Variability of photosensitive period and voltinism among populations of a butterfly, Ypthima multistriata, inhabiting similar latitudes and altitudes
Abstract
In Ypthima multistriata Butler (Lepidoptera: Nymphalidae), there are univoltine and bivoltine populations in adjacent areas with similar climatic conditions. A previous study revealed that larvae of both univoltine and bivoltine populations diapause under a constant short day (i.e., a constant short light period; L13:D11), but not under a constant long-day condition (L16:D8). However, in both types of populations, adults of an overwintering generation appear and oviposit in June and soon thereafter larvae hatch. Therefore, the younger larvae (at least the first instars) of both types of populations experience a long day; nevertheless, the larvae of univoltine populations diapause in nature. To resolve this inconsistency, we set up two hypotheses: (1) the photosensitive stage of larvae is the second instar or later, and (2) the photosensitive stage of univoltine populations is later than that of bivoltine populations. To test these hypotheses, we performed rearing experiments with two univoltine populations and two bivoltine ones. The results indicated that the photosensitive stage was the second or third instar and that the photosensitive stage was later in one univoltine population than in the two bivoltine populations. Larvae of the other univoltine population diapaused under all conditions. The former result supports our hypothesis, and the latter result indicates that the response to photoperiod is different among univoltine populations. In addition, larval development was slower in one univoltine population than in the bivoltine populations, which also delays the timing of the diapause decision in this univoltine population. Larvae that experienced a long day during the first and middle instars but experienced a short day at the end of their larval stage developed faster than larvae that experienced a constant long day. This may be an adaptation to enable emergence before the start of a cold season that is unsuitable for reproduction.
Introduction
Diapause is one of the major adaptations that have evolved to enable survival during a season that is inappropriate for breeding or to adjust life cycles to seasonal variations (Tauber et al., 1986; Denlinger, 2002). Recently, insect diapause has attracted much attention from the point of view of climate change (Gomi et al., 2007; Sgro et al., 2016; Baumgartner & Tarrant, 2017; Lehmann et al., 2017) and from a genetic perspective (Winterhalter & Mousseau, 2007; Bryon et al., 2017). In many temperate insects, diapause induction is controlled by photoperiod, which is reasonable because it is a stable signal of predictable seasonal change (Beck, 1980). Therefore, it is important to investigate the response to photoperiod in order to understand the phenology of temperate insects.
In general, the number of generations of most temperate insects is correlated with latitude, because lower temperature elongates the larval developmental time: The lower the latitude, the greater the number of generations that can emerge in a year (Tauber et al., 1986; Mousseau & Roff, 1989; Gomi & Takeda, 1990; Tanaka, 1994). In addition, temperate insects must recognize the arrival of winter and must diapause. Generally, insects at higher latitudes must diapause earlier, and therefore, their critical photoperiod (daylength at which the diapause incidence becomes 50%) is longer than that of insects at lower latitudes (Danilevskii, 1965; Masaki, 1999).
To explain insect photoperiodism, however, a critical photoperiod is not sufficient because the photoperiodic reaction is determined by both the critical photoperiod and the photosensitive developmental stage (the timing of deciding whether to enter diapause or not). However, there have been only few studies comparing photosensitive developmental stages between populations within a species (Ichinose & Negishi, 1979; Eizaguirre et al., 1994).
Ypthima multistriata Butler (Lepidoptera: Nymphalidae) is a satyrine butterfly distributed widely in temperate areas from China to western Japan. This species lives in open forests or forest edges and overwinters in larval diapause (Fukuda et al., 1984). Unlike most temperate insect species, the number of generations of the butterfly differs even among populations located at similar latitudes and altitudes. There are four types of voltinism in Japan: bivoltine with adult emergence in June and September, univoltine with adult emergence in June, univoltine with adult emergence in July, and multivoltine (Noriyuki et al., 2011). Bivoltine is the most common voltinism in Japan. In the Tsushima Island population, adults emerge intermittently from May to October, and therefore, this population is regarded as multivoltine. Some populations with different voltinism patterns are geographically adjacent; for example, in the Seto Inland Sea in Japan, the population of Ieshima Island is bivoltine, whereas that of Tangashima Island located ca. 1 km away from Ieshima Island is univoltine (Figure 1). Therefore, one cannot explain the variations of voltinism in Y. multistriata only by climatic adaptation such as adaptation to temperature. As such a voltinism pattern is rare in insects, this phenomenon in Y. multistriata provides an interesting opportunity to uncover its mechanism and thereby may lead to a deeper understanding of voltinism.
