The photokinesis of oriental fruit flies, Bactrocera dorsalis, to LED lights of various wavelengths
Abstract
The oriental fruit fly, Bactrocera dorsalis Hendel (Diptera: Tephritidae), is an invasive pest of orchards around the world, particularly in Asian countries such as China. Light traps offer a potential means for pest monitoring and management. This study aimed to evaluate the sensitivity of the fly to light and investigate the impact of monochromatic light in the sensitivity spectrum on B. dorsalis. Six light wavelengths in LEDs – green (522 nm), yellow (596 nm), blue (450 nm), red (633 nm), purple (440 nm), and white (compound light) – were adapted to test responses of 5-, 10-, and 20-day-old B. dorsalis adults kept in laboratory conditions. We also tested the effects of green and red lights on pupal development and adults’ life activities. The results indicated a phototaxis preference rank in B. dorsalis adults to monochromatic LEDs with, in decreasing order, green, yellow, purple, blue, and red. Moreover, positive phototaxis significantly increased with age. Male adults are more sensitive than female adults to test lights, mainly at the age of 10 and 20 days. Emergence rates of pupae exposed to 12 and 24 h green light daily were 42 and 67%, respectively, whereas controls held in red light emerged at 33 and 37%, respectively. Furthermore, body weight, female fecundity, and mortality of B. dorsalis in night-time exposure of green light (from 21:00 to 09:00 hours; during daytime flies were illuminated by white LED light) were significantly higher than in red-light test groups and dark controls. In conclusion, B. dorsalis displayed preference toward green light, and fly age and gender seemed to significantly impact the phototactic behavior. Green LED light exposure during nighttime remarkably improved the emergence rates of B. dorsalis, and it enhanced growth, development, and ovipositing peak period, but decreased adult lifespan. This research lays a foundation for the development of new trap models, e.g., with green sticky cards or green light, for monitoring and control of B. dorsalis in the field.
Introduction
Sight plays an essential role in insect behavior – compound eyes process light stimuli from the environment, leading to food sources, reproductive partners, oviposition sites, hosts, prey, or detecting predators from a distance (Homberg & Paech, 2002; Stalleicken et al., 2006). When the photoreceptor cells of compound eyes receive motivational light stimuli, insects display positive or negative movement. Trapping technology based on chromatic cues is an important strategy in controlling Tephritidae (Diptera). Females of the olive fruit fly, Bactrocera oleae Gmelin, were more attracted to yellow or orange spheres than to red, blue, green, black, or white spheres (Katsoyannos, 1989). Yellow fruit-mimicking spheres of 7 cm in diameter were the most attractive to Mediterranean fruit fly, Ceratitis capitata Wiedemann (Katsoyannos, 1989; Cornelius et al., 1999). A previous study has shown that a green stimulus is the most suitable cue for trapping melon fruit fly, Bactrocera cucurbitae Coquillett (Xue & Wu, 2013). Li et al. (2017) found that yellowish green and yellow were the optimum colors for trapping Bactrocera tau Walker on sticky panels in the field.
The oriental fruit fly, Bactrocera dorsalis Hendel, is a devastating agricultural pest with health implications in the tropical and subtropical countries. Bactrocera dorsalis is highly polyphagous with multiple overlapping generations, which has been reported to damage more than 250 species of fruit and vegetable crops (Li et al., 2012; Shen et al., 2012). Females of B. dorsalis will lay eggs inside fruits, wherein larvae will feed until they come out and drop to hide in the soil for pupation. Therefore, the application of chemical control of the hidden immature stages is difficult and insecticides proved unsuccessful. Moreover, B. dorsalis field populations developed resistance against organophosphates, synthetic pyrethroids, and abamectin insecticides (Hsu & Feng, 2000; Jin et al., 2011; Vontas et al., 2011). The para-pheromone methyl eugenol (ME) is widely and effectively used to detect, control, and eradicate male adults of B. dorsalis worldwide (Vargas & Prokopy, 2006; Lin et al., 2012; Pagadala et al., 2012). However, ME was considered carcinogenic to humans by the National Toxicology Program of the United States Department of Health and Human Services, hampering its long-term use (Miller et al., 1983; Shelly, 1997; Smith et al., 2002; Zheng et al., 2012). In addition, ME can only trap sexually mature male flies, but will not affect immature forms or females. Thus, it is urgent to explore a more ecologically friendly, simple, and effective method for controlling B. dorsalis.
