Attractive males produce high-quality daughters in the bean bug Riptortus pedestris
Abstract
There are numerous studies on the genetic benefit of female mate choice. Fisherian benefits are detected frequently, in which attractive males benefit females by increasing the mating success of sons. In contrast, good-genes benefits are relatively small or undetectable, especially as males often face a trade-off between the expression of secondary sexual traits and viability. In this situation, the effects of good genes might be masked in their sons and, therefore, should be investigated in daughters. A previous study has shown that attractive males produce attractive sons (i.e., Fisherian benefit); the present study aimed to verify the existence of good-genes benefits by revealing whether attractive males of the bean bug Riptortus pedestris (Fabricius) (Hemiptera: Alydidae) produced high-quality daughters. Male attractiveness was measured using courtship latency, and the fitness of the females and their offspring was measured based on their lifetime reproductive success as well as the longevity of the daughters. There was no evidence that females directly benefited from mating with attractive males. Whereas male attractiveness (i.e., courtship latency) did not affect nymphal viability or the longevity of daughters, the attractive males with lower courtship latency could produce the daughters with higher lifetime reproductive success. These results suggest that female mating preference in R. pedestris evolved via Fisherian and good-genes benefits.
Introduction
Female mate choice is one of the main sources of sexual selection driving the evolution of male sexual traits such as ornament and courtship displays (Andersson, 1994). In addition, female mate choice based on male sexual signaling may be advantageous either via direct and/or indirect benefits (Andersson, 1994). The direct-benefit hypothesis assumes that attractive males have highly exaggerated sexual traits that reflect the quality and/or quantity of their resources, and females can increase their fecundity and/or longevity by mating with attractive males (Hoelzer, 1989; Andersson, 1994). Nuptial gifts, access to territory, defense from predators, and cooperation in parental care are typical examples of direct benefits (Price et al., 1993; Møller & Jennions, 2001).
The effects of indirect benefits of mate choice are expected to be smaller than those of direct benefits (Kirkpatrick, 1996; Kirkpatrick & Barton, 1997), and meta-analyses appear to confirm this (Møller & Alatalo, 1999; Jennions et al., 2001; Møller & Jennions, 2001). Nevertheless, when direct benefits are absent, indirect benefits are required to maintain female preference in two general ways (Andersson, 1994; Kirkpatrick, 1996). First, when male sexual traits reflect male attractiveness and are heritable, females mating with such males can indirectly increase their fitness by increasing the reproductive success of their sons (i.e., Fisher's runaway; Fisher, 1930; Lande, 1981). Second, male sexual traits may indicate heritable condition and viability (i.e., good genes). In this situation, females can indirectly increase their fitness by increasing the viability of their offspring (reviewed in Jennions & Petrie, 2000; Andersson, 2006).
Although mate-choice benefits via the Fisherian process have been well demonstrated in numerous taxa (e.g., Brooks & Endler, 2001; Taylor et al., 2007; Potti & Canal, 2011; Suzaki et al., 2013; Okada et al., 2014), relatively few studies have successfully detected the benefits related to good male genes (but see Moore, 1994; Hoikkala et al., 1998; Hoefler et al., 2009; Garcia-Gonzalez & Simmons, 2011; Simmons & Holley, 2011). A meta-analysis of indirect benefits suggested that the effect of good genes on the evolution and maintenance of female preference is relatively smaller than that of the Fisherian model (Møller & Alatalo, 1999; Prokop et al., 2012). One possible reason for this difference is that sons generally face a resource allocation trade-off between survival and reproductive success (Droney, 1998; Kokko, 2001; Getty, 2002; Simmons, 2012). In this case, the good-genes benefit is masked because of biased resource allocation toward sexual traits (Kokko et al., 2002, 2003; Cameron et al., 2003; Hunt et al., 2004). Therefore, the effects of good genes could also be investigated in daughters, in which such biased resource allocation is less likely to mask these effects (Jennions & Petrie, 2000; Hunt et al., 2004).