Based on rearing experiments, Noriyuki et al. (2011) found that (1) both univoltine and bivolutine populations of the butterfly entered diapause in response to constant short days (L13:D11), whereas they avoided diapause in response to constant long days (L16:D8), and (2) the critical photoperiod was longer in the univoltine than in the bivoltine populations. Certainly, a longer critical photoperiod increases the opportunity to diapause; however, it is difficult to completely explain the variation of voltinism in Y. multistriata by a critical photoperiod. In the wild, the overwintering generation reproduces in June in both bivoltine and univoltine populations, and therefore, all of these newly hatched larvae should be exposed to the longest days (ca. L16:D8) in a year. However, diapause is induced in the univoltine populations in nature. This is incompatible with Noriyuki et al.’s (2011) first finding. To resolve the inconsistency, we should consider the seasonal change of daylength. In the northern hemisphere, daylength reaches its maximum in June and then decreases; however, the rearing experiment to determine the critical photoperiod used constant daylength throughout the larval period.
We accordingly made two predictions. (1) As the photosensitive stage of Y. multistriata is the second instar or later, larvae in univoltine populations do not make the diapause decision under the longest days (L16:D8) in nature. (2) As the photosensitive stage is later in univoltine than in bivoltine populations, larvae of univoltine populations make the diapause decision under a shorter day than those of bivoltine populations. To test these predictions, we performed a rearing experiment to clarify the photosensitive stage and the developmental pathway using univoltine and bivoltine populations of the butterfly.
Materials and methods
Collection sites
For our experiments, we used two bivoltine populations (Yao and Ieshima) and two univoltine (June) populations (Tangashima and Kakogawa). These populations are located at similar latitudes and at altitudes of <50 m (Figure 1). We collected six females in Tangashima and three in Kakogawa in June 2016, and four females in Yao and one in Ieshima in September 2016. Ypthima multistriata is an endangered species listed as ‘vulnerable’ (the danger of extinction is increasing) in the Japan Red List (Ministry of the Environment of Japan, 2017), and therefore, we refrained from collecting many females.
Rearing procedure
Eggs were obtained from wild females and their descendants. The second-generation larvae were used only for the experiment to investigate the effect of temperature (see below), and all the other experiments were performed using the first-generation larvae. Each female was kept in a transparent plastic jar (240 ml) with a small gramineous plant (as an egg-laying bed). The eggs produced by each female were allocated equally to each experimental condition, and hatched larvae were reared individually in a Petri dish. The egg period was about 1 week in all populations. Although numerous gramineous and Cyperaceae species are available as food plants for Y. multistriata (Fukuda et al., 1984), we provided leaves of the grass Pleioblastus chino Makino var. viridis that grows naturally in the Osaka Prefecture University campus (135°50'E, 34°32'N) because it sprouts in spring and autumn, with similar timing to the adult emergence of Y. multistriata. We provided fresh leaves throughout the experiment, and the leaves were replaced at least every 2 days.
