Limb ablation and regeneration in Harmonia axyridis: costs for regenerators, but benefits for their progeny
Abstract
Previous work revealed that Harmonia axyridisPallas (Coleoptera: Coccinellidae) in Beijing, China, were capable of regenerating a forelimb amputated in the fourth instar; 75% of surviving individuals fully regenerated the limb during pupation. In this study, we tested a population of H. axyridis invasive in North America and found that virtually 100% of beetles surviving the operation successfully regenerated the limb. Ablated/regenerated beetles spent longer in pupation, and emerging females were smaller than controls. However, reproductive success was unaffected in all pairwise crosses of control/regenerated adults; there were no differences in pre-oviposition period, the time required to produce 10 clutches, 10-day fecundity, or the fertility of eggs, whereas ablated/regenerated parents paid a developmental cost, their progeny obtained benefits. Offspring of crosses that included a regenerated parent tended to have faster larval development than the control cross, although not all were significantly different from controls. However, when either or both parents were ablated and regenerated, their daughters were heavier than controls at emergence. Limb regeneration during pupation appears to activate a physiological cascade which increases the magnitude of beneficial parental effects normally conferred to progeny, possibly via pleiotropic effects. The invasive North American H. axyridis population appears to have higher regeneration capacity than the Chinese population tested previously, although how regeneration capacity might be associated with invasiveness remains unclear. Limb regeneration ability may be a side effect of selection on other traits that confer high fitness under either natural or sexual selection, as it seems unlikely to confer fitness benefits directly in this species.
Introduction
The ability of insects to regenerate a limb lost during an immature stage has been documented in a wide range of hexapod orders. These include Blattodea (Roberts, 1973; Tan et al., 2013), Coleoptera (Mitten et al., 2012; Lee et al., 2013), Hemiptera (Luscher, 1948; Knobloch & Steel, 1988), Lepidoptera (Yang et al., 2016), Mantodea (Karruppanan, 1998), Odonata (Parvin & Cook, 1968), Orthoptera (Maleville & Reggi, 1981; Luedke & Lakes-Harlan, 2008; Yang et al., 2016), and Phasmatodea (Chen et al., 1999; Maginnis, 2006a). In holometabolous insects, the pupal stage provides an opportunity for regeneration of a leg lost during larval development, as the entire body undergoes metamorphosis at this time. Apart from the obvious benefits of regeneration, the process involves physiological costs in addition to those associated with structure loss. The energetic burden of regeneration is termed the ‘regenerative load’ (Juanes & Smith, 1995) and it can have negative impacts on growth, reproduction, and behavior (Maginnis, 2006b). An improved understanding of the adaptive value of limb regeneration in specific ecological contexts could provide key insights into its evolution and maintenance.
Wigglesworth (1972) demonstrated that limb regeneration capacity is an exclusive property of the insect epidermis. In general terms, the base of each appendage is surrounded by a ‘regenerative field’ where epidermal cells are able to dedifferentiate following appendage removal, proliferate into a blastema, and then regenerate the appendage (Heming, 2003). The molecular genetic basis of insect limb development has been most studied in Drosophila melanogaster Meigen (e.g., Grubbs et al., 2013; Cordoba et al., 2016), beginning with the seminal work of Nüsslein-Volhard & Wieschaus (1980). The little work that does exist on appendage development in Coleoptera suggests some contrasts with the dipteran system (Angelini et al., 2009).
Limb regeneration ability could be subject to selection either directly, if the ability itself conferred a selective advantage, or indirectly, if the ability was linked to other traits that elevate fitness. Empirical evidence for direct natural selection of larval insect leg regeneration can be difficult to obtain, because it requires estimating the frequency of limb loss in natural populations of immature insects (e.g., Maginnis, 2008). However, physiological cascades associated with limb regeneration may have important consequences for development or life history that can be revealed by careful laboratory observations. For example, tail regeneration in the lizard Liolaemus nitidus (Wiegmann) is linked to an increase in metabolic rate (Naya & Bozinovic, 2006). Epigenetic factors appear to be involved in regulating the leg regeneration process in Gryllus bimaculatus DeGeer (Hamada et al., 2015). Wang et al. (2015) demonstrated that regenerating males of Harmonia axyridis Pallas (Coleoptera: Coccinellidae) were preferred as mates by females over non-ablated controls, even though they are visually indistinguishable to the observer, suggesting some linkage between regeneration and other traits that might have a chemical basis and thus be distinguishable by females. We infer that limb regeneration can evolve in response to either natural or sexual selection, and that the ability may be an indirect target of selection if it represents a pleiotropic side effect of other traits that are strongly correlated with fitness.
