How to become larger: ontogenetic basis of among-population size differences in a moth
Abstract
Evolutionary studies on animal body size have primarily focussed on selective pressures operating during the adult life. In contrast, ontogenetic pathways leading to differently sized adults have received less attention. In the present study, based on a common garden experiment, we report considerable genetic differences in body size among European populations of Ematurga atomaria (L.) (Lepidoptera: Geometridae). In terms of body mass, the moths from a southern (Georgian) population are twice as large as their northern (Estonian) conspecifics. Detailed monitoring of larval growth schedules revealed that the size difference arises through a longer development period of the Georgian larvae, with no difference in the number of instars. Differential (instantaneous) growth rates of the larvae do not differ between the populations. Eggs and newly hatched larvae are larger in the Georgian population but the difference vanishes in the second instar. The larger size of the Georgian moths is regained through higher relative mass increments during each of the three final instars. Such gradual ‘accumulation of the difference’ confirms the idea about constraints on substantial evolutionary changes in growth patterns within a single instar. The larger Georgian moths were found to be considerably more fecund which implies a strong selection for large female size. It remains unclear which counteracting selective pressures have favored the smaller size of Estonian conspecifics. As the associated difference in egg size appears not to be carried over to larger larval size, the adaptive value of larger eggs is not likely in contributing to the prospective large adult size. The larger eggs of the Georgian population should have an adaptive value per se, or represent a mechanistic consequence of larger maternal body size.
Introduction
Body size is a central life-history trait due to the multitude of effects it has on various aspects of individual fitness (Roff, 1992, 2002; Stearns, 1992). Nevertheless, understanding the adaptive evolution of body size and related traits has turned out to be surprisingly complicated (Blanckenhorn, 2000; Tammaru et al., 2002; Berger et al., 2006, 2012; Ringsby et al., 2015; Rollinson & Rowe, 2015). Whereas ecological selective pressures on animal body sizes are becoming increasingly well understood, the role of physiological and developmental mechanisms which underlie adaptive size differences has attracted less attention. This is unfortunate because focussing solely on costs and benefits of adult body size would dismiss those selective factors which operate during the immature development (see Kingsolver et al., 2012; for an insect example). In insects, developmental-biological studies on size determination concentrate primarily on the last instar (Stillwell & Davidowitz, 2010; Shingleton, 2011; Nijhout et al., 2014), with the contribution of earlier ontogenetic stages remaining less known (but see Stillwell et al., 2014; Grunert et al., 2015).
Knowing ontogenetic patterns which lead to size differences among individuals may help us to locate constraints on growth trajectories. Not all modifications of the growth curve which are favored by selection can be realized. In the case of arthropods, insects in particular, discontinuity of growth due to discrete instars (Nijhout, 1981; Sehnal, 1985) is seen as a major source of constraints on realizable shapes of larval growth curve (Sehnal, 1985; Ayres & MacLean, 1987; Higgins & Rankin, 1996; Esperk & Tammaru, 2004; Maino & Kearney, 2015; Tammaru et al., 2015). Detailed information about the ontogenetic differences accompanying differences in body size may help us to delimit the space available for adaptive responses in growth-related traits.
There are just three basic ways to become larger. The ultimately larger individual (1) can be larger from the beginning (egg, newly hatched larva), (2) grow faster, or (3) have a longer development time (Blanckenhorn et al., 2007; Tammaru et al., 2010). These options have been evaluated in the context of sexual size dimorphism (SSD) in insects. Females – usually the larger sex in insects – have been suggested to grow faster as larvae (Blanckenhorn et al., 2007) or to grow for a longer time (Fischer & Fiedler, 2001; Tammaru et al., 2010; Teder, 2014). The longer development period of females may be accompanied by an increase in the number of instars (Gomi, 2005; Esperk & Tammaru, 2006; Esperk et al., 2007; Etilé & Despland, 2008; Barraclough et al., 2014; Montezano et al., 2014). The possibility of being larger from the beginning, i.e., the option of a sex-related difference in egg size, appears to have received little attention. The works reporting such a difference appear to be limited to arthropods with a haplodiploid sex determination system (Jayasingh, 1980; Macke et al., 2011; Budrien≐ et al., 2013; Walzer & Schausberger, 2015), whereas there are scattered reports on the absence of sexual dimorphism in egg sizes in other arthropods (Kim, 1999; Yasuda & Dixon, 2002; Schenk & Söndgerath, 2005; Tammaru et al., 2010).