Experimental design
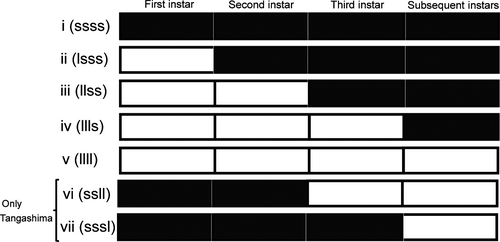
In our experiment, we used two incubators with different daylength: One was set on long day (L16:D8, averting diapause), and the other was set on short day (L13:D11, inducing diapause). L16:D8 and L13:D11 reflect the natural daylength at the collecting sites in June and September, respectively. To examine the larval photosensitive stage, we reared larvae in five test groups (groups i, ii, iii, iv, and v) in which daylength conditions were different (Figure 2). The larvae were transferred from long to short day after ecdysis at the very beginning of different instar stages. The reciprocal experiment, from short-day to long-day condition, was performed only for the univoltine Tangashima population because we could obtain a sufficient number of eggs (test groups vi and vii; Figure 2) only for this population. For the experiment, the temperature of incubators was set at 22.5 °C (dark period) and 25 °C (light period) based on the day/night temperature cycles in the field.
We examined the effect of temperature on diapause induction with the univoltine Tangashima and bivoltine Yao populations. Since we could not obtain many samples of the Kakogawa and Ieshima populations, we could not perform this experiment for them. The larvae were reared at 22.5 and 25 °C under L16:D8 for both Tangashima and Yao populations. The sample size was 31 (22.5 °C) and 30 individuals (25 °C) in Tangashima, and 24 (22.5 °C) and 21 individuals (25 °C) in Yao.
In all experiments, we recorded the number of diapausing larvae. The developmental duration of each instar was recorded. However, we did not record the developmental time of diapausing larvae after the fourth instar because they did not pupate during our study.
Data analysis
Diapausing larvae of Y. multistriata continue to feed and molt through several supernumerary instars; however, they do not pupate. Therefore, the larvae that had not pupated for more than 110 days were considered to have entered diapause. We considered 110 days to be a long enough period for judging whether larvae had entered diapause, considering the seasonal occurrence of hatching of the bivoltine populations in June and September. Diapause as in this species has been observed in other lepidopteran and coleopteran larvae (Koštál, 2006; Shintani, 2011).
We judge the photosensitive stage based on the incidence of diapause of each test group; however, there is no established criterion to numerically evaluate it. Therefore, using the same logic that was used for determining the critical photoperiod, we calculated the estimated instar stage at which more than 50% larvae would diapause if they were transferred to L13:D11 (critical photosensitive stage, CPS). For this calculation, it was assumed that the incidence of diapause changes linearly during the progression between the two instar stages and that there is a time point at which the diapause rate becomes 50%. As there were individual differences in the number of instars, we defined the total number of days after the fourth instar as the length of the fourth larval period (all larvae reached the fourth instar, and some reached fifth or sixth instar; Table 1).
| Voltinism | Test groups and sex | No. samples | Instar | Mean no. instars (min–max) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 and subsequent | Total | ||||
| Univoltine | Tangashima v (llll) ♀ | 12 | 8.64 ± 0.50 | 9.36 ± 2.58 | 11.55 ± 1.75 | 61.09 ± 7.74 | 90.64 ± 7.67 | 5.5 (5–6) |
