Impact of Life History on Hippopotamus Skull Ontogeny
ABSTRACT
The biological processes underlying the wide phenotypic mammal diversity are still not thoroughly understood. In this study, we examined how major stages in the life history of the common hippopotamus (Hippopotamus Amphibius) influence its craniomandibular morphology throughout ontogeny. Using geometric morphometrics and life-history meta-analysis correlations, we characterized skulls from 198 individuals spanning 20 developmental stages. The most significant morphological changes were observed during early infancy (0–3 years), coinciding with lactation and weaning, and during puberty (10–15 years), coinciding with reproductive maturation. These findings align with growth patterns typical of social mammals exhibiting high sexual dimorphism. Notably, we identified a pattern previously undocumented in any other vertebrate: cranial morphology stabilizes earlier than the mandibular one. Specifically, late-stage (20–25 years) shape modification in the mandibles indicates progressive reconfiguration of masticatory biomechanics as well as a continuous change of dental occlusion throughout life. This pattern is common in both male and female individuals and may be related to shifts in diet rather than sexual selection. This study provides the most comprehensive ontogenetic dataset for a semi-aquatic, large semigraviportal mammal with a polygynous social structure, offering a valuable foundation for future evolutionary studies based on comparative analyses.
Summary
-
Hippopotamus undergoes major skull changes in infancy (0–3 years) and puberty (10–15 years).
-
Cranial shape stabilizes earlier than mandibular one, a unique trait.
-
Late-life (20–25 years) mandibular shifts suggest ongoing dietary adaptations.
1 Introduction
Skull morphology in vertebrates is closely linked to vital functions such as feeding, breathing and communication, and is affected by a complex interplay of intrinsic and environmental factors (Goswami et al. 2023). Life-history traits (events that characterize the changes in life strategies during an individual's life cycle) have the potential to both shape and constrain craniomandibular phenotype, particularly during size-shape allometric changes throughout growth (Goswami et al. 2023; Zelditch et al. 2004; Tanner et al. 2010). Many processes of phenotypic change have been shown to be closely related to life-history modifications throughout an individual's life, including changes in specific systems such as the nervous system or general body variations such as body mass (Bogin 1999). Many of these novel adult phenotypes are the results of allometric modifications happening in existing characters via ontogeny (Gould 1968), that can be driven by both intrinsic (such as sexual dimorphism (Shea 1986; Badyaev 2002) and extrinsic (such as ecomorphology (Svanbäck and Eklöv 2002); Klingenberg 2005; Rivera et al. 2024) patterns. Prominent among these are those related to the attainment of sexual maturity and reproduction, including the end of the immature stage, the onset of puberty, and associated ethological changes (Laws 1968; Dittrich 1976). However, our understanding of the impacts of life-history traits on phenotypic variation through ontogeny in non-primate mammals is extremely limited (Goswami et al. 2023; Tanner et al. 2010; Purvis and Harvey 1995; Galatius et al. 2011).
In this regard, the common hippopotamus (Hippopotamus amphibius) presents an interesting case study due to its unique morphology and ecology, with no analogues among extant large mammals (Eltringham 1999). The polygynous social structure typical of hippopotamuses (Eltringham 1999; Verheyen and Verheyen 1954; Lewison 1998; Klingel 2013; Shannon et al. 2021) implies a great impact of sexual selection on their behavior (Laws 1968; Shannon et al. 2021). Their morphological adaptations reflect not only a semi-aquatic lifestyle, but also the evolutionary pressures on an ecological-evolutionary strategy reserved for few mammalian groups (Weston 1997; Boisserie 2002; Reidenberg 2007). In turn, the need to support a body weight that can exceed 3000 kg in their terrestrial incursions (Coughlin and Fish 2009) is reflected in adaptations of the skeleton that denote both semigraviportal (Hutchinson and Pringle 2024) and aquatic habits (Reidenberg 2007; Barklow 2004). The role of hippos as ecosystem engineers of freshwater habitats throughout the Quaternary reinforces the particular interest for a proper understanding of their autecological processes (Hyvarinen et al. 2021; Voysey et al. 2023).
The scarcity of detailed information on the evolutionary processes that led to the unique ecology of the genus Hippopotamus is remarkable (Hyvarinen et al. 2021; Weston and Boisserie 2010). Nevertheless, the craniomandibular fossil record of this genus in Africa and Europe is relatively extensive (Harris 1991; Mazza 1995), including individuals at various ontogenetic stages (Martinez-Navarro et al. 2022; Fidalgo et al. 2024) and covering a time range spanning virtually the entire Quaternary (Fidalgo et al. 2021; Pandolfi et al. 2023; Martino et al. 2024a). However, understanding the evolutionary processes underlying the phenotypic changes identified, as well as assessing possible divergent ontogenetic trajectories, requires a detailed analysis of hippopotamus living representatives (Weston 1997; Boisserie 2002). The possibility of investigating relevant evolutionary processes, such as brain development (Weston and Lister 2009) and variation in craniofacial morphology (Weston 2003; van der Geer et al. 2018; Pandolfi et al. 2020; Martino et al. 2024b), depends on the availability of robust ontogenetic data that allow to test hypotheses within phylogenetic, chronological, geographical and ecological contexts. Furthermore, the correct association between life-history events and phenotypic changes during development offers an opportunity to explore the presence of distinct ethological patterns in hippopotamuses throughout their evolutionary history (Martinez-Navarro et al. 2022; Weston 2003; Martino et al. 2024b). On the other hand, numerous efforts have been made to understand ontogenetic processes in cetaceans (Galatius et al. 2011; Mattson et al. 2006; Daniel and James 2013; Fortune et al. 2020; Lanzetti et al. 2022a, 2022b, 2023), the group of living mammals most closely related phylogenetically to hippopotamuses (Gatesy 1997; Nikaido et al. 1999). The availability, for the first time, of large craniomandibular ontogenetic series of hippopotamus would allow comparison of the evolutionary constraints affecting its development with those observed in cetaceans, thus providing a basis for assessing key episodes in mammalian evolution, such as the divergence of aquatic habits in Cetartiodactyla (Reidenberg 2007; Boisserie et al. 2011; Orliac et al. 2023).