In the present study, we assessed whether attractive males of the bean bug Riptortus pedestris (Fabricius) (Hemiptera: Alydidae) sire superior daughters. Courtship latency, which is the time from the initiation of male courtship to successful genital insertion, was used as a measure of male attractiveness. This is because female R. pedestris never open their genital opening after male courtship ends and, therefore, females can decide whether to mate with courting male or not (see details below). Males with lower courtship latency are regarded as attractive because attractive and preferred males generally mate sooner than unattractive males (e.g., Shackleton et al., 2005; Taylor et al., 2007, 2008; Okada et al., 2013). Despite the significant genetic variance in male attractiveness and the production of attractive sons, mating with attractive males neither increases lifetime reproductive success (LRS, i.e., fecundity) nor the longevity of R. pedestris females (Suzaki et al., 2013). Furthermore, there is no information on the relationship between male attractiveness and daughter quality. Thus here, we aimed to also investigate the effect of male attractiveness on female direct and indirect fitness, i.e., on the mother's LRS, nymphal survival, and the daughter's LRS and longevity.
Materials and methods
Insect culture
The stock population was cultured from approximately 50 R. pedestris individuals collected in Fukuyama City, Hiroshima, Japan, in late autumn 2006 (Kimura et al., 2008). Insects were reared on soybean (Glycine max [L.] Merr.) and red clover (Trifolium pratense L.) (both Fabaceae) seeds, and on water containing ascorbic acid (0.05%) (Kamano, 1991). Food and water were replaced once every 2 weeks. The stock population was maintained at 1500–2000 nymphs per generation and kept in plastic cups (9.5 cm diameter, 4 cm high) with a standing density of 10–20 individuals per cup. Newly emerged adults were individually separated into Petri dishes (9 cm diameter, 1.5 cm high) on the day of emergence to ensure that they remained virgins. Thus, adults did not interact with conspecifics until the experiments. Rearing and experiments were performed in a chamber maintained at 25 °C with a L16:D8 h photoperiod (light phase: 07:00–23:00 hours).
Measurement of male attractiveness
Numata et al. (1986) described the following highly stereotypical sequence of courtship behaviors in this species under laboratory conditions. After mounting on the female's back, the male taps her antenna with his foreleg while shaking his body and when the female accepts the male's mating attempt, she opens her ovipositor valves and the male inserts his genitalia. After genital connection, the male turns around, and the pair stands in an end-to-end position.
As mentioned above, in this study courtship latency (the time from initiation of courtship to the commencement of copulation) was used as an indicator of male attractiveness and a male with lower courtship latency was treated as an attractive male and vice versa (Suzaki et al., 2013). Male courtship latency is significantly repeatable (Suzaki et al., 2013) and is not affected by the female body size (GLMM: F1,86.59 = 0.692, P = 0.41; Appendix S1 and Figure S1). Our previous study also revealed that male courtship latency is not significantly associated with female fecundity and longevity (Suzaki et al., 2013). Thus, it is not likely that males of R. pedestris prefer high-quality females and courtship latency can be used as a proxy of their attractiveness (Suzaki et al., 2013).
A 15- to 20-day-old virgin male and a 7- to 10-day-old female were randomly chosen and placed in a plastic cup (7.8 cm diameter, 4.3 cm high) lined with filter paper, where their interaction was continuously recorded using a digital video camera (HDR-PL590V; Sony, Tokyo, Japan) until copulation was terminated. Courtship latency was then measured using the video-recorded data. Although we recorded copulation from the beginning until the end, we did not use copulation duration to analyze the female fitness because it does not affect female fitness in R. pedestris (Appendix S1 and Figure S2). In accordance with the method used in our previous study (Suzaki et al., 2013, 2015), if bugs did not copulate within 2 h, they were discarded. After mating, the pair was immediately separated to avoid additional mating, and the female was isolated to assess her fitness. All observations were conducted between 15:00 and 23:00 hours.