The recent discovery that many species of Coccinellidae possess varying levels of limb regeneration ability during pupation (Wang et al., 2015; Wu et al., 2015; Saxena et al., 2016) has raised various questions about the biological and ecological significance of limb regeneration within the family. If limb regeneration evolves in response to natural selection, we might expect its adaptive value to vary among ecological contexts. This could be tested by comparing the regenerative abilities of species with herbivorous or fungivorous habits to those that are predaceous, on the hypothesis that the latter will be a greater risk of limb loss due to development in more competitive environments. However, insights into the physiological consequences of regeneration for life history and fitness can be revealed by simple comparisons of regenerating beetles vs. unoperated controls, or vs. non-regenerating beetles when these are obtained. The latter can be especially informative if the decision to regenerate or not represents a significant bifurcation in developmental pathways, in which case it should result in distinct life-history consequences (Maginnis et al., 2015). Alternatively, if regeneration is the norm, a failure to regenerate may simply reflect a physiological constraint such as poor condition.
Working with Coccinella septempunctata L., Wu et al. (2015) found that complete regeneration was achieved by over 80% of individuals when complete ablation of a leg occurred in the second instar, with a declining proportion of individuals regenerating the leg completely when ablation occurred in subsequent instars; only about 38% of individuals regenerated legs ablated in the fourth instar, but it was not clear if these data included partially as well as fully regenerated individuals. Saxena et al. (2016) examined Menochilus sexmaculatus (Cheilomenes sexmaculata) (Fabricius) and found that only 32% of individuals fully regenerated limbs when they were severed proximal to the coxa in the fourth instar, and that the proportion regenerating was lower when ablation was conducted in the third instar. In contrast, Wang et al. (2015), working with a Chinese population of H. axyridis, observed 83% survival of larvae subjected to foreleg amputation in the fourth instar, with 75% of individuals regenerating the leg. These contrasting results regarding the effect of life stage on regenerative capacity indicate significant species-specific variation, not only in regenerative capacity, but also in associated life-history consequences.
A preliminary experiment with our locally invasive population of H. axyridis indicated that almost 100% of fourth instars regenerated forelegs that were amputated proximal to the coxa. This result denied us the opportunity to contrast regenerating with non-regenerating individuals in subsequent experiments, as the only contrasts possible were with non-ablated controls. We hypothesized that processes associated with the recovery from ablation and limb regeneration would require the up-regulation of many developmental genes in the pupal stage, with the potential for a cascade of secondary effects on subsequent physiology and life history. As parental effects in coccinellids are quite sensitive to parental condition (e.g., Vargas et al., 2012a,2012b, 2013, 2014), we also hypothesized that physiological and pleiotropic effects associated with regeneration would be transgenerational and that regenerative costs would be evident in the life histories of progeny.
Materials and methods
Insect colony
A colony of H. axyridis was established from adult beetles collected from a maize field at the Agricultural Research Center, Hays (KS, USA), in June 2016. The adults were held in 1-l glass mason jars, ca. 160–180 beetles per jar, filled with strips of wax paper to serve as harborage and provisioned daily with eggs of the Mediterranean flour moth, Ephestia kuehniella Zeller (Lepidoptera: Pyralidae), with water provided on a cotton wick. Jars were kept in a growth chamber set to 18 ± 1 °C, 50–60% r.h., and L16:D8 photoperiod. Previous work has shown these conditions to be ideal for maintaining beetles in reproductive diapause for extended periods. All life stages of experimental insects were held in a climate-controlled growth chamber set to 23 ± 1 °C, 50–60% r.h., and L16:D8 photoperiod.
Limb amputation
In a preliminary experiment, we amputated 100 H. axyridis fourth instars to estimate the percentage that would regenerate. For operation, we chilled larvae on a cold plate for several minutes before using fine scissors to amputate the femur of the right foreleg proximal to the coxa. Surprisingly, leg regeneration during pupation was complete in all operated insects. This informed us that contrasts between regenerated vs. unregenerated insects would not be possible, but rather only contrasts between amputated vs. unamputated controls.