This study presents for the first time a complete craniomandibular ontogenetic series of the common hippopotamus, and investigates these in detail using 3D geometric morphometrics. Previous work on hippopotamus skull morphology has proposed in a preliminary way that life-history traits can influence phenotypic changes throughout development (Weston and Lister 2009; Weston 2003; van der Geer et al. 2018; Martino et al. 2024b). The end of lactation and the onset of sexual behavior have been proposed as key physiological and phenotypic change points (Martino et al. 2024b). However, the previous study dealt with a smaller dataset and did not test the influence of life-history patterns on the general shape of the skull. In this study, we examined the correlation of life-history traits with skull shape during ontogeny. Specifically, we evaluated changes in cranium and mandible shape in relation to size and life-history traits from a meta-analysis. Our dataset included 183 crania and 172 mandibles (198 individuals) representing all 20 ontogenetic stages proposed by Laws (Laws 1968). The main hypothesis investigated in this study focus on whether the craniomandibular phenotypic changes in Hippopotamus amphibius individuals throughout their lifetime are affected by their unique ecological characteristics, diverging from typical growth patterns observed in other mammals [see stages of mammalian growth in Bogin (1999)]. The analysis employed the von Bertalanffy growth curve to define these ontogenetic patterns (von Bertalanffy 1957). In cases where a process modifying the typical generalized mammalian growth pattern has been detected, the possible involvement of other phenomena causing intraspecific phenotypic variation, such as sexual maturation or changes in ecological habits, has been discussed in detail.
2 Results
2.1 Cranioandibular 3D Geometric Morphometrics
3D geometric morphometric analyses using a combination of fixed and sliding/semi-landmarks (see Section 4; Extended Data Figures S1 and S2) revealed that in shape, the first principal component (PC1) explains almost half the variation in the cranium and a third in the mandible (Figure 1a,b). In both structures, PC1 is strongly correlated with age and size (Figure 1). The remaining principal components (PC) show relatively little variation and seem to be associated with minor differences between individuals of the same age classes.
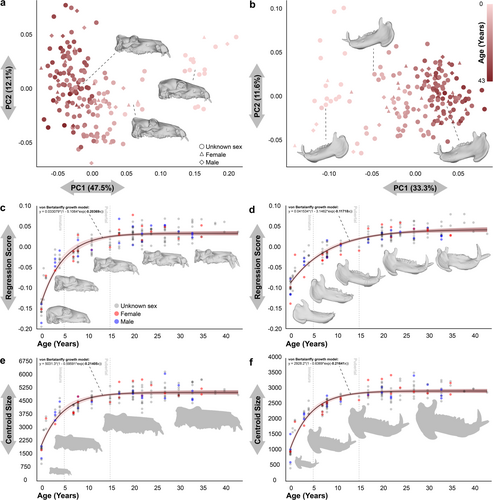
The pattern of change with age in cranial shape conforms to the typical ontogenetic pattern in mammals, comprising an initial stage of very rapid change, usually associated with lactation, a second stage of reproductive or pubertal development, and an asymptotic phase at sexual maturity (von Bertalanffy growth coefficient = K = 0.20; Figure 1c) (Bogin 1999). The significantly lower growth rate and a delay in the stabilization of the mandibular shape stands out (K = 0.12; Figure 1d). This mandibular development during maturity highlights the presence of another underlying biological phenomenon, which departs from classical ontogenetic patterns in mammals and has a high impact on shape variability. The growth in size of both the cranium (K = 0.21; Figure 1e) and the mandible (K = 0.22; Figure 1f) show a standard pattern, with more than half of the accumulated change in the immature stage, and a last phase of change in values and degree of variability in the pubertal stage before sexual maturity.
Shape and size in both crania and mandibles are highly correlated (OLSR, R2 = 0.92, p < 0.0001; R2 = 0.81, p < 0.0001, respectively) (Extended Data Figure S3a,b). In the case of the mandible, the allometry is noteworthy, with slower and more gradual variation in shape by age than in the cranium (Figure 1d,e). The allometric pattern present in the mandible is peculiar, fitting significantly better to a quadratic function than to a linear correlation (Linear regression model Akaike IC = 4.1 < Quadratic model Akaike IC = 6.2) (Extended Data Figure S3b). Interestingly, despite these differences in the rate of variation with respect to age, there is a greater correlation of shape against size for the mandible than the cranium (Extended Data Figure S3c,d). Strongest correlation is between the size of the crania and mandibles (OLSR, R2 = 0.99, p < 0.0001; Extended Data Figure S3c), although in the case of shape the degree of correlation is also high (OLSR, R2 = 0.86, p < 0.0001; Extended Data Figure S3d).
The series of craniomandibular morphological modifications in the ontogeny of this species can be observed in Extended Data Figures S4, S5, S6, S7, S8, and S9, and Extended Data—Supporting Information S20: 3D Models. These phenotypic changes are mainly focused on the relative proportion of the neurocranium to the chewing apparatus (maxillae, premaxillae and mandibles in general) and the overgrowth of the superstructures (apophyses, crests and other craniomandibular projections), going from very globular crania with very high neurocranial proportions in juveniles/newborns to extremely antero-posteriorly elongated crania in adults. Other notable changes with ontogeny are the great projection of the orbits, the occipital condyles, the occipital superstructures, the zygomatic processes, the mandibular angles and, in general, the alveolar areas of the entire anterior dentition (incisors and canines).