Besides the among-sex differences, among-population differences in adult body size are ubiquitous in insects. In addition to specialized studies on latitudinal gradients (Pincheira-Donoso, 2010; Huston & Wolverton, 2011; Shelomi, 2012), different sizes are frequently reported for different geographic populations in taxonomic handbooks (e.g., Mikkola & Jalas, 1977, 1979). Common garden studies proving genetic background of such differences are not abundant but these still cover a wide variety of insect taxa, e.g., antlions, beetles, lepidopterans, grasshoppers, and damselflies (Arnett & Gotelli, 1999; Saastamoinen et al., 2013; Barton et al., 2014; Parsons & Joern, 2014; Stoks et al., 2014; Sniegula et al., 2016). Nevertheless, though there are studies reporting among-population differences in various parameters of the immature stages (e.g., Fox, 1993; Fox & Caldwell, 1994; Tikkanen et al., 2000; Armbruster & Conn, 2006; Nilsson-Örtman et al., 2015), we are unaware of attempts to subject the ontogenetic patterns behind among-population size differences to a systematic scrutiny.
In the present study, we focus on the ontogeny of among-population size differences in the geometrid moth Ematurga atomaria (L.) (Lepidoptera: Geometridae). First, we demonstrate the genetic component in such differences using a common garden design. Furthermore, we provide a detailed analysis of immature development to investigate whether the size difference is already present in the eggs, and/or whether it arises during the larval stage, either through higher growth rates, or longer development of the ultimately larger insects. To be able to discuss the findings in the evolutionary-ecological context, we also evaluate size-dependent fecundity and longevity of the differently sized moths.
Materials and methods
Study insects
Ematurga atomaria (Lepidoptera, Geometridae, Ennominae) is a medium-sized (wing span 2–3 cm) day-flying moth frequently abundant in various habitats of temperate Eurasia. The larvae are cryptic (Sandre et al., 2013), highly polyphagous (Porter, 1997), and solitary external feeders. The species is univoltine at northern latitudes, with the pupa as the overwintering stage, whereas two generations annually occur in more southern areas (Leraut, 2009), lowland Georgia included (T. Tammaru, pers. obs.). Larval development consists of five instars (Vellau & Tammaru, 2012; Vellau et al., 2013).
The laboratory stock was formed by offspring of wild-caught females (four or more per population) from lowland (400 m a.s.l.) and alpine (1 500 m a.s.l.) Georgia (Caucasus) (41°N), as well as several locations in Estonia (58°N) (see Table 1 for exact data). In the course of field collections in 2012–2013, the lowland Georgian specimens were noticed to be considerably larger than their Estonian conspecifics, with the alpine Georgian moths taking an intermediate position.
| Population | Location | No. broodsa | No. mating pairsb | No. individualsc | ||
|---|---|---|---|---|---|---|
| Common gardend | Monitoring developmente | Adult fecundity and longevityf | Common garden | Monitoring development | ||
| Estonia (EST) | Tähtvere (58°22′N, 26°42′E) | – | 2 | 6 | – | 13 |
| Karilatsi (58°7′N, 26°55′E) | 2 | 8 | 20 | 28 | 67 | |
| Tõstamaa (58°20′N, 23°59′E) | 2 | 6 | 19 | 42 | 37 | |
| Lowland Georgia (GEOL) | Marelisi (41°57′N, 43°16′E) | 6 | 11 | 35 | 66 | 98 |
| Alpine Georgia (GEOA) | Gveleti (42°43′N, 44°37′E) | 3 | – | – | 67 | – |
| Hybrid (HYBR) | Karilatsi/Marelisi | 2 | – | – | 18 | – |
- a Number of broods (offspring of one female) used in the respective experiments.
- b Number of mating pairs used in the adult longevity experiment.
- c Number of larvae that successfully pupated and were used in the analyses.
- d Experiment designed to test for genetic differences in growth parameters.
- e Experiment designed to monitor among-population differences in body size throughout immature development.
- f Experiment designed to study adult longevity and the relationship between number of eggs laid and pupal mass.