Bactrocera dorsalis adults’ diurnal host-finding behavior is strongly affected by visual cues. Therefore, the best strategy to attract and lure oriental fruit flies is by using traps based on visual stimuli (Prokopy & Owens, 1983; Wu et al., 2007). By hanging fruit-mimicking spheres of different colors in guava (Psidium guajava L.) trees, Vargas et al. (1991) demonstrated that yellow or white spheres would be useful devices for monitoring the oriental fruit fly. In subsequent field tests, Cornelius et al. (1999) found that yellow spheres captured more female oriental fruit flies than yellow rectangular blocks, or than spheres of other colors. Huang & Lin (2004) found that female flies of B. dorsalis prefer to oviposit into mature yellow fruit in the field. Unfortunately, these colors were not quantified. Wu et al. (2007) found that green light stimuli (500–570 nm) enhanced the attractiveness of B. dorsalis by use of green paper, whereas blue light stimuli (380–500 nm) diminished the attractiveness by using electroretinogram technology. It should be noted that there was considerable color difference between printed paper sheets. The use of monochromatic light sources may solve this issue. However, no research has yet examined the light responses of B. dorsalis.
A light-emitting diode (LED) is a semiconductor device that produces highly monochromatic light with little heat, it has a long lifetime, low energy consumption, and a high degree of safety (Duehl et al., 2011; Cho & Lee, 2012; Jeon et al., 2012). The solid state physics of LEDs are an advantage for controlling pest behavior and more efficient in practical application. LED traps have been manufactured based on insect responses toward the lighting and were widely used for monitoring and control of pests such as Culicoides brevitarsis Kieffer (Bishop et al., 2006), Tribolium castaneum Herbst (Duehl et al., 2011), Euscepes postfasciatus Fairmaire (Katsuki et al., 2012), and Helicoverpa armigera Hübner (Yoon et al., 2012). A variety of abiotic factors can influence the efficiency of light traps, including the light wavelength, intensity, time of exposure, and the spectral composition of the light (Shimoda & Honda, 2013; Sun et al., 2014). Also, the responsiveness of insects to light appears to differ between the sexes. Due to their flying capability (Venter et al., 2009), structure of compound eyes (Lau & Meyer-Rochow, 2007; Meyer-Rochow & Lau, 2008), and physiological status (Bourg & Badia, 1995; Belmain et al., 2000; Castrejon & Rojas, 2010), the photoresponsive behavior of female and male adults is inconsistent. For instance, males and gravid females of Indian meal moth, Plodia interpunctella (Hübner), exhibited preference to blue light, unlike virgin females (Cowan & Gries, 2009). Females of Loxostege sticticalis (L.) and Anomala corpulenta Motschulsky displayed stronger phototactic responses than males (Jiang et al., 2010, 2015). When reaching the age of 12 days, adult males of Xestobium rufovillosum De Geer displayed a preference for light, whereas females increasingly preferred the dark (Belmain et al., 2000). The study of innate phototactic behavior can provide the basis for designing electric insect killers (Shimoda & Honda, 2013).
The phototropic responses of B. dorsalis males and females of different ages toward multiple LED wavelengths, and the influence of monochromatic lights on biological parameters have not been studied before. This work tests the preference of B. dorsalis to six LED wavelengths in the laboratory. Our results may aid in developing sustainable, secure, and more efficient light traps for monitoring B. dorsalis.
Materials and methods
Insects
The B. dorsalis strain was maintained in a laboratory in South China Agricultural University, at 27 ± 1 °C, 75 ± 1% relative humidity, and L14:D10 photoperiod. Hatched larvae were maintained on an artificial diet including sugar (9%), yeast (15.1%), nipagen (0.15%), sodium benzoate (0.15%), wheat germ oil (0.15%), citric acid (1.7%), and water (73.8%) (Chang et al., 2006). Adult flies were reared in cages and fed another artificial diet consisting of 1:1 yeast extract:dry sugar (wt/wt).