| Tangashima v (llll) ♂ | 22 | 8.39 ± 1.16 | 8.65 ± 0.98 | 11.83 ± 1.27 | 53.17 ± 9.96 | 82.04 ± 9.56 | 5.36 (5–6) | |
| Tangashima iv (llls) ♀ | 16 | 8.5 ± 1.26 | 8.56 ± 0.89 | 12.19 ± 0.83 | 32.44 ± 7.01 | 61.69 ± 6.94 | 5 | |
| Tangashima iv (llls) ♂ | 14 | 8.5 ± 1.16 | 8.93 ± 1.00 | 12 ± 2.00 | 29.57 ± 4.67 | 59 ± 5.57 | 5 | |
| Bivoltine | Yao v (llll) ♀ | 12 | 6.5 ± 0.80 | 6.75 ± 0.97 | 11.08 ± 1.73 | 19.83 ± 6.26 | 44.17 ± 7.41 | 4 |
| Yao v (llll) ♂ | 12 | 5.75 ± 0.75 | 6.42 ± 0.90 | 9.92 ± 1.68 | 17 ± 4.88 | 39.08 ± 5.96 | 4 | |
| Yao iv (llls) ♀ | 15 | 5.4 ± 0.91 | 6.2 ± 0.68 | 10.67 ± 1.88 | 16.87 ± 3.02 | 39.13 ± 3.85 | 4.13 (4–5) | |
| Yao iv (llls) ♂ | 13 | 5.54 ± 1.13 | 6.15 ± 1.14 | 9.69 ± 1.32 | 14.15 ± 1.46 | 35.54 ± 2.22 | 4 | |
| Yao iii (llss) ♀ | 13 | 5.69 ± 1.18 | 6.38 ± 1.12 | 11 ± 0.91 | 14 ± 1.00 | 37.08 ± 1.50 | 4 | |
| Yao iii (llss) ♂ | 14 | 6.14 ± 0.77 | 5.64 ± 0.74 | 10 ± 1.30 | 13.36 ± 4.80 | 35.14 ± 5.40 | 4.07 (4–5) | |
| Ieshima v (llll) ♀ | 6 | 6.33 ± 0.52 | 7.83 ± 0.41 | 11.5 ± 2.17 | 30.33 ± 10.33 | 56 ± 9.96 | 4.33 (4–5) | |
| Ieshima v (llll) ♂ | 4 | 6.5 ± 0.58 | 7.75 ± 0.50 | 13 ± 2.16 | 27.25 ± 14.64 | 54.5 ± 13.96 | 4 | |
| Ieshima iv (llls) ♀ | 6 | 6.33 ± 1.21 | 7.67 ± 0.82 | 10.17 ± 2.93 | 22.33 ± 4.32 | 46.5 ± 2.07 | 4.5 (4–5) | |
| Ieshima iv (llls) ♂ | 5 | 7.2 ± 1.10 | 7.2 ± 1.64 | 10.6 ± 1.52 | 20.6 ± 3.91 | 45.6 ± 2.51 | 4 | |
Generalized linear mixed models (GLMMs) were constructed to analyze diapause incidence and developmental time. The diapause incidence was analyzed using GLMMs with logit link function. GLMMs were constructed for each test group; however, we could not construct GLMMs for test groups i and v because either almost all larvae diapaused (i) or did not diapause (v). The voltinism of the population was included as an independent variable. Each mother butterfly was included as a random factor. For this analysis, we used the glmmML function in the package glmmML (Brostrom, 2018) for R v.3.5.0 (R Core Team, 2018). Developmental time of non-diapausing larvae was analyzed using a linear mixed-effects model. Models were constructed for each instar. Voltinism of the population, test group, and sex were included as independent variables. Each mother butterfly was included as a random factor. For this analysis, we used the lme function in the package nlme (Pinheiro et al., 2018) for R v.3.5.0 (R Core Team, 2018). In the GLMMs, the Kakogawa and Ieshima populations were excluded because they were not suitable for analysis (almost all larvae entered diapause in all experimental groups in the Kakogawa population and we could collect only one mother butterfly in the Ieshima population). All statistical analyses were performed for the test groups in which larvae were transferred from long-day to short-day condition.
Results
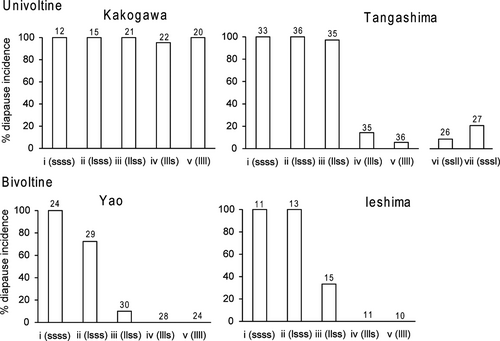
The photosensitive stage occurred in the second or third instar in the univoltine Tangashima and the bivoltine Yao and Ieshima populations; however, in the univoltine Kakogawa population, almost all larvae entered diapause in all experimental groups (Figure 3). The value of CPS was 3.6 in the Tangashima, 2.4 in the Yao, and 2.75 in the Ieshima population. A significant effect of voltinism was found in the GLMM for test group iii, but not for test groups ii and iv (Wald Z-statistical test: P<0.001; Table 2). In the univoltine Tangashima population, when larvae were transferred to a long photoperiod after they completed the second or third instar, most larvae entered direct development (Figure 3).
