Multimodal imaging evaluation of a primary cardiac lymphoma in an immunocompetent patient
Abstract
Primary cardiac lymphoma (PCL) is a rare extranodal lymphoma that involves only the heart and/or pericardium. It is fatal in the absence of a prompt diagnosis and adequate therapy. Here, we report an immunocompetent 55-year-old Chinese man with palpitations and occasional chest distress for 1 month. Multimodal imaging work-up showed a tumor infiltrated into the myocardium, which greatly contribute to a timely pathology diagnosis of PCL.
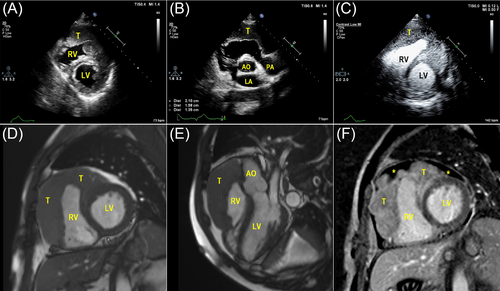
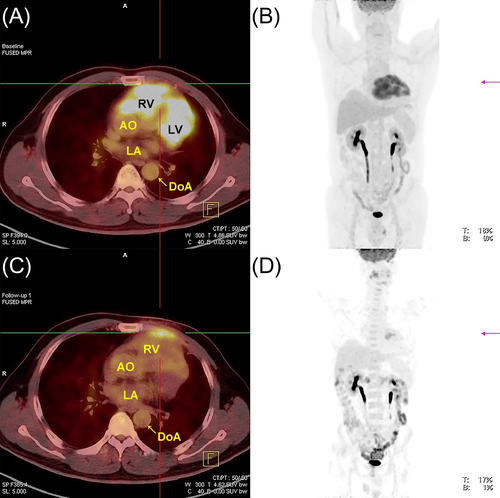
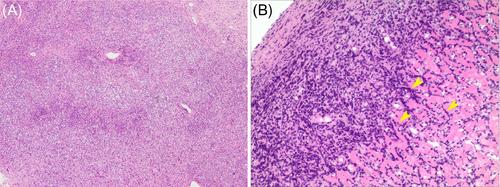
A 55-year-old man presented in our department with palpitations and occasional chest distress for 1 month. Biochemical examinations were all within normal limits and test for HIV was negative. On the day of hospitalization, an electrocardiogram showed a Q wave in the II, III, and aVF leads. Transthoracic echocardiography (TTE) demonstrated pericardial effusion with a multi-segmental infiltrated ill-defined hypoechoic mass that extensively affected the mitral and papillary muscle level of the left ventricular (LV) anterior wall (Figure 1A, Movie S1), the middle to upper part of the right ventricular (RV) free wall, the RV outflow tract, and the initial segment of the main pulmonary artery (Figure 1B). The thickness of the infiltrated wall ranged from 2.8 to 3.4 cm. The mitral and tricuspid valves were not affected, but the main pulmonary artery was narrowed where the peak systolic velocity measured 1.7 m/s. The myocardial contrast echocardiography (MCE) study revealed rich perfusion in the thickened RV wall myocardium similar to other myocardial segments (Figure 1C, Movie S2). Initially, a cardiac tumor was suspected. Cardiac magnetic resonance imaging (CMRI) confirmed the extent of the mass infiltration (Figure 1D and E, Movies S3 and S4) of the TTE and showed the impairing motion of the lateral and inferior RV walls. Post-contrast-enhanced CMRI revealed non-homogenous gadolinium enhancement of the tumor, areas of non-enhancement implied pericardial effusion (Figure 1F, Movie S5). No evidence of mediastinal lymphadenopathy was observed. Furthermore, positron emission tomography/computed tomography (PET/CT) scan revealed elevated 18fluorodeoxyglucose accumulation with high standardized uptake values (maximum 9.8) in the RV free wall and the LV anterior wall, suggesting a malignant nature of the neoplasm, coronal views showed no evidence of distant metastatic disease (Figure 2A and B). The patient finally underwent an open chest biopsy through median sternotomy. The morphology and immunohistochemistry of excised masses revealed complete destructive replacement by CD20 + B-lymphocytes (Figure 3). A diagnosis of primary diffuse large B cell lymphoma of the RV was made. The patient was started on a standard systemic chemotherapy protocol of 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and showed a good response to the chemotherapy (Figure 2C and D). One year later, the patient was in complete clinical remission.
PCL is extremely rare, representing only 1.3% of all primary cardiac tumors and 0.5% of all extranodal lymphomas.1 Patients usually die within a few months after being diagnosed.2 Prompt diagnosis and early R-CHOP chemotherapy treatment appear to be the only chance for cure.3 However, the clinical manifestations may be heterogeneous.4 Multimodal approaches greatly aid in the diagnosis and characterization of cardiac masses. Electrocardiogram and echocardiography represent a quick noninvasive approach to evaluate patients with lung- and heart-related symptoms. MCE can be used to evaluate the blood supply of the mass; in general, PCL exhibits rich perfusion in the thickened myocardium that distinguishes the mass from normal myocardium. In comparison, CMR techniques yield accurate and reproducible data regarding soft-tissue and tumorous infiltration of the myocardium and pericardium, myocardial perfusion, myocardial fibrosis, LV and RV size and function, and valvular regurgitation. PCL demonstrates heterogeneous enhancement after intravenous contrast material administration and less likely than sarcomas to have necrosis, involve the valves, and extend into heart chambers. MRI is performed for diagnosis, while CT is used for serial monitoring of the treatment response. PET/CT is crucial in determining whether a mass is benign or malignant, providing additional information about the metabolic activity of lesions. Certain features, such as high 18fluorodeoxyglucose avidity on PET, suggest a malignant tumor rather than a benign tumor. The final diagnosis must still be confirmed by biopsy.
CONFLICT OF INTERESTS
The authors declare no conflict of interests.