Measurement of female fitness
Mated females (n = 75) were transferred to Petri dishes (9 cm diameter, 2 cm high) containing an excess of food and water with a 1-cm3 cotton wool piece as an oviposition substrate. Females were allowed to feed and oviposit ad libitum. Females were transferred to a new Petri dish every week, at which time the eggs in each Petri dish were counted. The egg count ceased after 5 weeks and the count obtained was treated as the LRS. Because the fecundity in the first 5 weeks was positively and significantly correlated with both fecundity in the 10 weeks when most of the sperm transferred by the first mated male may be exhausted (Sakurai, 1998a,b) and lifetime fecundity (Figure S3), the 5-week fecundity was used as a proxy of the LRS. One female was accidentally killed only 2 weeks after the onset of the experiments and she was excluded from the analysis of direct benefit. However, she oviposited sufficient eggs for use in the subsequent experiments, therefore her offspring were not discarded from the assessment of indirect benefits.
Offspring fitness
To investigate the effect of male attractiveness on offspring fitness, 20 eggs per female were randomly chosen from the eggs laid during the first 2 weeks to examine the viability and they were reared until eclosion, under the conditions described above. Thirty to 40 eggs per female were kept as a reserve, and used in the subsequent experiments after all the offspring used in the assessment of viability died before sexual maturity. Newly emerged daughters were reared individually until subsequent experiments. To assess the LRS of the daughters, one 7-day-old daughter of each female was randomly selected and placed in a mating arena with a virgin male randomly selected from the stock culture, and their mating behavior was video-recorded. After copulation, males and daughters were immediately separated and the LRS of daughters was monitored for 5 weeks, similar to that of the mother. To assess longevity, one virgin 7-day-old daughter of each female was randomly chosen and placed in a Petri dish containing only water. The daughters’ survival was assessed every day until their death.
Statistical analysis
To investigate whether male attractiveness affected female and daughter productivity, a generalized linear model with Poisson distribution, log link, and overdispersion test was used. The analyses considered the LRS of the females or daughters as the dependent variables and male attractiveness as the independent variable. For daughter's longevity, we used Cox's proportional hazards analysis (Cox, 1972). Daughter's longevity was used as the dependent variable, and male attractiveness was used as the independent variable. To test whether offspring viability was affected by male attractiveness, we conducted logistic regression analysis with offspring viability from egg-to-adult emergence (live or dead) as the dependent variable and male attractiveness as the independent variable. All statistical analyses were conducted using the JMP v.9.0.2 software for Windows (2010; SAS Institute, Cary, NC, USA).
Results
Female fitness
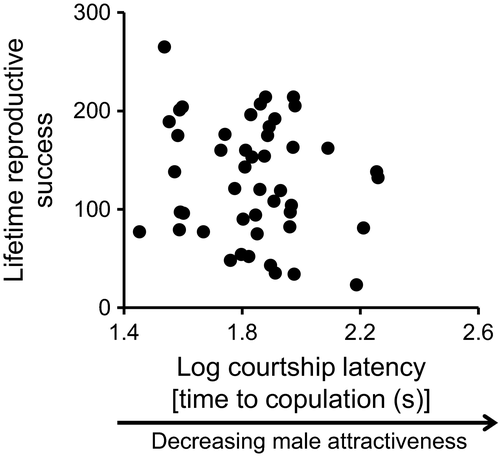
Sire attractiveness (i.e., courtship latency) lasted on average 82 s, and total and weekly LRS were 130 and 26 eggs (Table 1). Among the 75 females tested, 60 successfully oviposited after copulation and 48 produced more than 20 viable eggs, which was sufficient to measure offspring viability, and had oviposited for 5 weeks (except one female, as mentioned above). The generalized linear model showed that male attractiveness (i.e., courtship latency) had no significant effect on female LRS (coefficient ± SE = −0.00223 ± 0.0020, n = 48; χ2 = 1.325, d.f. = 1, P = 0.25; Figure 1). This trend did not change even when females who laid <20 eggs were included (−0.00073 ± 0.0020, n = 60; χ2 = 0.117, d.f. = 1, P = 0.73).