| Model | Coefficient | SE | Z | Pr (>|z|) |
|---|---|---|---|---|
| Test group ii | −11.61 | 89.82 | −0.13 | 0.90 |
| Test group iii | −5.72 | 1.18 | −4.84 | <0.0001 |
| Test group iv | −10.87 | 101.7 | −0.11 | 0.92 |
The GLMM analyses of developmental time indicated that the developmental time was longer in the univoltine Tangashima population than in the bivoltine Yao population in all instars (i.e., also in total developmental time) of both sexes (Tables 1 and 3). The Ieshima population displayed an intermediate value between the Tangashima and Yao populations. The difference between populations became larger after the fourth instars relative to the first three instars. The larvae in the univoltine Tangashima population reached the fifth or sixth instar, whereas the larvae in the bivoltine populations reached only the fourth or fifth instar (Table 1). In addition, a significant effect of test group was found for the fourth and subsequent instars (Table 3). In the Tangashima and Yao populations, the larval developmental time of the fourth and subsequent instars was longer in the constant long-day groups than in the short-day experienced groups in the third or subsequent instars (Table 1). The same trend was observed in the Ieshima population. However, it might not have been the experience of short daylength but rather a difference in diapause incidence which caused this result. That is, the incidence of diapause in the group that experienced short days in the third or subsequent instar was higher than that in the constant long daylength group (Figure 3), which means that the shorter larval developmental time of the former might have been caused by excluding larvae with longer developmental time as diapausing larvae. In order to rule out this possibility, we excluded the three individuals with the longest developmental time in test group v in the Tangashima population and the three individuals with the longest developmental time in test groups iv and v in the Yao population to equalize the fraction of larvae that was excluded from the analysis. The same GLMM analysis yielded an unchanged result (significant for the fourth and subsequent instars; P<0.001).
| Instar | Variable | Coefficient | SE | d.f. | T | P |
|---|---|---|---|---|---|---|
| 1 | Test group v | 0.3 | 0.19 | 129 | 1.55 | 0.12 |
| Test group iii | 0.37 | 0.26 | 129 | 1.43 | 0.15 | |
| Sex | −0.12 | 0.17 | 129 | −0.7 | 0.48 | |
| Voltinism | −2.71 | 0.19 | 8 | −14.1 | <0.001 | |
| 2 | Test group v | 0.3 | 0.22 | 129 | 1.4 | 0.16 |
| Test group iii | −0.21 | 0.3 | 129 | −0.71 | 0.48 | |
| Sex | −0.3 | 0.2 | 129 | −1.5 | 0.14 | |
| Voltinism | −2.45 | 0.22 | 8 | −11.32 | <0.001 | |
| 3 | Test group v | 0.0038 | 0.27 | 129 | 0.014 | 0.99 |
| Test group iii | 0.14 | 0.37 | 129 | 0.38 | 0.7 | |
| Sex | −0.67 | 0.25 | 129 | −2.65 | <0.01 | |
| Voltinism | −1.62 | 0.42 | 8 | −3.88 | <0.01 | |
| 4 and subsequent | Test group v | 15.12 | 1.46 | 129 | 10.37 | <0.001 |
| Test group iii | 3.8 | 1.99 | 129 | 1.91 | 0.058 | |
| Sex | −2.44 | 1.35 | 129 | −1.8 | 0.074 | |
| Voltinism | −26.57 | 2.03 | 8 | −13.07 | <0.001 |
Under constant L16:D8 at 22.5 and 25 °C, all individuals avoided diapause in both the Yao and Tangashima populations.