2.2 Modeling and Meta-Analysis
The ontogenetic patterns presented by each trait can be easily divided according to their growth coefficient in the von Bertalanffy growth curve. In those models with a coefficient greater than 0.15, there is a tendency for an abrupt change early in life (immature stage), which gives way to an attenuated change during puberty to reach the asymptotic stage practically coinciding with the onset of adulthood (Figure 2). This is the case for cranium shape and size, mandible size, height, or cranial capacity. The same pattern is observed in the degree of fusion of the mandibular symphysis (Extended Data Figure S10d) (Laws 1968). In the majority of these cases, the sharpest change in values occurs mainly in the suckling period, which never exceeds 2 years of age (Martino et al. 2024b).
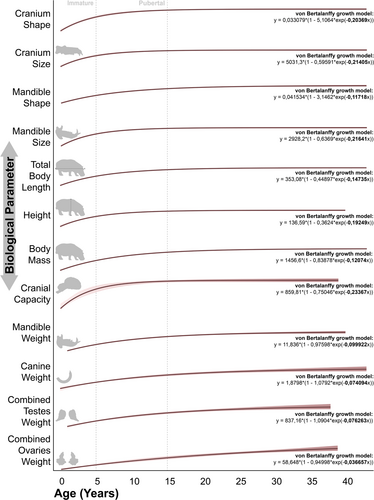
On the contrary, in the traits with von Bertalanffy coefficients lower than 0.15, more gradual change is observed throughout ontogeny, with practically no rate changes between the life-history stages mentioned above (Figure 2). In some cases, this pattern is related to a late stage of phenotypic change that is mainly observed in males and is related to the display of secondary sexual characters, as in the case of the weight of the mandible and lower canines (Extended Data Figure S11) (Laws 1968; Shannon et al. 2021). In the case of the gonads this pattern is especially different from the general ontogenetic pattern (see original data in Extended Data Figure S10l,m) and it is very likely that the data fit more appropriately to another model of change due to the very late onset of expression of the hormones that determine the changes in these organs (Emerson 2000). More particular is the case of the change in total body length and mass of individuals, which despite not showing a pronounced sexual dimorphism (Shannon et al. 2021), present new stages of change later in the life of the organisms, moving away from the typical generalized mammalian growth pattern (Figure 2). Interestingly, the growth pattern of total body length and mass of individuals seems to be the most similar to the one we observed in the case of mandibular shape (Figure 2).
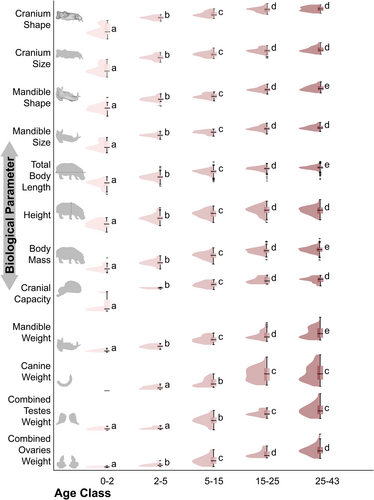
In most of the data evaluated, we found the typical generalized mammalian pattern of change between age classes, being especially significant between the pre- and post-weaning stages, notably between late infancy and puberty and with some between puberty and adulthood, which should not show significant changes throughout its duration (Figure 3). Specific cases such as combined testes weight, which does not change significantly until puberty, deviate from this pattern. Other peculiar cases are those of mandibular and canine weights, which show change during adulthood (at least statistically significant in the case of the mandible) and a large bimodality in late adulthood (Figure 3; Extended Data Figure S11). This bimodality is easily explained by sexual dimorphism in the development of secondary sexual characters. Interestingly, we also found significant changes within adulthood in the case of mandibular shape, total body length, and body mass, in this case without associated data bimodality (Figure 3; Extended Data Figure S11). For a detailed summary of the factors assessed in the consideration of the ontogenetic pattern, see Extended Data Table S1.
3 Discussion
3.1 Cranial Phenotype and Life-History Traits Across the Ontogeny in Hippos
Our results highlight a dramatic change in craniomandibular anatomy along with other body parameters (such as body size or, especially, cranial capacity; Laws 1968; Shannon et al. 2021; Boisserie 2002; Weston and Lister 2009; Martino et al. 2024b) in the early stages of infancy, between birth and 3 years of age. A relationship of this early reconfiguration of cranial structure has been attributed to factors such as weaning, which occurs before the age of 2 years (Martino et al. 2024b) and mainly between 6 and 8 months (Eltringham 1999; Lang 1975). Indeed, as observed in our results, many of the modifications in the craniomandibular anatomy at this stage of development (e.g., enlargement of the muscle insertion zones in the temporals, reconfiguration of the alveolar space in the mandible or the projection of the gonial angle) seem to be related to the structure and functionality of the masticatory apparatus (Hiiemae 1978; Weijs 1980), so this potential relationship seems to be well founded.
A marked increase in cranial capacity occurs during infancy (Weston and Lister 2009), which is accompanied by a rapid change in facial structures such as the relative projection of the orbits or the alveolar areas of the canines and incisors. Early structuring of the central nervous system is a typical and well-known process in mammals (Bogin 1999) and is often linked to the sudden need for independence of the offspring after weaning for survival (Herring 1985). More specific is the splachnocranial projection, linked to positive craniofacial allometry (CREA) following the suggestion that larger species/individuals/ontogenetic stages in mammals tend to become long-faced as they diverge in size (Cardini and Polly 2013; Andrea 2019). In turn, these anatomical changes may be related to the semi-aquatic lifestyle of hippopotamuses (Reidenberg 2007). Although there are multiple hypotheses that assign characteristics more suited to an aquatic lifestyle to infant individuals than to adults (e.g., greater buoyancy or webbed feet) (Coughlin and Fish 2009; van der Geer et al. 2015). Indeed, Verheyen (Verheyen and Verheyen 1954) reported observations of infant individuals actually swimming. Likewise, the elevation of the orbits and the projection of the nasals with their involvement in the elevation of the nostrils, which in turn define the adult cranial shape of the common hippopotamus, are features that have been typically related to a more aquatic behavior (Boisserie et al. 2011; van der Made et al. 2015).