The larvae were reared in the laboratory for at least two generations before the common garden experiment (see below), and four generations before larval development was monitored. The larvae were kept at 20 °C, being housed individually in plastic jars. Fresh leaves of a host plant were provided every 2nd day. The offspring of females were randomly mated within locations so that inbreeding was avoided. In addition, some first-generation hybrid broods were produced by mating lowland Georgian individuals to Estonian ones to confirm the genetic component in body size differences. During stock maintenance and experiments (see below), L10:D14 photoperiodic regime was used to ensure that each larva would develop into a hibernating pupa rather than produce second-generation adults. This was necessary to avoid any bias related to different development pathways chosen by the larvae (e.g., Gotthard et al., 1999; Gotthard, 2008; Friberg et al., 2012).
Common garden rearing
Parameters of larval growth were compared for E. atomaria representing lowland Georgia, alpine Georgia, Estonia, and hybrids of Estonia and lowland Georgia. The larvae of different origin were reared under a common garden design in controlled laboratory conditions at the University of Tartu, Estonia. Rearing E. atomaria under common garden conditions was part of a more comprehensive study on latitudinal gradients in multiple lepidopteran species. Accordingly, some of the data on E. atomaria have previously been used in another context (Meister et al., 2017).
The larvae were let to molt into their final (fifth) instar strictly simultaneously (see Meister et al., 2017, for more methodological details). Synchronized rearing allowed us to standardize environmental conditions to the maximal possible extent. Larvae representing all populations and broods (= offspring of a single female) were randomly divided between rearing chambers with different temperatures (16, 20, and 24 °C), and were offered various host plants [Salix alba L. (Salicaceae), Trifolium repens L. (Fabaceae), and Vaccinium myrtillus L. (Ericaceae)]. Such a crossed-factor design was chosen to reveal potential among-population differences in the parameters of larval growth as such (consistent differences across all experimental environments), and to separate those from possible population-specific plastic responses to temperatures or host plants (environment-specific among-population differences). The latter were not of specific interest in the present study. The position of larvae representing different populations, and feeding on different host plants were randomized on rearing trays.
Differential (= instantaneous) growth rate (DGR) was calculated on the basis of body masses recorded for each larva on the 2nd and 3rd day of the development in the final instar (see Meister et al., 2017; for a discussion of the method). DGR was calculated as (final mass1/3 – initial mass1/3)/time (mg1/3/days) with the cube root transformation applied to linearize larval growth curves (Tammaru & Esperk, 2007). Duration of the development in the last instar was recorded. The pupae were weighed and sexed by means of morphological inspection; see Table 1 for sample sizes.
To explore the among-population differences in growth parameters (pupal mass, development time in the final instar, growth rate), general linear mixed models (GLMMs, Proc MIXED; Littell et al., 1996) were constructed with population (see Table 1 for clarification), temperature treatment, host plant, and sex as categorical fixed factors, whereas brood was included as a random factor. For estimating denominator degrees of freedom (d.d.f.) the Kenward–Roger method was used; accordingly, when testing for the effect of population, the number of d.d.f. were derived from the number of broods, accounting for non-independence of individual measurements. The data presented in the graphs are population averages with the effects of additional factors (temperature, host plant, sex) removed (‘least square means’ option of GLMM), full ANOVA tables are to be found in the Supplementary material (Table S1–S6). All statistical analyses were conducted in SAS v.9.4 (SAS Institute, Cary, NC, USA).
Monitoring larval development
To study the ontogeny of the among-population size difference, the development of E. atomaria individuals was monitored from the egg to pupal stage in a separate rearing experiment in 2016. This comparison included representatives of the lowland Georgian and Estonian populations, i.e., we compared those populations which had the highest genetically based difference in body size. Eggs (n = 335; 27 broods) and newly hatched larvae (n = 215; 27 broods) were weighed. For a sample of larvae (98 from lowland Georgia, 117 from Estonia) reared simultaneously, final masses of all instars were recorded and development periods within each instar were determined in the course of daily inspections.
The larvae were weighed during the pre-molt growth stasis, a stage that can be recognized by visual inspection with some experience. The larvae were reared from hatching to pupation under 20 °C on a high-quality host plant, V. myrtillus (Vellau et al., 2013). Positions of the vials in rearing trays were randomized with respect to population and brood.
Focal traits (egg size, larval body mass in the beginning of each instar, pupal mass) were analyzed as dependent on two fixed factors (population and sex) using Proc MIXED; brood was included as a random factor, with degrees of freedom estimated by Kenward–Roger method. Full ANOVA tables are to be found in the Supplementary material (Tables S7–S24).