| n | Mean ± SE | |
|---|---|---|
| Sire | ||
| Courtship latency (s) | 48 | 81.75 ± 7.91 |
| Dam | ||
| Total lifetime reproductive success (no. eggs) | 47 | 129.91 ± 8.60 |
| Lifetime reproductive success per week (no. eggs) | 47 | 25.98 ± 1.72 |
Offspring fitness
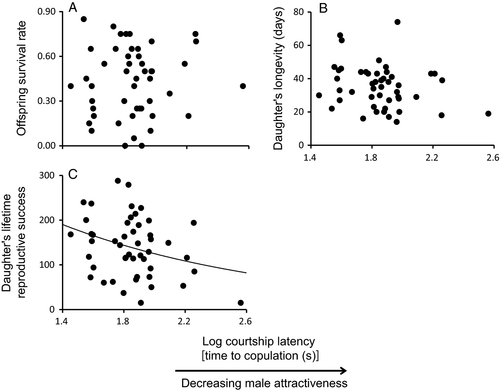
Offspring viability was on average 43%, total and weekly LRS of dams were 140 and 28 eggs, and longevity was 35 days (Table 2). Offspring viability from egg-to-adult emergence was not affected by male attractiveness (coefficient ± SE = −0.00098 ± 0.0012; χ2 = 0.671, d.f. = 1, P = 0.41; Figure 2A). Cox's hazard model showed that male attractiveness had no effect on daughter longevity (0.00589 ± 0.0030; χ2 = 3.129, d.f. = 1, P = 0.077; Figure 2B). In contrast, the time males took to achieve copulation (i.e., courtship latency) was negatively correlated with the lifetime reproductive success of their daughters (−0.00382 ± 0.0016; χ2 = 6.642, d.f. = 1, P = 0.010; Figure 2C). In other words, more attractive males sired daughters that went on to have higher reproductive success than the daughters of less attractive males.
| n | Mean ± SE | |
|---|---|---|
| Offspring | ||
| Egg-to-adult viability | 48 | 0.43 ± 0.03 |
| Daughter | ||
| Total lifetime reproductive success (no. eggs) | 48 | 140.04 ± 9.60 |
| Lifetime reproductive success per week (no. eggs) | 48 | 28.01 ± 1.92 |
| Longevity (days) | 48 | 35.35 ± 1.87 |
Discussion
Abundant evidence indicates that the lifetime fitness of females increases after receiving male-derived resources (Hoelzer, 1989; Andersson, 1994). However, in the present study, the direct benefit of mating with an attractive male was not detected in R. pedestris. This result is consistent with that of our previous study (Suzaki et al., 2013).
When direct fitness effects are small or absent, indirect benefits can sufficiently maintain mate preference (Kirkpatrick, 1996). Indeed, mating with an attractive male provided indirect benefits to R. pedestris females because male attractiveness was positively associated with the LRS of the daughters and had no negative effects on nymphal viability or the longevity of the daughters. This indicates that attractive males can produce superior daughters.
In R. pedestris, male attractiveness is genetically associated with male courtship quality and hindleg size (Suzaki et al., 2013), and previous studies have suggested that both traits are condition-dependent (Suzaki et al., 2013; Kim & Lim, 2014). Because the LRS of the females also depends on condition (e.g., Madsen & Shine, 1996; Blanckenhorn, 2000; Bonduriansky & Head, 2007; Saastamoinen et al., 2010; Katsuki et al., 2012), daughters produced by attractive fathers might be able to invest their resources in reproduction, which is consistent with the good-genes theory (Andersson, 1994).