In order to obtain insects for the main experiment, a series of 30 mated females from the stock colony were isolated in plastic Petri dishes (5.5 cm diameter), and provisioned with frozen eggs of E. kuehniella plus water on a small cube of sponge, both refreshed daily. Eggs were collected daily by transferring beetles to clean dishes. Following eclosion of eggs, 240 neonates were isolated in Petri dishes (as above) and labeled according to maternal lineage to prevent the subsequent pairing of siblings. Larvae were reared out on eggs of E. kuehniella and water (as above). A total of 208 larvae of the 240 molted successfully to the fourth instar and were divided into two equal groups of 104, ensuring that maternal lineages were equally represented in both groups. Larvae of the treatment group were amputated, as described above, within 24 h of molting to the fourth instar, whereas the other group was left unoperated as controls. Upon emergence, adults were isolated in Petri dishes (as above) and provisioned with water and E. kuehniella eggs ad libitum, both refreshed daily.
Reciprocal crosses
Once adult beetles were 7–8 days old, all available insects were paired with non-siblings in one of four reciprocal crossing treatments representing all possible male–female combinations of regenerated and control beetles. Mating treatments (n = 22 pairs per treatment) were established as follows: T1, ♀REGEN × ♂REGEN; T2, ♀REGEN × ♂CONTROL; T3, ♀CONTROL × ♂REGEN; and T4, ♀CONTROL × ♂CONTROL. Following termination of copula, females were again isolated in Petri dishes (as above) and provided with E. kuehniella eggs and water, refreshed daily. Males were isolated in separate Petri dishes and used to remate the same females every other day. All eggs were collected daily and held until eclosion so that incubation period and egg fertility could be recorded. Oviposition by females was quite erratic, so females were held until they laid 10 clutches, or failed to do so in 60 days. Data from couples that did not produce 10 clutches in a 60-day period were discarded. We also reared a series of 10 larvae from the second clutch of each female, isolating neonates in plastic Petri dishes (5.5 cm diameter) and rearing them as previously described. Larvae were examined twice daily and all developmental transitions were recorded until emergence of adults. Within 24 h of emergence, adults were sexed and their fresh weight was obtained by weighing them on an analytical balance.
Data analysis
Developmental and reproductive data that passed Levine's test for equality of variances and Shapiro–Wilk test of normality were analyzed by one-way ANOVA followed by the Bonferroni test (α<0.05) when more than two groups were compared. We used a test of proportions weighted by sample size (STATISTICA v.5.5; StatSoft, Tulsa, OK, USA) to determine whether differences in count data (larval mortality, sex ratio) were significant.
Results
Regeneration
Of the 104 larvae ablated, nine died in the pupal stage (8.7%), significantly more than the two (of 104, 1.9%) in the control treatment (test of proportions: P = 0.030). Two males in the amputation treatment did not regenerate the limb completely and their data were excluded from analysis, but the remaining 93 beetles regenerated a limb that was normal in appearance. Interestingly, one female individual appeared to overcompensate for the treatment and regenerated a slightly longer than normal limb. The sex ratio was 42.2% female in the control treatment, not significantly different from 44.2% in the ablation treatment (test of proportions: P = 0.78).
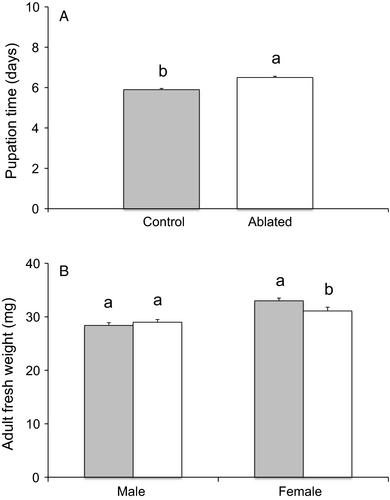
Larvae surviving amputation spent longer in pupation than control larvae (F1,193 = 48.17, P<0.001; Figure 1A). The fresh weight of regenerating males at emergence was not different from that of control males (F1,108 = 0.73, P = 0.40), but control females weighed more than regenerating females (F1,83 = 4.97, P = 0.028; Figure 1B).