Fitness consequences of large adult size
To address the benefits of large body size, we compared adult longevities and fecundities between the representatives of lowland Georgian (large moths) and Estonian (small moths) populations. Mating couples were formed placing males and females from the same population into 100-ml plastic jars (see Javoiš et al., 2011; for details of the method). Longevities of both the male and female partner were determined by daily inspection and analyzed by a two-way ANOVA with population and sex as fixed factors. The number of eggs laid was determined by counting the newly hatched larvae (E. atomaria lays eggs in clusters making directly counting the eggs difficult) and those eggs which failed to hatch. To determine the total number of eggs produced by the female, dead moths were dissected and eggs remaining in their abdomina were counted. Egg production (eggs laid + eggs found in the abdomina) was first compared among the populations using a one-way ANOVA, thereafter an ANCOVA model was constructed with population and the year of the experiment (2016 and 2017) as fixed factors. Furthermore, pupal mass was included as a continuous covariate to test for the differences in fecundity with the effect of body size being accounted for; population*pupal mass interaction was examined to test for differences in the mass vs. fecundity relationship among the populations. Full ANOVA tables are to be found in the Supplementary material (Tables S25–S30).
Results
Among-population differences in body size
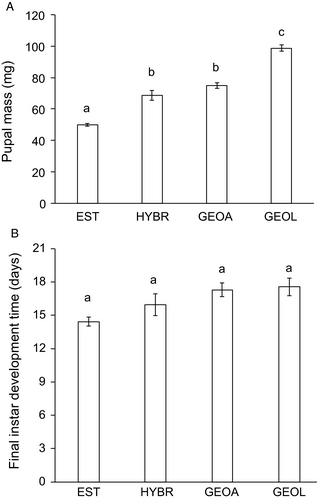
In the common garden rearing of E. atomaria larvae, pupal mass was confirmed to be significantly different among the populations (Figure 1A, Table 2), with as much as 68% of the variance attributable to the geographic origin of the insects. Pupal mass of lowland Georgian moths was 2.0× higher on average than that of their Estonian conspecifics, with the alpine Georgian population taking an intermediate position. The hybrids of Estonian and lowland Georgian moths had body sizes close to the average of the parent populations, confirming the genetic character of the differences. Neither population*temperature nor population*host plant interaction attained significance in the reduced model (Table 2) indicating that the difference in body size is consistent across the environments. The significant population*sex interaction refers to a stronger female-based SSD in both Georgian populations, as compared to the Estonian ones. In the monitoring experiment, pupal masses were 1.6× higher for lowland Georgian insects, compared to Estonian ones.
| Factor | Effect | Full model | Reduced model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | P | ω2 | d.f. | F | P | ω2 | ||
| Pupal mass | Population (Po) | 3,12 | 75 | <0.0001 | 0.68 | 3,12 | 77 | <0.0001 | 0.69 |
| Sex (S) | 1,191 | 62 | <0.0001 | 0.049 | 1,204 | 61 | <0.0001 | 0.054 | |
| Host plant (H) | 2,190 | 10.1 | <0.0001 | 0.015 | 2,201 | 12 | <0.0001 | 0.020 | |
| Temperature (T) | 2,188 | 7.6 | 0.0007 | 0.0012 | 2,200 | 12 | <0.0001 | 0.022 | |
| Po*H | 6,190 | 2.1 | 0.055 | 0.0062 | |||||
| Po*T | 6,188 | 2.2 | 0.047 | 0.0030 | |||||
| Po*S | 3,193 | 7.8 | <0.0001 | 0.017 | 3,205 | 7.1 | 0.0001 | 0.016 | |
| Development time | Po | 3,8.8 | 2.7 | 0.11 | 0.064 | 3,9 | 2.8 | 0.10 | 0.064 |
| S | 1,190 | 1.8 | 0.19 | 0.0005 | |||||
| H | 2,188 | 4.0 | 0.021 | 0.014 | 2,199 | 4.5 | 0.012 | 0.017 | |
| T | 2,185 | 38 | <0.0001 | 0.19 | 2,195 | 53 | <0.0001 | 0.27 | |
| Po*H | 6,188 | 2.8 | 0.014 | 0.031 | 6,198 | 2.3 | 0.034 | 0.025 | |
| Po*T | 6,185 | 1.5 | 0.19 | 0.0050 | |||||
| Po*S | 3,192 | 1.9 | 0.13 | 0.010 | |||||
| DGR | Po | 3,11 | 0.26 | 0.85 | 0 | ||||
| S | 1,196 | 0.64 | 0.42 | 0 | |||||
| H | 2,192 | 25 | <0.0001 | 0.14 | 2,209 | 40 | <0.0001 | 0.23 | |
| T | 2,189 | 18 | <0.0001 | 0.10 | 2,207 | 22 | <0.0001 | 0.12 | |
| Po*H | 6,192 | 0.78 | 0.59 | 0 | |||||
| Po*T | 6,195 | 0.47 | 0.83 | 0 | |||||
| Po*S | 3,195 | 1.8 | 0.15 | 0.0084 | |||||
- ‘Population’ refers to the geographical origin (see Table 1). Reduced model was obtained through backward elimination (α = 0.05). Effect sizes are presented as estimates of the proportions of variance accounted for by respective factors (semi-partial ω2; some negative estimates are replaced with zeroes; SAS Proc GLM). See the Material and methods section and Meister et al. (2017) for the definition of DGR.