The second moment in the life cycle of hippopotamuses that raises several questions is the correlation between phenotypic changes and the attainment of sexual maturity (Martino et al. 2024b). The exact age of sexual maturation in hippopotamuses is indeed highly variable among individuals, especially if their development has occurred in different contexts (Dittrich 1976; Sayer and Rakha 1974). Even so, it seems clear that in captivity first offspring can be observed in both females and males at an average age of 5 years (Dittrich 1976). However, if we focus on hippopotamus individuals inhabiting nature reserves, everything seems to point to an onset of puberty around 6–7 years of age in the wild with the attainment of maturity and conclusion of puberty in all individuals at around 15 years of age (Sayer and Rakha 1974; Marshall and Sayer 1976; Smuts and Whyte 1981). Very similar information is provided by data on the weight and production of the testes and ovaries, which begin their pronounced development in 5 years old hippopotamus individuals (Smuts and Whyte 1981). In the case of the testes, the onset of spermatogenesis coincides with this age and growth changes speed at the end of puberty, whereas in the ovaries, growth continues with the same trend throughout the life of the individual.
According to our results, a cranial phenotype very similar to that of the adult is structured in the pubertal stage, reaching stabilization of shape and size with the definitive maturity of all individuals. This phenomenon does not extend to the mandible, which seems to follow a similar dynamic of morphological change to that found in infancy. Individual shoulder height seem to respond in a very similar way to the cranium, reaching adult size by the end of the pubertal stage (Shannon et al. 2021; Marshall and Sayer 1976). As in most mammals (Kilborn et al. 2002), it is during puberty and around the final establishment of maturity that the full development of much of the musculoskeletal system is reached, with the fusion of most of the epiphyses and sutures of the post-cranial skeleton, with the exception of some vertebral discs and bony sutures of the girdles (Extended Data Figure S12) (Weston 1997). All this information points to a high correlation between the development of reproductive traits and musculoskeletal system that enables independence and reproduction in individuals between 5 and 15 years of age (taking into account the large amplitude of this interval due to the incorporation of data from zoo and wild individuals). At the social level, these still young males are structured in subgroups within the same group (bachelors) as long as they maintain a non-confrontational profile with the territorial males, delaying the age of the first courtship displays (Eltringham 1999).
With the onset of adulthood, dental eruption and the fusion of the mandibular symphysis reach the adult phenotypical stage (Laws 1968; Boisserie 2002). In many species, sexual dimorphism develops late in ontogeny and mainly in size differences between the sexes (Lindenfors et al. 2007; Pérez-Barbería et al. 2002; Roylance-Casson 2021). This phenomenon is strongly related to the expression of different development rates (e.g., in the rate of body growth or in the development of hypertrophy of an anatomical element) during ontogeny between females and males (Isaac 2005). In hippopotamuses, this difference in the development of most body structures between sexes does not seem to occur, with sexual dimorphism mainly reflected in the bimodality of canine and mandibular weights in adults [for more information see Shannon et al. (2021)]. It is expected that the greatest development of sexual dimorphism in canines and mandibles occur at the same age when young adult males acquire territorial behaviors and intentions to dominate the groups in which they have grown up (Eltringham 1999). If these observations were confirmed, it would be very interesting to test the relationship between the triggering of canine hypertrophy by late hormone expression and the onset of territorial ethological patterns (Emerson 2000).
In the general pattern of ontogenetic change in mammals (Bogin 1999; Shea 1983, 1985a, 1985b; Leigh and Shea 1995), and specifically in hippopotamuses (Weston 2003), much of the process of phenotypic growth modification has an allometric explanation. It is precisely the different allometry patterns between the elements of the craniomandibular system that are most affected during life-history and evolutionary processes, generating the diversity of forms that we currently find in mammals (Lanzetti et al. 2022a, 2023; Goswami and Prochel 2007). In our case, particularly striking are the differences in the variation rate in the shape and size observed between the mandible and the cranium. This result suggests the presence of a mismatch in the growth rate between these two structures. This discrepancy could be related to the pronounced sexual dimorphism of the mandible and, in particular, of the lower canines. Surprisingly, when looking at the data on mandible shape and size, it is not possible to discern any sexual bias or bimodality in the distribution of the data at any ontogenetic stage (see Figure 3 and Extended Data Figure S11). In the case of the mandible, the quadratic model to which its allometry fits could be explained by a first phase in its ontogeny in which the variation in size did not entail any change in its shape. This process could be explained, in part, by a phase of growth that was not associated with any dental emergence event or its associated morphological reconfiguration (Laws 1968; Weston 1997; Avedik and Clauss 2023). Likewise, in the mandibular shape, a final stage of change seems to emerge before the stabilization of the mature adult phenotype. This last stage of change is not observed in the shape and size of the cranium nor in the height of the individuals. Instead, a similar pattern is observed in total body length or body mass. It is easy to relate the continuous change during adulthood of these latter parameters to the pattern of food intake (Lui and Baron 2011; Mumby et al. 2015). If we look in more detail, the main anatomical changes that occur in the mandible during adulthood are centered on the increased development of the muscular attachments of the masseter muscles that produce an extension of the gonial angle. These morphological changes have major implications for the masticatory biomechanics that are progressively reconfigured with the continuous modification of dental occlusion (Avedik and Clauss 2023). It is important to mention intraspecific agonistic behavior as another possible biological feature related to this morphological trait. Although territorial males are the main source of agonistic behavior, females readily respond to it. Both males and females have constantly growing canines and incisors that they use in fights, and this ethological phenomenon may be an important driver of mandibular morphology and its change over time (Herring 1985; Avedik and Clauss 2023).
