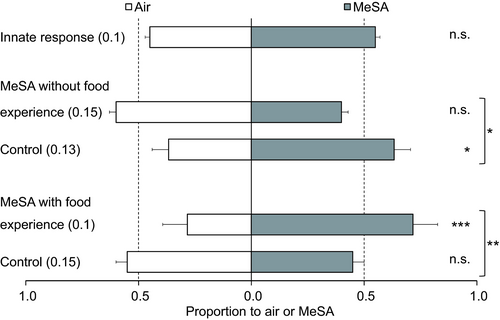
For lacewing immatures that were trained with the association of MeSA and food, there was a significant difference among replicates (replicate*volatile: χ2 = 6.88, d.f = 2, P = 0.032; Figure 2); in two of the three replicates, the predators displayed a strong preference for MeSA, whereas no preference was found in the third replicate. Nevertheless, there was a strong overall preference for MeSA (χ2 = 11.6, d.f. = 1, P<0.001). The control group of this treatment did not show attraction or repellence to MeSA (χ2 = 0.60, d.f. = 1, P = 0.44). The response of the control group differed significantly from the group with experience (experience*volatile: χ2 = 8.90, d.f. = 1, P = 0.0029; Figure 2).
There was no significant difference in response of the control groups vs. the group of naïve predators (Figure 2, bars 1 + 3 + 5: χ2 = 4.10, d.f. = 2, P = 0.13) but the response of the two experienced groups differed significantly (bars 2 vs. 4: χ2 = 12.4, d.f. = 1, P<0.001).
Release-recapture experiment
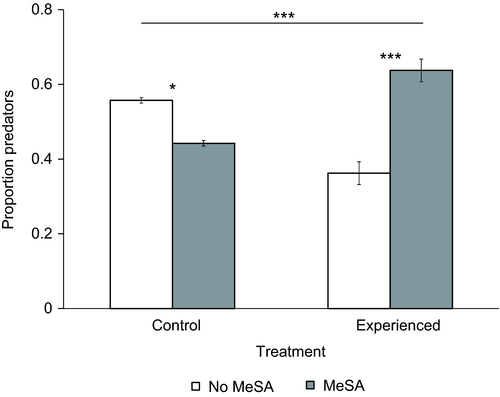
The release-recapture experiment revealed that the group without experience (control) had a significant preference for patches without the volatile (χ2 = 5.30, d.f. = 1, P = 0.021; Figure 3). In contrast, immatures trained with the association of MeSA with food were recaptured significantly more on discs with MeSA (χ2 = 30.6, d.f. = 1, P<0.0001; Figure 3). A higher proportion of individuals from the experienced group was recaptured on discs with MeSA than from the control group (χ2 = 30.8, d.f. = 1, P<0.0001; Figure 3). Experience with the volatile did not affect the proportion of individuals that were recaptured from the total number of predators released (F1,6 = 0.029, P = 0.96). On average, 49.5% of all released predators were recaptured. The other predators were still walking in the area or outside the experimental area. Most predators were recaptured within 6 h after their release.
Discussion
It is known that predatory arthropods may use volatiles to explore their environment, but there is little knowledge of the ability of immatures to learn and use these cues. Here we show that lacewing immatures can learn the association between volatiles and the presence or absence of food. Because immatures are the most voracious stages in several groups of predators, such as in lacewings, some species of Syrphidae, and Coccinellidae (Gilbert, 1981; Hodek & Honêk, 1996), it is important to study the behavior of these immatures, and more specifically, whether they also use volatiles to find plants with prey, and whether they are able to learn or search randomly (Hajek, 2004).
We chose MeSA as a volatile cue because it is produced by many plant species upon attack by herbivores (Dicke et al., 1990; Turlings & Erb, 2018). After exposure to MeSA associated with food, C. cubana immatures were attracted to this compound. In contrast, immature lacewings that were exposed to MeSA in the absence of food (hence, they became hungry) were not attracted or repelled by it. Such associative learning was reported for adult predatory arthropods like mites and bugs (Drukker et al., 2000a,b; de Boer & Dicke, 2004a), but little is known about changes in immature predator behavior as a result of a previous experience with volatiles. Earlier studies have shown habituation and learning in mosquito larvae (Ferrari et al., 2008; Baglan et al., 2017) and in juvenile crickets (Hale & Bailey, 2004), or the effects of pre-imaginal experience on behavior as adults (Isingrini et al. 1985; Gutiérrez-Ibáñez et al., 2007; Blackiston et al., 2008; Signorotti et al. 2014). To our knowledge, this is the first study that demonstrates that immature predators are able to learn the association of a volatile cue with food and use this to locate food. We think that this use of volatiles is not restricted to MeSA, but further experiments will have to confirm whether immature lacewings can also learn the association between other volatiles and unconditioned cues.