Among-population differences in development time
In the common garden experiment, larval development times in the final instar were consistent with the differences in body size: the largest lowland Georgian individuals took the longest to develop, whereas the development times were the shortest for the smallest Estonian individuals; the hybrids were again intermediate in this respect (Figure 1B). The alpine Georgian population had slightly shorter development time compared to the lowland Georgian population. Over the four groups, the difference in the development time of the last instar was non-significant (Table 2), but note a highly significant difference in the growth monitoring data (below).
Total larval development time recorded in the monitoring experiment was 1.2× longer for lowland Georgian than for Estonian moths (mean ± SEM = 43.0 ± 1.2 vs. 36.5 ± 0.98 days; F1,10 = 16.9, P = 0.002).
Among-population differences in growth rate
Differential growth rate (DGR) in the last instar, recorded in the common garden experiment, did not differ significantly among populations. The proportion of variance in DGR explained by population was estimated to be zero (Table 2). Similarly, population*sex, population*host plant, and population*temperature interactions were not significant, despite the strong and significant main effects of temperature and host plant.
Monitoring development: origin of the size differences
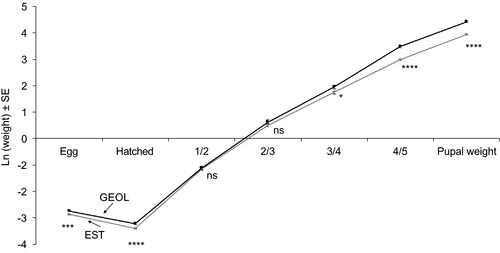
In the sample of individuals for which the development was monitored in detail, all the larvae developed through five instars. Comparing the populations with largest (lowland Georgia) and smallest (Estonia) individuals, egg mass was 1.1× higher in the former, on average, similar to the mass of newly hatched larvae (1.2×) (Figure 2). Curiously, the size difference was lost by the time the larvae molted from first to second instar. The among-population body mass difference (lowland Georgia larger than Estonia) reappeared when the larvae were molting from third to fourth instar, and was present thereafter until pupation. Consistently, relative mass increase within the first instar was 1.3× higher for Estonian than for lowland Georgia larvae (Figure 3A), whereas the opposite was true in the succeeding instars (1.1–1.2× higher within-instar increase in Georgian insects).
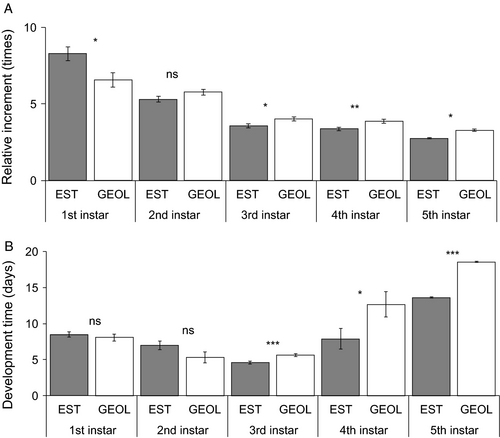
Starting from the third instar, within-instar development time was significantly higher for the lowland Georgian population – relative differences for instars 3, 4, and 5 were 1.2, 1.6, and 1.4×, respectively (Figure 3B). In the first two instars, the development time was longer in the Estonian population (1.1 and 1.3×, respectively), but these differences were not significant. All these patterns were highly consistent between the two sexes (Tables S31–S37; Figures S1 and S2) and reasonably consistent among broods (Figure S3).