3.2 General Growth Patterns
In general, four distinct patterns of change during the lifespan of individuals could be characterized in the hippopotamus body structures assessed. The first is that of the internal organs and skeletal system, with a rapid initial phase of change followed by attenuated change during puberty and a final asymptotic phase in adulthood. This pattern is common for such body systems in all mammals (Tanner et al. 2010; Bogin 1999; Shea 1986; Galatius et al. 2011; Mattson et al. 2006; Fortune et al. 2020; Shea 1983, 1985a, 1985b) and, as expected, fits most hippo traits evaluated in the meta-analysis. The development of the nervous system seems to fit this model best, mainly due to the aforementioned early stabilization of the encephalic structure (Weston and Lister 2009). Directly connected to the latter is cranial development (Lyras 2019).
The second pattern occurs in the elements associated with secondary sexual characteristics and presents a pattern with changes in infant age and puberty that are slower than in the previous case. An extra stage of morphological change is also visible within adulthood, the latter characterized by bimodality in the data reflecting sexual dimorphism. This phenomenon is typical of mammals that show high rates of sexual dimorphism in the later stages of sexual maturity (Shea 1986; Emerson 2000; Lindenfors et al. 2007). The origin of the latter is rooted in the differential selection patterns experienced by female and male individuals (Badyaev 2002; Isaac 2005). These patterns are theoretically more pronounced in organisms with polygynous mating systems that occupy open habitats (Pérez-Barbería et al. 2002; Jarman 1974) which provide environmental conditions more suitable for this new phase of energetic investment (German and Hochberg 2020). As we observed, in the case of H. amphibius this pattern is characteristic of the lower canines but not of the body size, highlighting its ecological peculiarities compared to other ungulates (Shannon et al. 2021).
The third case is present in body sizes and characteristics that are neither skeletal nor closely associated with internal organ maturity, showing ontogenetic changes similar to the second case, but without expressing bimodalities in the data at any stage. This phenomenon is due to a gradual variation in the characteristics of all individuals throughout their lives (Bogin 1999; von Bertalanffy 1957; Jeglinski et al. 2012), with these changes being mainly associated with an increased intake or accumulation of fat in tissues (Lui and Baron 2011). This phenomenon is documented in the post-maturity body mass gain in large mammals with competitive social interaction such as Asian elephants (Mumby et al. 2015) and it is not surprising that hippopotamuses, with their semigraviportal nature (Hutchinson and Pringle 2024), exhibit similar characteristics. More curious is the presence of a similar pattern in mandibular morphology, which in this case we assimilate to changes in the biomechanics of mastication.
The last of the patterns is presented by the gonads, which do not begin to develop until late in puberty and continue to undergo substantial changes throughout the individual's life. Unsurprisingly, this dynamic is associated with a large increase in physiological pathways related to sex hormones production (Bogin 1999; Emerson 2000).
In conclusion, the analysis of a large cranial dataset has provided extensive information on the ontogenetic pattern of hippopotamuses, the only large semigraviportal social mammal with semi-aquatic habits. Specifically, we gain insight into the main stages that characterize the ontogenetic change in Hippopotamus amphibius. As in the vast majority of social mammals, the first years of life are the key for the establishment of the adult craniomandibular structure. These changes are strongly conditioned by the development of the central nervous system and changes in feeding and ethological habits. The sexual maturation phase in turn leads to the stabilization of most phenotypic characteristics of adult individuals, including cranial morphology, full skeletal development, and maturity of the reproductive organs. One of the most striking results obtained is the discovery of an extended/additional late phase of modification of the mandibular phenotype that, interestingly, is not related to the development of sexual dimorphic characteristics in males (i.e., hypertrophy of the lower canines). It is possibly linked to occlusal reconfiguration in adulthood due to dental wear and to an increased food intake, leading to a marked development of the insertions of the temporalis and masseter muscles. This pattern can also be seen in the change in mass and total length of individuals during adulthood. These results are particularly important since hippopotamuses are a peculiar component of the present, as well as the past, megafauna. Future comparative work including these data together with studies of phylogenetically close groups (such as the pygmy hippopotamus, Choeropsis liberiensis) or their extinct representatives (such as hippopotamuses or anthracotheriids, which have a good fossil record) will allow to rigorously address issues such as the ontogenetic origin of adaptations to aquatic life in mammals or the impact of changes in global climate on niche modifications in megafauna through their development.
4 Materials and Methods
4.1 Materials
To representatively assess the change in the craniomandibular phenotype of Hippopotamus amphibius during its life cycle, 198 individuals (183 crania and 172 jaws) have been digitized in 3D. This sample includes individuals of all ontogenetic stages, mainly wild individuals, although some come from zoos. It also includes individuals whose sex in life is known (24 known females and 24 known males) and others for which it has not been possible to obtain this information. The specimens have been digitized by various specialists, using photogrammetric methodologies, structured light scanners, laser scanners, or computed tomography. The materials come from 20 institutions of importance in the study of the natural sciences: D'Arcy Thompson Zoology Museum (Dundee, Scotland, UK), Darwin Museum (Moscow, Russia), Institut Català de Paleoecologia Humana i Evolució Social (Tarragona, Spain), Lapworth Museum of Geology (Birmingham, England), LSA Museum of Zoology University of Michigan (Michigan, USA), Museo de Anatomía Comparada de Vertebrados (Madrid, Spain), Museum of Natural History Wroclaw (Wroclaw, Poland), Museum of Zoology (Cambridge, England), Museum of Vertebrate Zoology (Berkeley, UK), Museu de Ciències Naturals de Barcelona (Barcelona, Spain), Museo di Storia Naturale di Firenze (Florence, Italy), The Natural History Museum (London, England), Muséum National d'Histoire Naturelle (Paris, France), National Museum of Nature and Scien (Tokyo, Japan), Universidade Nova (Lisbon, Portugal), Royal Museum for Central Africa (Tervuren, Belgium), Staatliches Museum für Naturkunde Stuttgart (Stuttgart, Germany), Museo Anatómico Universidad de Valladolid (Valladolid, Spain), University of Wyoming Museum of Vertebrates (Wyoming, USA), Museum für Naturkunde (Berlin, Germany). The data associated with each specimen included in the study can be found in Extended Data Tables S2 and S6.