Discussion
Variation of photoperiodic responses in populations with different voltinism
Our predictions were that the photosensitive stage of Y. multistriata is the second instar or later, and it should be later in univoltine populations than in bivoltine populations. The results of our experiment supported the hypotheses, as (1) most larvae of both Yao and Tangashima populations entered diapause in test group ii, (2) diapause incidence of the Yao population was lower than that of the Tangashima population in test group iii, and (3) most larvae of both populations did not diapause in test group iv. These results indicated that the CPS was the second or third instar, and it was later in the univoltine Tangashima population than in the bivoltine Yao population. The bivoltine Ieshima population displayed the same trend as the bivoltine population of Yao: The CPS value was smaller than that of the Tangashima population. In addition, we found that larvae of the univoltine Kakogawa population diapaused in all conditions, including a constant long-day condition.
Under L16:D8 at constant 22.5 and 25 °C, all individuals avoided diapause in the Tangashima and Yao populations. Therefore, it is likely that a difference in the photocycle rather than the temperature cycle affected the incidence of diapause in our experiments.
In the converse experiment (test groups vi and vii), most larvae of the Tangashima population developed directly, indicating that the larvae continued to have sensitivity to the long-day condition after the CPS. Therefore, we probably should interpret the CPS value as a developmental point at which larvae are no longer sensitive to a diapause-inducing signal. Friberg et al. (2011) used the concept ‘point of no return’; that is, the diapause decision point after which larvae cannot switch developmental pathways regardless of external factors. It may be a more suitable concept than photosensitive stage also for Y. multistriata. Like the three species studied by Friberg et al. (2011), Y. multistriata (of the Tangashima population) can switch their developmental pathway more flexibly from diapause to direct development than in the opposite direction. Hence, the point of no return after which larvae cannot change their developmental pathway from diapause to direct development is later than that of the opposite direction.
We found an inconsistency between our results and those of Noriyuki et al. (2011). Noriyuki et al. (2011) stated that diapausing larvae stop feeding on plants, whereas in our study, all larvae continued to feed but some did not pupate. We regarded such larvae as diapausing. The photoperiod and temperature conditions in our experiments were almost the same as those in the experiments of Noriyuki et al. (2011). We cannot explain this inconsistency.
Variation of photoperiodic responses in populations with the same voltinism
We found that the photoperiodic reactions were different not only between populations with different voltinism but also between populations with the same voltinism. Larvae of the univoltine Tangashima population exhibited sensitivity to photoperiod, whereas almost all larvae of the univoltine Kakogawa population exhibited insensitivity to long photoperiod and entered diapause. Larvae of the Kakogawa population may have a longer critical photoperiod than L16:D08 or they lack photoperiodism, as is also found in univoltine populations in other insects (Pruisscher et al., 2017; Schebeck et al., 2017). Wickman et al. (1990) showed that some larvae of the partially bivoltine satyrine butterfly Coenonympha pamphilus L. from the same population entered diapause regardless of the photoperiod.
Under all conditions, the value of CPS and developmental time were slightly different between the Yao and Ieshima populations. Larvae in the Ieshima population showed an intermediate value of CPS between those of the Tangashima and Yao populations. These results suggest that even within populations displaying the same voltinism, the mechanisms of diapause are different. This may indicate that voltinism in Y. multistriata evolved independently in each population. Noriyuki et al. (2010) pointed out the same possibility based on phylogenetic analysis using mitochondrial DNA. It is known that photoperiodic reactions change rapidly in populations of various invasive insects (Gomi & Takeda, 1990; Gomi, 1997; Sadakiyo & Ishihara, 2011; Reznik et al., 2015). In contrast, Friberg et al. (2011) revealed that three distantly related butterflies all make diapause decisions with similar timing.
Variation of developmental time in populations with different voltinism
The developmental time was longer in the univoltine Tangashima population than in the bivoltine Yao population in all instars. The bivoltine Ieshima population showed the same trend as the bivoltine population of Yao: The developmental time was shorter than that of the Tangashima population. The largest difference of developmental time was seen after the fourth instar (i.e., after the CPS) because of the difference in the number of instars.