Adult longevity and egg numbers by populations
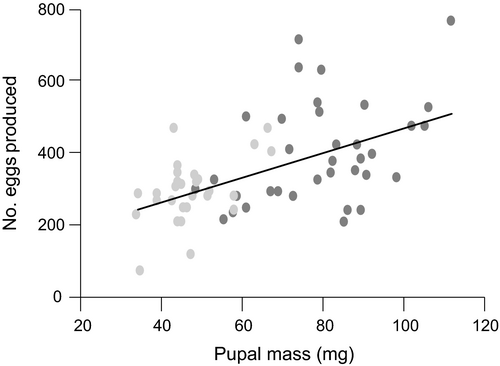
The larger Georgian moths had a 1.2× higher adult longevity (6.4 vs. 5.5 days; 2-way ANOVA: F1,103 = 6.3, P = 0.014) than the smaller Estonian ones; sex did not have an effect (F1,103 = 0.67, P = 0.41). When population*sex interaction was added to the model, it did not attain significance (F1,102 = 0.09, P = 0.76). Total egg production per female was 1.4× higher in the Georgian population (1-way ANOVA: F1,61 = 11.4, P = 0.0013; Figure 4). Overall, the females succeeded to lay 69% of their total egg supply in the experimental conditions. There was no difference among the populations in terms of oviposition success (eggs laid/eggs produced; Kruskal-Wallis test: χ2 = 0.0003, P = 0.99). When female body mass was accounted for, i.e., in the ANCOVA model with pupal mass as a covariate and study year as an additional factor, Georgian moths were found to produce fewer eggs (LSMEANS of populations 319 vs. 389, respectively). However, the effect of population remained non-significant (F1,59 = 2.91, P = 0.09) in this analysis. Similarly, the population*pupal mass interaction did not attain significance (F1,58 = 2.32, P = 0.13) when added to the model.
Discussion
Differences among populations
Our study revealed clear genetically based differences in adult body size among geographical populations of a geometrid moth. Pupal masses were almost twice as high in lowland Georgian populations of E. atomaria than in Estonian populations, situated about 1 900 km to the north. Moths originating from an alpine habitat in Georgia (about 100 km apart from the lowland site) had pupal masses intermediate of the two extreme populations.
Among-population size differences were persistent across host plants and rearing temperatures (no population*environment interactions). This justifies the interpretation that the difference is in ‘size as such’, and does not indicate local adaptations to host plants or thermal conditions. In particular, any dependence of the size difference on rearing conditions would have allowed us to alternatively interpret the pattern as evidence of population-specific plastic responses. For example, had the Georgian moths been larger under higher temperatures only, this could have indicated an adaptation of these populations to warmer conditions, and not that the Georgian moths are larger in general.
Although phenotypic size differences are frequently found among insect populations, controlled common garden rearing experiments are necessary to reveal the genetic background. For Lepidoptera in particular, several studies report genetically based geographical differences in body size. Larger sizes at lower latitudes – the pattern characteristic of E. atomaria – has been found frequently (Blanckenhorn & Demont, 2004; Nygren et al., 2008; Meister et al., 2017) but not universally (Chown & Gaston, 2010; Kivelä et al., 2012; Barton et al., 2014; Horne et al., 2015). With one notable exception (Arnett & Gotelli, 2003), the among-population difference documented in most previous studies were substantially smaller than the almost two-fold mass difference detected for E. atomaria in the present work. E. atomaria offers therefore a case most suitable for studies on causes and consequences of genetic within-species size differences.
As compared to body sizes in the adult stage, less data is available on among-population differences in egg size. This also applies to studies on the relationship between egg size and adult body size at the level of among-population comparisons. Similar to our results, egg size has been found to have a negative association with latitude in Lepidoptera (García-Barros, 1992) and Heteroptera (Blanckenhorn & Fairbairn, 1995). However, positive associations of egg size with latitude have been recorded more often (Blau, 1981; Harvey, 1983; Solbreck et al., 1989; Azevedo et al., 1996; Parry et al., 2001; Berthiaume et al., 2009). The among-populations difference in egg mass of 1.1×, documented in the present study, is well within the range of values reported previously (up to 1.5×; Solbreck et al., 1989). Furthermore, in addition to positive correlations between egg and body size across species (Berrigan, 1991; Davis et al., 2012), positive among-population correlations between these traits have sometimes been reported at the within-species level (e.g., Blau, 1981; García-Barros, 1992; Czesak & Fox, 2003).