Some of the specimens included in the analyses showed a certain degree of fragmentation, which has been corrected by merging the symmetrical anatomical part, if preserved (Lautenschlager 2016; Moya-Costa et al. 2019). Blender v.3.1.2 software was used for the symmetrisation of the specimens. The alignment and fusion of the fragments were performed in CR Studio v.1.7.5.086 software. The age estimation of each individual was carried out following the criteria of emergence and dental wear proposed by Laws (Laws 1968), as long as the mandible was present, and those proposed by Martínez-Martinez-Navarro et al. (2022) for isolated crania. The analyses included individuals from the 20 ontogenetic stages defined by Laws (Laws 1968).
4.2 3D Geometric Morphometrics
4.2.1 Templates and Measurement of Specimens
For the quantification of the shape and size of the digitized crania and mandibles has been carried out a study protocol based on 3D Geometric Morphometrics (Lawing and Polly 2010). For this purpose, landmark and semilandmark configurations have been developed to depict the shape of the structures that exhibit the greatest ontogenetic, phylogenetic, and evolutionary signal according to different proposals (Extended Data Figure S1) (Laws 1968; Weston 1997; Boisserie 2002; Weston and Boisserie 2010; Mazza 1995; Martinez-Navarro et al. 2022; Weston 2003; van der Geer et al. 2018; Pandolfi et al. 2020; Martino et al. 2024b; Lydekker 1915; Caloi et al. 1980; Faure 1985; Oliver 1993; Zorić et al. 2018). The anatomical characterization, together with details of each landmark and semilandmark, is described in Extended Data Table S3. Measurement of each specimen was carried out in Viewbox v.4.0 software (dHAL, Kiffisia, Greece), following the methodology described in Bastir et al. (2019).
4.2.2 Sliding Semilandmarks
To ensure mathematical homology between the semilandmarks measured on the different specimens, a sliding process to the template individual has been carried out in the same software beforementioned (Bookstein 1991, 1997; Gunz et al. 2005, 2009; Gunz and Mitteroecker 2013; Mitteroecker and Gunz 2009). This methodology is based on the application of algorithms that optimize as much as possible the mathematical correspondence between the points that define two lines or two surfaces. The algorithm used in this case is the bending energy minimization algorithm, based on thin-plate spline deformation (Bookstein 1997; Gunz and Mitteroecker 2013; Bookstein 1996; Bookstein et al. 2002; Green 1996; Perez et al. 2006). Once all specimens had been measured, another resliding protocol towards the average individual was carried out in RStudio (v.2023.03.0+386), using the function relaxLM from package Morpho v.2.11 (Schlager 2017). This process avoids the error that occurs when using only one individual as a reference for the sliding of semilandmarks, which is necessary to make them homologous across the entire sample (Gunz et al. 2005).
4.2.3 Missing Data
To include the largest possible sample of individuals, the missing structures of specimens with missing parts after virtual restoration have been estimated mathematically (crania: 38.8% of individuals, 3.2% of landmarks; Jaw: 10.5% of individuals, 1.1% of landmarks). The protocol used for the estimation of missing landmarks is based on TPS interpolation (Gunz et al. 2009). This protocol has been carried out in RStudio (v.2023.03.0+386) using the function estimate.missing from package geomorph v.4.0.5 (Baken et al. 2021; Adams et al. 2024).
4.2.4 Intraobserver Error
To quantify the intraobserver measurement error associated with landmark digitization, the MNCN_M22039 3D skull (designated as the “problem specimen”) was digitized six times on 6 different days by the same researcher. Then, these six measurements from the problem specimen were compared with 25 other digitized skulls randomly selected from our sample, all of them measured twice. The shape differences were assessed through procrustes distances, defined as the square root of the summed squared distances between procrustes registered landmark configurations and their shape coordinates (Gunz et al. 2009; Mitteroecker and Gunz 2009; O'HIGGINS 2000). An acceptable intraobserver error requires that the largest procrustes distance between the repetitions of the problem specimen has to be smaller than the smallest procrustes distances between the compared specimens (Morecroft et al. 2010).
Extended Data Table S4 and Figure S2a show that the highest procrustes distance between the five repetitions of the same specimen (MNCN_M22039; Pd: 0.0276) is much lower than the smallest procrustes distance between random specimens (NHMF_2462sk; Pd: 0.0741). Therefore, the intraobserver error is acceptable. In addition, in the 27 specimens measured more than once, all repetitions have the smallest procrustes distances of all the specimens evaluated. To observe the possible effect of this error in the whole sample, the procrustes distances of all the specimens are included in Extended Data Table S4 and the graphical representation of the whole sample and the remeasured specimens in Extended Data Figure S2b.
4.2.5 Generalized Procrustes Analysis and Principal Component Analysis (PCA)
Whole 3D GM data processing and analysis, and the production of graphical results were implemented in the software MorphoJ 1.07a (Klingenberg 2011), Past 4.03 (Hammer et al. 2001), and RStudio (v.2023.03.0+386). For each dataset, landmark configurations were subjected to a Generalized Procrustes Analysis: they were rotated, translated to a common centroid, and scaled to unit centroid size (CS) to remove any variation unrelated to shape (Bookstein 1991; Gower 1975). To assess variability in the crania and jaw morphology, independent PCA dimensionality reduction analysis was performed, detailing the variability picked up by the first three PCs in brackets in the title of the axes (Figure 1). The analyses include the graphical expression of the shape change associated with their axes. For the evaluation of the size of each specimen, the CS and the LogCS of those 3D models that were correctly scaled were used.