In the outdoor experiment, we found that the proportion of predators that arrived on patches with MeSA was significantly higher when predators had experienced this volatile in association with food than without experience. However, we had also expected that the numbers of predators that arrived on the patches would be higher in the experienced groups than in the control groups, because predators with experience should be more effective at finding the food. However, the replicates with the experienced group and the control group could not be carried out at the same time for logistic reasons, and the variation in the numbers of predators recaptured varied considerably among replicates (28–62% for experienced predators; 26–70% for the control group), which was likely due to differences in abiotic conditions. This large variation may have masked any effect of increased efficiency of the experienced predators. Future experiments should therefore aim at testing experienced and control groups simultaneously.
Plants attacked by herbivores are known to emit volatile compounds that are used by predators to locate prey (Dicke & Sabelis, 1988; Dicke et al., 1990; Turlings et al., 1990). These volatiles consist of a mixture of compounds that may vary with the species of host plant, even when attacked by the same herbivore, and with the species of herbivore, even when they attack the same plant species (Takabayashi et al., 1991; De Moraes et al., 1998). Moreover, the mixtures can vary with plant genotype, plant age, and abiotic conditions (Takabayashi et al., 1994; Gouinguené & Turlings, 2002). The role of plant-produced volatile compounds in the attraction of predators was extensively studied under laboratory conditions, but in nature, predators are likely to be exposed to even larger variations in volatiles from plants with prey and from the natural background. Because of this variation, natural enemies must cope with numerous signals that are associated with the presence of their prey (Sabelis et al., 1999a,b, 2007). It has been suggested that animals can cope with this variation by learning the association between volatile blends and the presence of food (Lewis & Tumlinson, 1988; Lewis & Takasu, 1990; Turlings et al., 1993; Drukker et al., 2000a; Hilker & McNeil, 2008; Janssen et al., 2014). Predators may have to switch among prey or host plants during their lives, so they need to associate new volatiles with prey availability (Dicke & Sabelis, 1988).
Our findings also have consequences for the use of volatiles to lure natural enemies in the field. Several studies have shown that the use of synthetic volatiles or the induction of herbivore-induced plant volatiles (HIPVs) can attract natural enemies (James, 2003, 2005; James & Price, 2004; Simpson et al., 2011). The authors suggested that using these compounds in the field could improve biological pest control, but mixed results have been obtained thus far (Rodriguez-Saona et al., 2012). Many plants attacked by herbivores already emit HIPVs that attract beneficial insects, so in this situation the application of HIPVs is unnecessary. When plants are not attacked by herbivores, application of HIPVs can attract those natural enemies, however, we demonstrated here that if such volatiles are not associated with a reward (i.e., food), the predators may learn the association between the volatile and the absence of food and will subsequently be less attracted to the volatile. Thus, application of volatiles under field conditions to attract predators without the presence of a reward needs to be approached with caution, a reservation voiced by several other authors (Turlings & Ton, 2006; Kaplan, 2012; Turlings & Erb, 2018).
In conclusion, our data demonstrate that lacewing immatures have the ability to learn the association between volatiles and the presence or absence of food and that they use volatiles when dispersing from a plant to find a new food patch. Further studies are needed to investigate the importance of this learning during the foraging of predatory arthropods under more natural conditions.
Acknowledgements
Financial support and scholarships were provided by the Federal Agency for Support and Evaluation of Graduate Education (CAPES), the National Council of Scientific and Technological Development (CNPq) and the Minas Gerais State Foundation for Research Aid (FAPEMIG). CMO and AMGB were supported by CAPES, AP and MV were supported by CNPq, CAPES, FAPEMIG and AJ received a scholarship from FAPEMIG (CBB-30003⁄09). The authors declare no conflicts of interest. We thank the two anonymous reviewers for constructive comments.