Monitoring the immature development of the insects allowed us to shed light on the ontogenetic determination of the among-population differences. Overall, the case of E. atomaria confirms the emerging understanding that whenever larger body size is ‘needed’ in insects, it is achieved by means of a longer growth period and not through an increase in DGR (Fischer & Fiedler, 2001; Tammaru et al., 2010; Esperk et al., 2013; Meister et al., 2017; for some examples). In E. atomaria, the 1.6-fold among-population difference in pupal masses corresponded to a 1.2-fold increase in larval development time (‘monitoring experiment’). Unlike in many other cases (Esperk et al., 2007), the longer development time in the southern E. atomaria is not accompanied by a difference in the number of instars which appears to be invariable in the species studied. Instead, the larger size of Georgian moths is attained through higher relative mass increments in several instars. This is analogous to the pattern of the ‘accumulation of size difference’ in the case of SSD (Tammaru et al., 2010) and supports the idea about physiological constraints on within-instar growth patterns. In particular, it appears that larger size cannot be attained by a major modification of growth patterns within one single instar, such a difference has to be accumulated in the course of several instars. Recent studies have suggested that oxygen limitation may constitute the proximate basis of the constraint on within-instar mass increment (Greenlee & Harrison, 2004; Callier & Nijhout, 2011; Kivelä et al., 2016a,b).
A peculiar pattern found in E. atomaria is, however, the ‘disappearance’ of the size difference in live mass during the early larval development. Both the eggs and neonate larvae were larger in the lowland Georgian population but the difference was lost by the time the larvae molted from their first to second instar. We are unaware of any studies showing an analogous pattern in insect growth trajectories. This phenomenon may deserve further attention, irrespective of whether such pattern will appear to be unique to E. atomaria or not. In particular, attention is needed to the question to which extent the (disappearance of) size differences is related to a change in cell number, as opposed to a change in cell size (Arendt, 1997; Azevedo et al., 2002; Blanckenhorn & Llaurens, 2005; Vijendravarma et al., 2011; Chapman et al., 2013). Such detailed information is currently not available for E. atomaria, therefore appropriate caution is needed when interpreting the disappearance of the among-population difference in live larval masses as the absence of the effect of egg size on further development. For example, differences in egg size could still be carried over between developmental stages by means of a difference in cell number.
Evolutionary ecology of growing larger
Estonian (58°N) representatives of E. atomaria were smaller as adults than their Georgian conspecifics further south (41°N). The most popular explanation for such a ‘converse Bergmann's’ (Blanckenhorn & Demont, 2004) latitudinal difference relies on the idea that selection on short development time – due to seasonal time constraints at higher latitudes – has a correlated effect on body size (Masaki, 1967; Roff, 1980; Iwasa et al., 1994; Chown & Gaston, 2010). This explanation is indirectly supported by the frequently found parallel latitudinal clines in juvenile development time (Masaki, 1967; Telfer & Hassall, 1999; Kivelä et al., 2011; Välimäki et al., 2013; this study). In the specific case of E. atomaria, support is also provided by the fact that the moths were much smaller – as compared to the lowlands – in the alpine habitat of Georgia, similar to Estonia in terms of season length.
Nevertheless, the primary role of season length as a selective factor for low body size in northern and alpine populations of E. atomaria appears unlikely. This is because this species is strictly univoltine in northern Europe and the length of, e.g., Estonian summer is more than sufficient for completion of its single generation. The hypothesis ‘time constraint limits body size in the northern areas’ therefore does not appear plausible (see Tammaru et al., 2001; for an example of another geometrid species with a similar ecology). Moreover, the broadly polyphagous larvae cannot be limited by the availability of host plants in suitable phenological stage, considering in particular the fact that some of the preferred host plants [Calluna vulgaris (L.) Hull] are evergreen (see Vellau & Tammaru, 2012, for more species-specific discussion).
Predation pressure on larvae is potentially a powerful selective factor on development time and body size in insects (Teder et al., 2010). Mortality through bird predation may well reach values as high as 5% per day, with just moderate variations in this figure being sufficient to cause optimal body sizes to differ about twofold (Remmel et al., 2011). Accordingly, natural selection may favor a smaller body size in the north/mountains, if predation risk in such habitats is systematically higher than in lowland habitats of southern Europe. Currently, there appear to be no data available to evaluate whether this is actually the case (but see Roslin et al., 2017). However, if an adaptive response to varying predation levels were the primary reason for the among-population differences in E. atomaria body size, it would be hard to explain why E. atomaria is the only species that is so strongly affected. Indeed, the abundance of insectivorous birds in particular should have a similar effect on many lepidopteran species with folivorous larvae, yet these large latitudinal differences in body size are rare among the European representatives of the order. In addition to predators, parasitoids can have the potential to inflict selection on growth parameters of their hosts (Solbreck et al., 1989; Teder et al., 1999; Kingsolver et al., 2012). With no information on geographic differences in parasitoid complexes of E. atomaria available, there is currently no way to assess whether parasitoids contribute to the geographically different optima of body size in this species.