Reproduction of reciprocal crosses
Two couples in T3 and four couples in T4 failed to produce 10 clutches within 60 days and their data were excluded from analysis. There were in total seven, four, nine, and four infertile couples (either no eggs were laid, or none of them hatched) in treatments T1-T4, respectively, and their data were also excluded from analysis. This reduced the total number of couples in the experiment from 88 to 54. There were no effects of treatment on female pre-reproductive period (F3,50 = 1.75, P = 0.17), the time females required to produce 10 clutches (F3,50 = 2.18, P = 0.10), 10-clutch fecundity (F3,50 = 0.64, P = 0.58), or egg fertility (F3,50 = 1.47, P = 0.23; Table 1).
| Dependent variable | Cross | |||
|---|---|---|---|---|
| ♀REGEN × ♂REGEN | ♀REGEN × ♂CONTROL | ♀CONTROL × ♂REGEN | ♀CONTROL × ♂CONTROL | |
| Pre-oviposition period (days) | 24.4 ± 3.7 | 19.9 ± 3.6 | 20.4 ± 4.4 | 32.6 ± 5.8 |
| Reproductive period (days) | 34.7 ± 5.8 | 21.5 ± 2.2 | 32.9 ± 6.2 | 24.1 ± 3.5 |
| Fecundity (no. eggs) | 257 ± 20 | 249 ± 18 | 285 ± 33 | 245 ± 12 |
| Egg fertility (% hatching) | 43.6 ± 4.1 | 46.7 ± 2.7 | 54.3 ± 7.7 | 48.6 ± 3.9 |
- No differences were significant among crosses for any variable (Bonferroni test: P>0.05).
Development of progeny
Among the progeny of the four reciprocal crosses derived from mating regenerated and control beetles, a total of five larvae underwent a supernumerary fifth instar (two females in T2, one male and one female in T3, and one male in T4) that required 3 days to complete in one case, and 4 days in the others. These periods were included in the total larval development time for these individuals. Larvae in the control treatment suffered 19.1% mortality, compared to 4.4–5.6% in other treatments, but the differences among treatments were not significant (F3,40 = 2.04, P = 0.12).
Treatment did not affect the time required for eggs to hatch (F3,50 = 2.81, P = 0.10), but did decrease total larval development time (F3,392 = 6.14, P<0.001; Table 2). Differences among treatments were also significant for duration of the first instar (F3,392 = 6.74), second instar (F3,392 = 6.26), third instar (F3,392 = 6.56, all P<0.001), fourth instar (F3,392 = 5.61, P = 0.001), and pupation time (F3,392 = 2.84, P = 0.038; Figure 2). In most cases treatment led to a decrease in developmental time compared to the control. Male fresh weight at emergence was unaffected by treatment (F3,191 = 0.50, P = 0.68) but female weight was enlarged (F3,197 = 4.82, P = 0.003).
| Dependent variable | Cross | |||
|---|---|---|---|---|
| ♀REGEN × ♂REGEN | ♀REGEN × ♂CONTROL | ♀CONTROL × ♂REGEN | ♀CONTROL × ♂CONTROL | |
| Egg incubation period (days) | 3.48 ± 0.06 | 3.47 ± 0.04 | 3.53 ± 0.05 | 3.45 ± 0.04 |
| Total larval period (days) | 11.18 ± 0.08ab | 11.22 ± 0.12ab | 10.88 ± 0.08b | 11.58 ± 0.14a |
| Pupation period (days) | 6.16 ± 0.05 | 6.32 ± 0.06 | 6.26 ± 0.05 | 6.13 ± 0.06 |
| Male fresh weight (mg) | 29.36 ± 0.44 | 29.01 ± 0.42 | 29.15 ± 0.41 | 28.58 ± 0.52 |
| Female fresh weight (mg) | 33.28 ± 0.60a | 33.28 ± 0.54a | 33.66 ± 0.47a | 31.09 ± 0.48b |
- Means within a row followed by different letters were significantly different among crosses (Bonferroni test: P<0.05).

Discussion
The body mass of females was reduced following ablation/regeneration of a foreleg in the fourth instar, but this effect was not evident in males. This result is consistent with coccinellid females having higher resource requirements than males due to the demands of egg maturation, which renders them more sensitive to resource limitation. Interestingly, female body size was increased relative to controls by parental effects from an ablated/regenerated parent, regardless of which parent was treated. So a significant cost of ablation/regeneration borne by mothers resulted in a benefit for their daughters.
Another immediate cost of ablation/regeneration was an extension of pupation time by a little more than half a day, but remarkably, there was no apparent impact of the procedure on any measure of reproductive success. Although there are usually significant physiological trade-offs associated with limb regeneration (Maginnis, 2006b), this is not always the case. For example, Maginnis & Redmond (2009) failed to find any somatic cost of limb regeneration in either males or females of the twostriped walkingstick, Anisomorpha buprestoides (Stoll), although leg regeneration in the phasmid Sipyloidea sipylus (Westwood) resulted in disproportionately smaller wings (Maginnis, 2006a).