The GLMM analysis indicated that in the groups of the Yao and Tangashima populations that experienced short days in the third or subsequent instars (iii, iv), the developmental time (of fourth and subsequent instars) was shortened relative to that in the continually long-day group (v), although the cumulative temperature was higher in the continually long-day group than in the groups experiencing short days (v vs. iii and iv). The same trend was also observed in the Ieshima population. Therefore, the shortening of the developmental time appears an effect of experiencing a short photoperiod at the end of the larval period. Similar results have been reported in other lepidopteran insects (Eizaguirre et al., 1994; Gotthard et al., 1999; Goehring & Oberhauser, 2002). For larvae that do not enter diapause, shortening of the developmental time by a short photoperiod may be an adaptation to emerge before the cold season, which is unsuitable for reproduction.
Factors that determine variation in voltinism
The photosensitive stage is later in univoltine than in bivoltine populations. Moreover, larvae of univoltine populations developed more slowly than those of bivoltine populations in each larval stage. This can be interpreted as ‘state-dependent development’ seen in other insects (Gotthard et al., 1999; Friberg et al., 2012). These findings indicate that larvae of univoltine populations determine whether to enter diapause or not at a later season (under shorter daylength) than those of bivoltine populations. Considering this together with the finding that the critical photoperiod is longer in univoltine populations (Noriyuki et al., 2011), we concluded that the differences of critical photoperiod, photosensitive stage, and/or developmental rate lead to variations of voltinism in Y. multistriata.
Ichinose & Negishi (1979) reported intraspecific variation of photosensitive stages in a Japanese butterfly, Papilio protenor Cramer: the photosensitive stage of P. p. demetrius (the temperate population) was the fourth or fifth instar, whereas that of P. p. liukiuensis (a subtropical population with more generations per year than the temperate ones) was the second or third instar. This shows the same trend as our experimental results, although we found intraspecific variation of photosensitive stage between adjacent populations located at similar latitudes and altitudes in Y. multistriata.
It is possible that something other than photoperiod induces larval diapause. In our experiment, we collected butterflies from the two univoltine populations in June and from the two bivoltine populations in September. Therefore, the mother butterflies will have experienced different temperature and daylength, indicating that there may be differences in the maternal effect on diapause response (Danks, 1987). In addition, the effect of food plant quality on diapause induction is well known in other insects (Hare, 1983; Hunter & McNeil, 1997; Ishihara & Ohgushi, 2006; Takagi & Miyashita, 2008; Friberg & Wiklund, 2010). In our experiment, we collected food plants in the field during the experiment. Therefore, it is possible that the plant quality differed between the experimental univoltine and bivoltine populations. In the study of Noriyuki et al. (2011), all mother butterflies were collected in June and reared on the same food plant; nevertheless, the photoperiodic reaction differed between univoltine and bivoltine populations. This indicates that a maternal effect or food plant quality not necessarily plays an important role in diapause induction. Moreover, if maternal effects or food plant quality are key factors for diapause incidence of Y. multistriata, the larvae of our experimental bivoltine populations, whose mothers were collected in fall and who were fed leaves collected in fall, would have been likely to enter diapause. However, our results showed the opposite pattern. Therefore, these confounding factors appear not to have had strong effects on the results of our study.
Adaptive significance of variation of voltinism
We clarified a proximate factor that causes variation in the voltinism of a butterfly, but an ultimate factor is still unknown. In the present study, the habitat type was somewhat different between populations with different voltinism patterns. The habitats of univoltine populations (June) were acid wetland, whereas those of most of the bivoltine populations were secondary environments where succession was prevented by human activity (Y Hasegawa, pers. obs.). Therefore, by investigating the differences of the habitat in detail, the adaptive significance of the puzzling voltinism distribution of the butterfly may be understood.
Acknowledgements
We are grateful to Y. Tateiwa for information on the habitat of Y. multistriata. This study was supported by JSPS KAKENHI Grant Number JP15K06934.