Mortality during the larval stage likely constitutes the primary cost of growing larger. The optimal timing of maturation (pupation) decision – the proximate determinant of adult body size – must also depend on fitness benefits of large body size. E. atomaria is a capital breeder (Davis et al., 2016): the females enclose from pupae with most of their eggs fully developed (Javoiš et al., 2011). This implies a strong correlation between female size and fecundity, and thus a strong fitness advantage of being a large adult (Honěk, 1993; Tammaru et al., 1996, 2002; Fischer & Fiedler, 2001). The results of this study show that the fecundity advantage of size extrapolates also to the among-population comparison: fecundity of the large Georgian moths considerably exceeded that of their smaller Estonian conspecifics, whereas the counteracting effect of geographically variable egg size proved to be too weak to substantially affect the relationship. The among-population difference in fecundity should translate to a corresponding difference in adult fitness as there was equal oviposition success (eggs laid/eggs produced) of the females from the two populations (Estonian and lowland Georgian), and just minor differences in longevity.
The among-population difference in final body size was associated with a corresponding difference in egg size. The difference in egg size was carried over to the size of newly hatched larvae. In the literature, geographic differences in insect egg size have been interpreted as adaptations to various levels of predation or starvation risk of the neonates (Solbreck et al., 1989), or extreme temperatures (Brittain et al., 1984; Azevedo et al., 1996). However, the adaptive value of laying larger eggs is also frequently seen in ‘being large from the beginning’ (Parry et al., 2001; Berthiaume et al., 2009). This implies that larger initial size may contribute to attaining the larger adult size in due time, especially when growing period is limited.
The fact that the larger size of Georgian larvae transiently disappeared in the course of larval development leads us to the conclusion that ‘being large from the beginning’ (in the sense explained above) is unlikely to constitute the adaptive significance of the larger eggs in that population. Indeed, vanishing of the size difference in the course of larval development suggests decoupling initial and final sizes of an individual (considering however that there may be carry-over effects in other parameters like cell number, see above). Instead, we may even speculate that egg size has been a primary target of natural selection, and the large adult size of the females has evolved to facilitate laying large eggs. This idea is supported by the strong relationship between body sizes and egg sizes found in a cross-species comparative study on Geometridae (Davis et al., 2012), suggesting a mechanistic link between maternal size and egg size. We hypothesize that selection for large size of eggs and new-born larvae in lowland Georgian populations appears plausible as they are faced with much higher temperatures and, most likely, also with more xerophytic vegetation which must be tougher to chew and digest. This hypothesis awaits experimental testing.
This study demonstrates that detailed monitoring of immature development of the insects can yield information which is useful also in the context of evolutionary ecology, and demonstrates the potential of such an integrative approach (see Esperk et al., 2013, for an analogous case). We are currently unable to identify selective pressures responsible for the observed among-population difference in body size. Nevertheless, knowing that larger sizes are attained by means of prolonged instars, we learn that increased juvenile mortality must form a substantial cost of large adult size. Moreover, we were able to refute the ‘being larger from the beginning’ hypothesis of the benefits of large egg size. We concluded that larger eggs should have an adaptive value per se, or they represent just a mechanistic consequence of larger maternal body size.
Acknowledgements
We thank Ando Vaan, Annika Suu, Eve Möls, Hedvig Liblikas, Liisi Laks, Niko Rainer Johansson, Sonja Viinamäki, and Tiina Stanevitš for help in the lab. We thank Freerk Molleman, Mari-Liis Viljur, Robert Barry Davis, Sami Mikael Kivelä, Sille Holm, Tiit Teder, Toomas Esperk, and Virve Sõber for comments on the manuscript, and George Japoshvili for his help with expeditions to Georgia. The study was supported by institutional research funding IUT (IUT20-33) of the Estonian Ministry of Education and Research. All data have been deposited in public digital repository figshare: https://doi.org/10.6084/m9.figshare.5002187.v6.