Materials
Based on previous research on phototaxis in Tephritidae (Wu et al., 2007; Xue & Wu, 2013; Li et al., 2017), six LED light colors (green, red, yellow, blue, purple, and white; Table 1) were selected and customized in YuanEr Technology, Shenzhen, China (Sun et al., 2014). Sartorius-BP121S type electronic analytical balance was purchased from Sartorius AG. The Electronic timing socket was purchased from Bull International Electrical Group.
| Color | Peak wavelength (nm) | Wavelength range (nm) | Illuminance (lx) | Irradiance (W m−2) | Radiant flux (W) |
|---|---|---|---|---|---|
| Green | 522 | 480–580 | 337.70 | 0.47 | 0.48 |
| Red | 633 | 580–670 | 279.63 | 0.63 | 0.64 |
| Yellow | 596 | 540–620 | 298.68 | 0.48 | 0.51 |
| Blue | 450 | 420–500 | 225.14 | 0.82 | 0.86 |
| Purple | 440 | 400–480 | 219.70 | 0.53 | 0.53 |
| White | 452 (main), 570 (secondary) | Broad spectrum | 327.50 | 0.60 | 0.63 |
- The LED lights spectral parameters test distance is 30 cm.
Phototaxis device
In view of the strong flight and climbing ability of B. dorsalis adults, we designed a phototactic test device (made by Guangzhou Qianhui Chemical Glass Instrument, Tianhe, Guangzhou, Guangdong, China) (Figure 1). Each device (25 × 25 × 30 cm) consisted of transparent acrylic to allow for light exposure, comprising three chambers: a phototactic response chamber, an activity chamber, and a dark chamber (Figure 1B–D). Chambers were separated by an opaque partition with nine insect entrance holes (2 cm diameter). The LEDs were connected to a rheostat to adjust light intensities. The light intensity of LEDs was measured using a CL-200F spectrum illuminometer (Kexing Photoelectric, Hangzhou, China) at the center of the chamber (30 cm from the light source). The precise spectral characteristics of LED lights were measured by a CL-200FZ photoelectric properties integrated test system, which consists of a CL-200F spectrum illuminometer, an integrating sphere, and system software (Kexing Photoelectric).

Phototactic response
Effect of green and red lights on pupa emergence
Pupae formed within 24 h were separated haphazardly into six groups of 50, each put into a 100-ml beaker. The beakers were placed inside corresponding dark boxes (30 × 30 × 30 cm). The flies were exposed to green or red LED light (at 200 lx) for 0, 12, or 24 h every day. We counted the emerged adults daily at 10:00, 15:00, and 20:00 hours until all adults emerged. Then we calculated the pupal emergence rate. All treatments were repeated 3×.
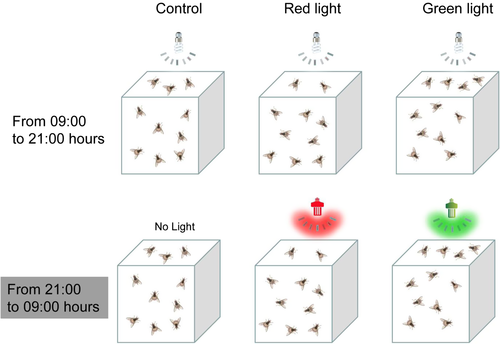
Influence of nighttime exposure to green or red lights on adult weight, mortality, and female fecundity
Groups of 200 newly emerged adults (within 24 h) with a 1:1 male:female ratio were selected haphazardly and placed in a cage (35 × 35 × 35 cm). Treatment groups were then illuminated with green or red LED lights (200 lx) daily, from 21:00 to 09:00 hours. Control groups were kept in the dark at the same time. From 09:00 to 21:00 hours, both experimental and control groups were illuminated by white LED light (200 lx; Figure 2). All flies were supplied the same food and water daily. Each treatment was repeated 3×. Individual adult weight was measured every 5 days. We recorded the number of dead flies at 10:00 hours every day until all flies were dead. Thus, adult weight and mortality rates for each treatment and control were calculated.
When tested flies reached sexual maturity (15 days of age), we calculated the female fecundity every 3 days. A plastic centrifuge tube (50 ml), with small oviposition holes punctured into it using a 19 gauge syringe needle, was used as an artificial egging device. Subsequently, we poured 1 ml self-made orange juice (as an oviposition stimulus) into the egging devices evenly, placed them in the flies’ cage at 15:00 hours, and allowed oviposition for 4 h. Immediately after the 4 h we counted the eggs and calculated the fecundity of female adults. Each experiment was repeated 3×.