4.2.6 Linear Regressions Shape-Size
To test the potential relationship between shape and size parameters of crania and mandibles were performed bivariate analyses including linear regression (Extended Data Figure S3). Regression score of procrustes coordinates against LogCS values was used as a representation of the shape variability (Drake and Klingenberg 2008; Klingenberg 2015). In the regression of the mandible shape against cranial shape, the regression score of the procrustes coordinates against age for each structure was used. To check the covariation between the variables, Ordinary Least Squares Regression has been performed. In the case of the mandible, its fit to a quadratic equation has also been evaluated together with the fit to the linear regression, comparing the quality of the fit with the Akaike IC index. Analyses were performed in MorphoJ 1.07a (Klingenberg 2011) and Past 4.03 (Hammer et al. 2001).
4.2.7 Warping
The anatomical visualization of the predominant craniomandibular changes in the ontogenetic series collected in the 3DGM analyses has been facilitated by warping the individual RMCA 2417 to the mean shape of each of the age classes (Bookstein 1989). Warping was carried out via TPS in RStudio (v.2023.03.0+386) using the function tps3d from package Morpho v.2.11 (Schlager 2017). The resulting 3D model of each age class can be seen in Extended Data Figures S4, S5, S6, S7, S8, and S11 and is available for download in the Extended Data—Supporting Information S20: 3D models.
4.3 Life-History Data
The proxies of the life-history traits have been obtained from different bibliographic sources and databases, which will be presented below. All the data extracted from the different sources of information and used in the analyses discussed here can be consulted in Extended Data Table S5.
General body parameters are represented by body length, height, and body mass, measured between 1961 and 1966 in Queen Elizabeth National Park (Uganda) by Prof. Richard Laws' team (Laws 1968) and published by Shannon et al. (2021).
Skeletal maturity data, such as fusion of mandibular symphysis and loss of the first deciduous premolar (dp1,) are taken from data from Queen Elizabeth National Park (Uganda) reported in Laws (Laws 1968). Estimates of bone fusion moments are taken from observations made by Weston (Weston 1997) on individuals from the National Museums of Kenya (Kenya).
Cranial capacity data are from the measurement of specimens in different European museums by Weston & Lister (Weston and Lister 2009).
The jaw weight and canine weight data, used and previously published by Shannon et al. (2021) to discuss sexual dimorphism, are part of the Queen Elizabeth National Park (Uganda) database generated by Prof. Richard Laws' team (Laws 1968).
Data relating to sexual maturity and changes in reproductive behavior are represented by: the percentage of pregnant and lactating females in the Luangwa valley (Zambia) between 1970 and 1971 (Marshall and Sayer 1976); the relationship between the percentage of pregnant and lactating females and the size of testes and ovaries is taken from specimens analysed between 1974 and 1975 in the Kruger National Park (South Africa) by Smuts and Whyte (1981); for the minimum age of generation of the first offspring, the data of Dittrich (1976) from the databases of various zoos have been considered; the absolute proportions of pubertal and mature individuals in a population according to age are from the Luangwa River (Zambia) and are taken from Sayer and Rakha (1974).
The graphical representation of each specific parameter has been done by means of scatter plots versus age of the individuals in Past 4.03 software (Extended Data Figure S10a–m,o) (Hammer et al. 2001), except in the case of the first offspring which have been represented as boxplots versus age (Extended Data Figure S10o). A summary chart has been generated to observe and discuss the ontogenetic coincidence of the different life-history events and processes with the developmental stages of craniomandibular morphology (Extended Data Figure S12).
4.4 Key Age Classes—Life-History Events
To assess the ontogenetic pattern of each trait under study, it was first necessary to determine key life-history events. The set of parameters related to hippopotamus reproduction assessed in this study characterizes quite clearly the three most important stages of non-human mammal development. First, in individuals of both sexes kept in captivity, the minimum and average age of reproduction are around 3 and 5 years, respectively, which indicates the ages for the onset of puberty (Extended Data Figure S10n) (Dittrich 1976). The onset of lactating or pregnancy in a small percentage of wild 5-year-old female individuals indicates the beginning of the pubertal phase, while the generalization of lactation and pregnancy in 14–15-year-old females marks the onset of adulthood (Extended Data Figure S10j,k) (Marshall and Sayer 1976; Smuts and Whyte 1981). It is not possible to observe these stages in the growth of the ovaries, which does not seem to follow a sigmoidal pattern (Figure 2; Extended Data Figure S10m) (Smuts and Whyte 1981). Males, on the other hand, do not appear to functionally generate sperm until 5 years of age (coinciding with the end of the infantile stage) and do not reach the most stable testicular size until 15 years of age (coinciding with the transition to adulthood; Figure 2; Extended Data Figure S10l) (Smuts and Whyte 1981). These stages can also be clearly seen in the pattern of fusion and maturation of the bones of the postcranial skeleton. A main skeletal maturation seems clear up to the age of 10 years and a final stage of skeletal growth closure with complete fusion of the epiphyses between the ages of 15 and 25 years (Extended Data Figure S12) (Weston 1997). In summary, the definition of the transition from infantile to pubertal age at around 5 years and the onset of adulthood at approximately 15 years seems to hold well in hippopotamuses.
4.5 Ontogenetic Modeling
Corresponding in this case L∞ with a, K with c, and t0 with (lnb)/c. In this paper, we focus especially on the values obtained for the growth coefficient (K in the original equation and c in the form we use here), which are comparable between the models obtained with each dataset and inform us of the dynamics of ontogenetic change presented by each character.