In contrast to the above supposition, it is also possible that non-genetic maternal effects affect daughter LRS. If a female that mated with high-quality males invests more resources in the eggs, the variation in daughter quality may be explained by differences in maternal investment (Sheldon, 2000). In this bug, larger females tend to produce larger eggs (S Toda, K Fujisaki & F Nakasuji, pers. comm.) and nymphs hatched from larger eggs have a higher survival rate and grow faster than those hatched from smaller eggs (Toda et al., 1995). We did not measure egg size or mass in this study; however, male attractiveness did not correlate with female body size (Figure S4), and offspring viability was unaffected by male attractiveness. Therefore, the result that attractive males sired daughters with high LRS was unlikely due to an artifact variation in the female body size that could be associated with maternal investment in eggs. Alternatively, although attractive males did not contribute to female LRS, there is a possibility that nutrients derived from attractive males increase egg quality (Vahed, 1998) and daughters sired by attractive males show higher LRS. However, male R. pedestris do not present their mates any nuptial gift and they do not produce spermatophores (Y Suzaki, pers. obs.). In addition, because males produce relatively large sperm and the female spermatheca is relatively small (Sakurai, 1998a), the spermatheca of the mated female is almost entirely filled by sperm, and there is likely to be insufficient room for transferring other nutrient content. Therefore, it seems that the nutrient seminal content transferred by attractive males did not affect LRS of the daughters. However, there is no direct evidence of the effect of the paternal and maternal investment on daughter LRS. Thus, the presence of non-genetic paternal or maternal investment, or both, that affects daughter quality should be investigated in future studies.
Increasing evidence suggests that alleles with positive effects on male (female) fitness have negative effects on female (male) fitness, generating negative father–daughter (and mother–son) fitness associations (i.e., intralocus sexual conflict; Rice & Chippindale, 2001; Fedorka & Mousseau, 2004; Pischedda & Chippindale, 2006; Foerster et al., 2007; Harano et al., 2010). When this conflict occurs, females might be exposed to indirect costs if they mate with males that are more attractive, because they produce low-quality daughters (e.g., Price & Burley, 1993, 1994). Nonetheless, there was no negative relationship between male attractiveness and daughter traits. In addition, because male attractiveness had no significant correlation with offspring sex ratio (Figure S5), daughters that sired attractive males likely do not have a lower nymphal mortality than do sons sired by attractive males. Therefore, R. pedestris daughters produced by attractive males are unlikely to have lower fitness owing to intralocus sexual conflict. However, because the sex of the nymphs could not be distinguished during the assessment of their viability, we were unable to assess whether daughters produced by attractive males exhibit low viability during the nymphal period.
We noted that the assessment of daughter fitness was conducted under uniform benign or harsh conditions, which are likely to mask true fitness variation (Hoffmann & Parsons, 1991). Furthermore, only one daughter per female was used for each measurement of daughter fitness. One daughter per female may not be an adequate sample size for investigating the indirect benefit because, although this study was conducted under laboratory conditions, it is assumed that a variety of factors affect daughter fitness. Thus, in future studies, the relationship between male attractiveness and daughter fitness should be investigated under various environmental conditions with different degrees of stress using more daughters per female.
In this study, we aimed to confirm the good-genes benefit of female mate choice in R. pedestris. We detected a positive correlation between male attractiveness and daughter quality, which suggests that females mated with attractive males can gain good-genes benefit by producing high-quality daughters. We used a no-choice test to assess the male attractiveness and female preference. Furthermore, we used courtship latency as a proxy of male attractiveness, because no-choice systems can exclude the effect of male–male competition and courtship latency is useful for calculating male attractiveness (Shackleton et al., 2005). However, a meta-analysis suggests that the strength of mate preference and assessment of mate quality are affected by experimental designs (Dougherty & Shuker, 2015). Therefore, additional studies are needed to investigate female mate preference and female fitness under various social and environmental conditions using several designs such as choice, no-choice, and random choice tests.
Acknowledgments
We would like to thank H Numata for providing the reagents and materials for this study and three anonymous reviewers for comments that greatly improved the manuscript. This study was partly supported by a Grant-in-Aid to YS from the Japan Society for the Promotion of Science Fellows (280154).