Wang et al. (2015) performed similar amputations on fourth instar H. axyridis from Beijing, China, and reported that regenerating beetles extended their pupation time by 4–5 h, relative to controls, whereas our beetles were delayed by about 12 h. The slightly lower temperature employed in our present experiment may account for some of this difference. Furthermore, Wang et al. (2015) observed that pupae of regenerating beetles were heavier than control pupae; we did not weigh pupae, but regenerating females weighed less at emergence than did controls. Progeny of the Chinese population that were sired by amputated males, whether they regenerated or not, had slower development in larval stages, and in total, compared to those sired by control males. In the present study, progeny of control females sired by regenerated males of the Kansas population (T3) developed faster than controls in the first and fourth instars, and in total larval development. Thus, a negative paternal effect in the indigenous population emerged as a positive paternal effect in the invasive population, contrary to our initial hypothesis. Collectively, these observations suggest some divergence between the indigenous and native H. axyridis populations with respect to the developmental responses to limb loss and the process of regeneration.
The daughters of ablated/regenerated parents all emerged at heavier weights than those of control parents in our experiment, and it did not matter which parent regenerated. Thus, another transgenerational impact of ablation/regeneration was positive, again contradicting our initial hypothesis. Coccinellids are known to exert maternal and paternal effects on their progeny, probably to raise their own fitness (Vargas et al., 2012a,2012b, 2014; Mirhosseini et al., 2014). If limb regeneration in H. axyridis cues an epigenetic cascade that involves the activation of many genes, some could be responsible for pleiotropic enhancement of parental effects. This is notable because parental stress or trauma normally translates into negative, rather than positive, effects for developing offspring, as was observed in the Chinese H. axyridis population (Wang et al., 2015). For example, restriction of larval diet diminishes the maternal effects normally conferred by female Hippodamia convergens Guerin-Meneville to their progeny (Vargas et al., 2012a). However, there is evidence that insects challenged by bacterial infection can use epigenetic mechanisms to improve the immunological resistance of their progeny, for example in tobacco hornworm (Rosengaus et al., 2017), although immunological challenges resulting in negative maternal effects have been also observed in mosquitoes (Vantaux et al., 2014).
Limb regeneration itself may not be a direct target of selection in H. axyridis, and the ability per se may not confer any direct fitness benefit in this species. Because larval development requires only 10–12 days, and larvae missing legs are not normally observed in the field (JP Michaud, pers. obs.), limb loss followed by survival to pupation is probably a rare event in nature. In contrast, limb loss in aquatic Crustacea can be common for various reasons, thus providing a selective advantage for limb regeneration (Maginnis et al., 2014). Similarly, in stick insects, leg loss during molting is fairly common, as is autotomy to escape predation (Maginnis, 2008), again providing selective advantages to regenerators. However, the campodeiform larvae of coccinellids possess legs that are far more robust than those of phasmids, and more strongly attached to thoracic segments; any molting complications almost invariably result in death. We speculate that the ability to regenerate limbs in H. axyridis is a side effect of other physiological traits expressed by high-fitness genotypes, possibly mediated by pleiotropic mechanisms, and that these traits are the true targets of selection. Interestingly, invasive populations of H. axyridis also have been shown to differ in larval egg cannibalism behavior relative to native populations, and those mass-reared commercially for biological control purposes (Tayeh et al., 2014), the former being the most cannibalistic. Similarly, the greater regenerative abilities observed in our beetles compared to the indigenous Chinese population (Wang et al., 2015) may reflect an association of both cannibalism and limb regeneration with generally higher fitness, and thus with successful invasion of novel environments, whether the traits themselves are direct targets of selection. Invasive H. axyridis populations appear to provide a good model system in which to explore the epigenetic mechanisms of limb regeneration in holometabolous insects, and their life-history consequences.
Acknowledgments
We are grateful to the Egyptian Cultural Affairs and Missions Sector, Ministry of Higher Education in Egypt, Mansoura University, and the Egyptian Cultural and Educational Bureau in Washington, DC, USA, for financial support for AHA. We thank Clint Bain for technical support in conducting the experiments. This is contribution no. 17-385-J of the Kansas Agricultural Experiment Station.