Statistical analysis
All analyses were carried out with SAS v.9.20 software (SAS Institute, Cary, NC, USA). Datasets of adult phototactic response rates to various LED wavelengths, weight, female fecundity, and mortality were checked for normality of distribution and homogeneity of variances with Shapiro–Wilk and Levene's tests, respectively. If data were normally distributed and had similar variances, then means were compared by one-way ANOVA. Significant ANOVA results were followed by mean separation by Duncan's multiple range test (α = 0.05). Non-normally distributed data were analyzed with a nonparametric Kruskal–Wallis test to compare medians (α = 0.05), followed by a Mann–Whitney test for pairwise comparisons, if applicable. The attractiveness of purple and blue light to flies, color preferences between female and male flies, and pupal emergence rates between green and red light treatment groups were compared with independent-samples t-test (α = 0.05). Results were plotted with Origin v.7.5 (OriginLab, Northampton, MA, USA).
Results
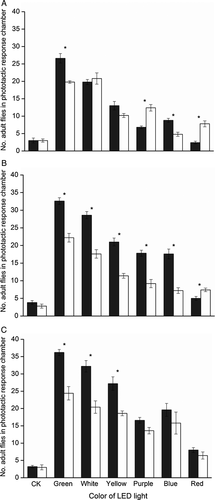
Phototaxis to different wavelengths of light
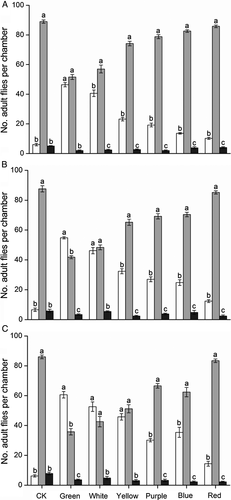
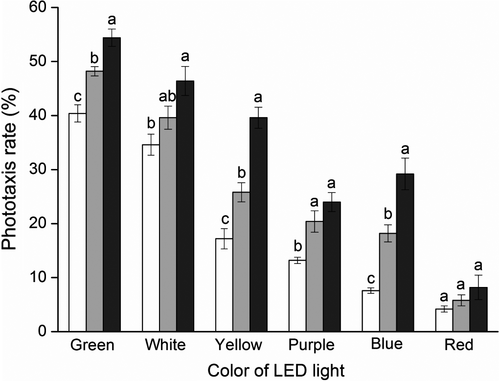
In control groups, the mean (± SEM) number of 5-, 10-, and 20-day-old B. dorsalis adults in the phototactic response chamber (Figure 1B) was 6.0 ± 0.84, 6.6 ± 1.12, and 6.2 ± 0.80, respectively – much lower than that in the activity chamber (Figure 1C), which was 89.0 ± 1.10 (F2,8 = 2 324.33), 87.6 ± 2.06 (F2,8 = 643.97), and 86.0 ± 1.22 (F2,8 = 1 351.28, all P<0.0001), respectively (Figure 3). After 30 min of conditioning in the dark, 5-, 10-, and 20-day-old adults displayed clear phototaxis toward all tested monochromatic and white lights, with the strongest behavioral response toward green, followed by white, then yellow. Green light attraction was significantly different to all other monochromatic lights (Figure 3). The white (composite) light also was strongly attractive to flies, and red light was the least attractive, with most flies staying in the activity chamber. There was no difference in attractiveness of purple and blue light to 10- (t = 1.72, d.f. = 4, P = 0.16) and 20-day-old flies (t = 1.19, d.f. = 4, P = 0.30).
Phototaxis at different post-emergence ages
Phototaxis increased with age, with newly emerged adults presenting lowest phototaxis (Figure 4). The mean (± SEM) phototactic responses of 10- and 20-day-old adults to green light were 48.2 ± 0.86 and 54.4 ± 1.60%, respectively – significantly higher than in 5-day-old adults (40.4 ± 1.60%) (F2,8 = 20.97, P = 0.0007). Phototactic responses of 20-day-old adults to white, yellow, purple, and blue lights were much higher than those of 5-day-old flies.
Phototaxis of males and females
Overall performance indicated that the number of males in the phototactic response chamber is higher than that of females, suggesting clear sexual differences (Figure 5). At 10 and 20 days old, males responded significantly stronger than females to green, white, and yellow light. The phototactic response of females to red light was stronger than that of males, significant differences were found in 5- (t = 4.63, d.f. = 4, P = 0.0098) and 10-day-old adults (t = 4.71, d.f. = 4, P = 0.0093). Although a tendency toward increased phototaxis reaction of older males is suggested, the difference is not significant (t = 2.36, d.f. = 4, P = 0.078).