The datasets evaluated correspond to the cranium and mandible shape (as regression scores procrustes coordinates/age), cranium and mandible size (as CS), total body length, height, body mass, cranial capacity, jaw weight, canine weight, combined testes weight, and combined ovaries weight (Figure 2).
To assess the presence of the theoretically expected ontogenetic stages of significant change in each body parameter evaluated, statistical tests were performed by grouping individuals into different age classes. Firstly, the pre-weaning stage (0–2 years), then the post-weaning infant stage (2–5 years), followed by the pubertal stage (5–15 years), and finally the adult stage. The latter has been divided into two age classes (15–25 years and 25–43 years) to allow us to evaluate the presence of a late stage of change that departs from the classical growth pattern in mammals (Figure 3). The frequencies of each value for each age class have also been arranged, making it possible to visualize the presence of bimodal distributions of the data (Figure 3). The generality of the data did not correspond to normal and homoscedastic samples, and small samples were included in the assessment, so non-parametric statistical tests were used. For analyses of the data, the Kruskal–Wallis test was applied, together with Mann–Whitney pairwise post hoc test. The results of the tests are specified in Figure 3 and in the Extended Data Table S6.
Author Contributions
Darío Fidalgo, Antonio Rosas, and Joan Madurell-Malapeira: conceptualization and writing. Darío Fidalgo, Faysal Bibi, Luca Pandolfi, Jean-Renaud Boisserie, Joan Madurell-Malapeira, and Antonio Rosas: research strategy development. Darío Fidalgo, Faysal Bibi, Luca Pandolfi, Roberta Martino, and Kheloud El Eshraky: data acquisition. Darío Fidalgo and Carlos A. Palancar: data analysis. Darío Fidalgo, Antonio Rosas Luca Pandolfi, and Joan Madurell-Malapeira: funding acquisition. All authors provided input to data interpretation and discuss the manuscript.
Acknowledgments
The authors would like to thank the editorial team of Evolution & Development, as well as the work of the two anonymous reviewers, who have helped significantly to improve the final version of this manuscript. We express our gratitude to University of Dundee Museum Collections (Dundee), Darwin Museum (Moscow), Institut Català de Paleoecologia Humana i Evolució Social (Tarragona, especially to Dr. Palmira Saladié and Dr. Josep Vallverdú), Lapworth Museum of Geology (Birmingham), LSA Museum of Zoology University of Michigan (Michigan, especially to Dr. Cody W. Thompson), Museo de Anatomía Comparada de Vertebrados (Madrid, especially to Mariano Padilla), Museum of Natural History Wroclaw (Wroclaw, especially to K. Stefaniak and J. Kotusz), Museum of Zoology (Cambridge), Museum of Vertebrate Zoology (Berkley, especially to Dr. Carol Spencer), Museu de Ciències Naturals de Barcelona (Barcelona, especially to Dr. Javier Quesada), Museo di Storia Naturale di Firenze (Florence, especially to P. Agnelli), The Natural History Museum (London, especially to Dr. Roberto Portela Miguez), Muséum National d'Histoire Naturelle (Paris, especially to Dr. Joséphine Lesur), National Museum of Nature and Science (Tokyo, especially to Dr. Kent Mori), Universidade Nova (Lisbon, especially to Eduarda Ferreira), Royal Museum for Central Africa (Tervuren, especially to E. Gilissen), Staatliches Museum für Naturkunde Stuttgart (Stuttgart, especially to Dr. Eli Amson and Dr. Stefan Merker), Museo Anatómico Universidad de Valladolid (Valladolid, especially to Dr. Francisco J. Pastor), University of Wyoming Museum of Vertebrates (Wyoming), Museum für Naturkunde (Berlin, especially to Dr. Christiane Funk, Enea Conte and Detlef Willborn). Sampling at The Natural History Museum in London was made possible thanks to funding from the SYNTHESYS+ program (H2020 Grant Agreement 823827, reference GB-TAF-TA4-007), at the Muséum National d'Histoire Naturelle in Paris thanks to the Institut Català de Paleoecologia Humana i Evolució Social project 200.400 Fund. I-Cerca 2020, at the Museum für Naturkunde in Berlin thanks to the Ministerio de Universidades grant EST23/00053 (associated with the project (FPU20/03389). Sampling at the Museums in Tervuren, Florence and Wroclaw was funded by SYNTHESYS+ [BE-TAF-136], by the University of Florence (“Progetto Giovani Ricercatori Protagonisti” initiative), by the European Union—Next Generation EU, call PRIN PNRR project P2022RZ4PL, and by the Wroclaw University of Environmental and Life Sciences (Visiting Professor), granted to L.P. and R.M. was funded by SYNTHESYS+ program (DE-TAF-TA4-063 2022) during her visit to the Staatliches Museum für Naturkunde of Stuttgart. The research activity of A.R. and D.F. was funded by Ministerio de Ciencia e Innovación, PID2021-122356NB-I00. D.F. was supported by the Ayuda del Programa de Formación de Profesorado Universitario (FPU20/03389) and is a PhD student at the Programa de Doctorado en Biología at the Universidad Complutense de Madrid. R.M. is granted by the Fundação para a Ciência e a Tecnologia (FCT) (2021.08458.BD).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the supporting material of this article. Information on all the data used and the results of the analyses performed can be found in the Extended Data Tables S2, S3, S4, S5, and S6. The graphical results of the craniomandibular phenotypic analyses can be found in Extended Data Figures S4, S5, S6, S7, S8, and S9, including a detailed atlas of each ontogenetic stage (Extended Data Figure S8). Note that 3D models of the average crania and mandible shapes of each ontogenetic stage will be uploaded to scientific online repositories (Extended Data—Supporting Information S20: 3D Models).