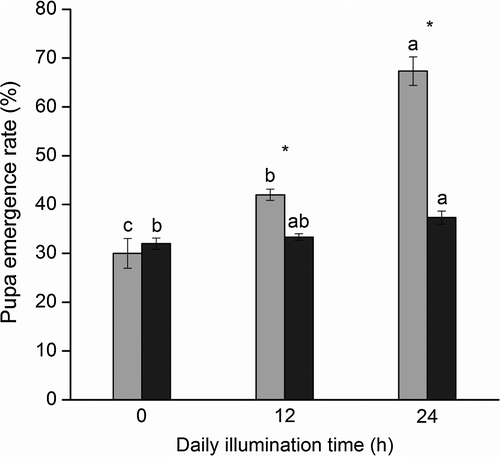
Influence of green and red light on pupa emergence
Emergence rates under green light exposure for 12 and 24 h daily were 21% (t = 13, d.f. = 2, P = 0.0059) and 45% (t = 7.21, d.f. = 2, P = 0.019) higher than under red light treatment, demonstrating that green light can significantly increase pupal emergence rates (Figure 6). Moreover, following the extending green light irradiation time every day, the emergence rates increased notably (F2,4 = 61.30, P = 0.0010).
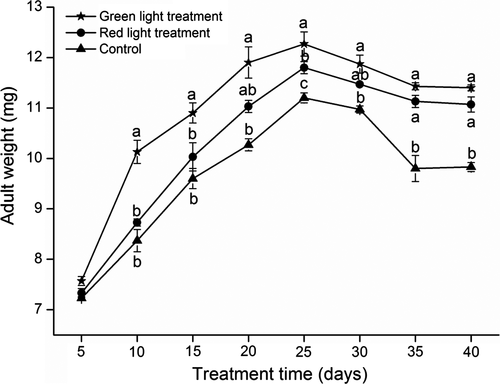
Influence of nighttime green and red light on fly weight and female fecundity
Nighttime exposure to green or red light resulted in increased adult body weight (Figure 7). After 5 nights of exposure, the mean (± SEM) fly weights did not differ [green, 7.6 ± 0.09 mg; red, 7.3 ± 0.09 mg; darkness (control), 7.2 ± 0.03 mg; F2,4 = 4.16, P = 0.11]. After 10–25 nights treatment, flies exposed to green light were significantly heavier than flies exposed to red or no light – after 25 nights exposure, flies exposed to green light weighed 12.3 ± 0.24 mg, those exposed to red light 11.8 ± 012 mg, and the control flies weighed 11.2 ± 0.10 mg (F2,4 = 30.88, P = 0.0037). These results demonstrate that both green and red nighttime irradiation have a positive impact on the growth and development of B. dorsalis adults, whereas the promotion of green exceeds that of red light.
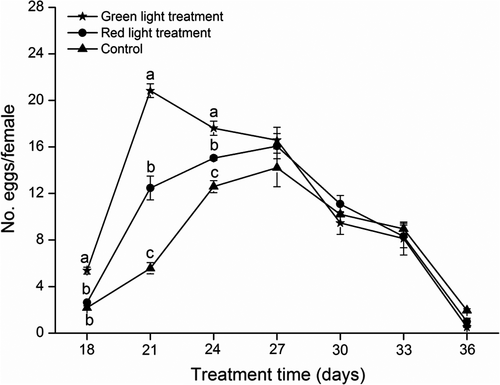
Green light promoted oviposition by female B. dorsalis (Figure 8). In the green light treatment groups, oviposition quickly increased after sexual maturation and peaked around the 21st day. In the red light test groups and in the controls, females took 27 days to peak oviposition after sexual maturation. The highest oviposition rate in the green light groups was 20.8 ± 0.59 eggs per female, which is significantly higher than the highest oviposition rate in the red light (16.1 ± 1.08) and control groups (14.2 ± 1.63) (F2,4 = 11.76, P = 0.021).
Influence of nighttime green and red light on adult mortality rate
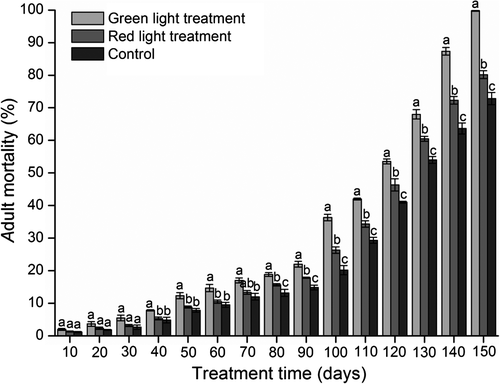
Nighttime exposure to green and red light decreased survival of B. dorsalis adults (Figure 9). Mortality of the two treated groups and the control group increased monotonically over time; the mortality rate (%) of flies exposed to green light was significantly higher than that of control flies from day 40 onwards, whereas the mortality rate of flies exposed to red light was significantly higher than that of the controls from day 80 onwards. After 80 days of treatment, mortality in the green and red light treatment groups had increased to 18.8 ± 0.60 and 15.7 ± 0.33%, respectively, and mortality in the control group had increased to 13.2 ± 1.09% (F2,4 = 20.99, P = 0.0076). After 150 days of treatment, all insects exposed to green light were dead, yet 19.8% of flies exposed to red light and 27.2% control flies were still alive (F2,4 = 223.96, P = 0.0001). Apparently nighttime exposure to green light decreases the lifespan of test flies more than red light treatment.
Discussion
Screening the sensitivity spectrum of insects to light can aid in controlling insect hazards and epidemics, e.g., the spread of arboviruses (Antignus, 2000). Most insects have highly conserved visual pigments, with sensitive spectral absorption between 350 and 700 nm, and they can perceive and respond to light in this range (Land, 1997). Insects differ in their sensitivity for specific wavelengths (Sun et al., 2014). In this study, we found both sexes in B. dorsalis have a wavelength preference, especially for green light, followed by white and yellow, and they are least sensitive to red light. This is in agreement with previous findings by Wu et al. (2007) who observed that B. dorsalis adults are more attracted to green and yellow plates than to red ones, and by Liang et al. (2016) who observed B. dorsalis adults have preference for green food items and are less interested in red food items.
Light irradiation was the required for adult B. dorsalis foraging, flight, and courtship (Liu & Ye, 2006). Our observations are consistent with the finding that B. dorsalis adults were most active in the day, and kept still at night (or in a dark room). Day-active phytophagous insects usually display the strongest phototactic reaction to wavelengths from 500 to 580 nm, while being generally blind to red (649 nm) and infrared light (Cohnstaedt et al., 2008; Chen et al., 2013). Insects indiscriminately respond to green objects including non-host plants (Couty et al., 2006). Wavelengths preferred by B. dorsalis adults would correspond to visible green hues. A similar tendency was observed with B. cucurbitae and B. tau (Xue & Wu, 2013; Li et al., 2017). The green-positive effect could be attributed to the green sensitivity of the 510-nm photoreceptor of B. dorsalis, which has been described as R8 cells based on the evidence from intracellular electrophysiological recordings (Wu et al., 2007). In nature the surface color of leaves, flowers, and ripe fruits of the host plants lays within the range of this sensitive short-wavelength spectrum – this may partly explain the attraction of green and yellow lights to tephritids (Prokopy & Owens, 1983; Drew et al., 2003; Blackmer et al., 2008; Yu, 2011; Tang et al., 2015). Based on field investigations, both Vargas et al. (1991) and Cornelius et al. (1999) demonstrated that yellow fruit-mimicking spheres were strong lures to B. dorsalis. Yang et al. (2011) studied the color preferences of B. dorsalis with traps containing a sweet bait hanging with differently colored balloons in a carambola garden. They found that the trap performed best where purple balloons were used. These findings do not seem to match our results. One reason may be that our research was conducted indoors in controlled conditions, where less interference takes place. Also, the color, shape, and size of traps are known to affect the response of oriental fruit flies. Differences in age, sex, and developmental status of their stock B. dorsalis may have been another reason for the inconsistent experimental result.
Age-related phototactic behavioral changes occur in B. dorsalis adults. The phototaxis rates of 20-day-old flies were higher than those of 5- and 10-day-old flies. This finding confirms the conclusions with other insects (Wei et al., 2000; Jiang et al., 2010; Zhu et al., 2014). Under various optical environments, the movements of blocking pigment granules in the pigment cells of insect compound eyes were closely related to the sensitivity to monochromatic light of different wavelengths and the status changes of compound eyes (Lau & Meyer-Rochow, 2007; Meyer-Rochow & Lau, 2008). This was a physiological basis for the specific sensitivity spectra of different insect species (Yu, 2011). When illuminating insects with dark compound eyes, blocking pigment granules inside pigment cells around the retinal cells move from the end of cornea to the basilar membrane. They surround the rhabdomere of each retinal cell. Before reaching the rhabdomere, the inciting light must go through dark pigment granules which absorb UV and visible light thus influencing spectral sensitivity of retinal cells (Chen et al., 1984). The phototactic rate of B. dorsalis increased with age probably because compound eyes may not yet be fully developed in the early imaginal state, when sensitivity of the blocking pigment cells may be weak, particularly for short wavelengths such as in the blue and red spectra. Also, age may affect phototactic tendencies due to a difference in the level of locomotor activity of flies (Elens, 1972). Males and females display higher flight activity and are generally more active with the increase in age (Cui et al., 2016). Mature male and female adults are sensitive to wavelengths as orientation cues during the search for food, reproductive partners and oviposition sites (Castrejon & Rojas, 2010).
The phototactic responses of B. dorsalis differed between the two sexes, with males being more sensitive to green, yellow, and white lights than females. Phototaxis differences between the sexes occur widely among insects (Meyer, 1978). Many studies reported a greater abundance of male moths compared to females in light trap catches (Kiss et al., 2003; Altermatt et al., 2009). The attractiveness of a blue-LED trap was higher to males of phlebotomine sand flies than to females during twilight (Silva et al., 2016). Male adults of Grapholita molesta Busck have a stronger phototactic reaction to blue and green light than female adults (Sun et al., 2014). Males of Carposina sasakii Matsumura preferred light at 333 nm, whereas females preferred 350 and 405 nm (Hou et al., 1994). This may be linked to gender differences in flight capability, body physiology, mating status, and compound eye structure, or to outside conditions such as light sources (Cowan & Gries, 2009; Cheng et al., 2011; Sun et al., 2014).
Insects are known to have a variety of biological and physiological responses to various wavelengths of the light spectrum, including attraction, repulsion, light adaptation, circadian rhythms, light toxicity, photoperiodicity, and others (Shimoda & Honda, 2013; Tariq et al., 2015, 2017). Circadian rhythms are daily behavioral rhythms of, e.g., locomotion, flight, feeding, and mating. During the night, after several minutes or a certain duration of exposure to artificial light, the timing of the diurnal/nocturnal behaviors of insects may shift and they may exhibit typical daytime behavior (Walcott, 1969; Okada et al., 1991). Some diurnal insects have been investigated that are active in crepuscular or nocturnal light with inherently diverse irradiance spectra, such as some bees and wasps (Warrant et al., 2004; Theobald et al., 2007). During our experiment, we observed that behaviors such as foraging, flight, and mating were activated in B. dorsalis adults when exposed to green and red lights in the night. As a result, we found that nighttime exposure to green light promoted B. dorsalis adult development, accelerating ovary development and maturation in a way that hastened the peak period of oviposition and increased fecundity. Zhou et al. (1995) pointed out that exposure to medium and long light wavelengths seemed beneficial to reproduction in B. dorsalis, as observed in our study. After exposure to green light the average lifespan of B. dorsalis adults was found to be decreased. This finding was similar to observations with Plutella xylostella L., G. molesta, and Tetranychus urticae Koch (Duan et al., 2010; Ismail et al., 2011; Yu, 2011).
Light traps are based on insect phototactic behavior, which has been considered as a ‘clean’ form of pest monitoring and control (Johansen et al., 2011). Some diurnal insects can be attracted to light sources at night (Shimoda & Honda, 2013). In addition, colored devices, such as yellow pan traps or yellow sticky traps, are widely used to capture diurnal pests including aphids, whiteflies, thrips, and leafminer flies (Mainali & Lim, 2010; Shimoda & Honda, 2013). Currently, various yellow sticky plates are widely employed in monitoring and control of B. dorsalis (Li et al., 2017). We found that its phototactic sensitivity to green light (522 nm) was greater than to yellow light. From a pest management perspective, our findings indicate that a stimulus in the green spectra may hold higher attractive potential to B. dorsalis, thus tests with green sticky traps or light traps are warranted.
Acknowledgements
We thank Eduardo Fox and Waqar Jaleel for linguistic correction. We are grateful to the members of our laboratory for their cooperation in oriental fruit fly rearing and treatment. This research was supported by grants from Guangdong Province College High-Quality Professional Foundation (No. 246) and National key research and development project of China (No. 2016YFC1201204).




