The effect of dietary intake changes on nutritional status in acute leukaemia patients after first induction chemotherapy
Abstract
This study aimed to evaluate how changes in dietary intake among acute lymphoblastic and acute myeloid leukaemia (ALL and AML) patients affect nutritional status after the first induction chemotherapy. Dietary intake was assessed using 24-h recall and a 136-item food frequency questionnaire. Nutritional status was assessed by Patients Subjective Global Assessment questionnaire before starting induction therapy and again after 1 month. All newly diagnosed acute leukaemia patients aged 15 years old and older who attended three referral hospitals for initiation of their induction chemotherapy were included in the sample selection provided that they gave informed consent. A total of 30 AML and 33 ALL patients participated in the study. Dietary intake and nutritional status worsened after the chemotherapy treatment. Dietary intake in terms of macronutrients, micronutrients, food variety and diet diversity score changed significantly after the induction chemotherapy. No significant relationship was found between the changes in dietary indices and nutritional status. Chemotherapy-related side effects as an additional factor to cancer itself could affect dietary intake of leukaemia patients. The effectiveness of an early assessment of nutritional status and dietary intake should be further investigated in order to deter further deterioration.
Introduction
According to a World Health Organization report, cancer incidence increased globally to reach 14.1 million new cases in 2012 and is estimated to rise to 21.4 million new cases by 2030 (Alwan 2010). Similarly, cancer incidence in Iran, as a developing country, has been increasing. According to the latest published information on common cancers in Iran, leukaemia is ranked the fifth most common cancer in men (Radmard 2010) and sixth in women (Sadjadi et al. 2005). The age-standardised incident rate (ASR) for leukaemia in Iran is significantly higher than that of the rest of the world. Among Iranian men and women, ASRs are 7.7 and 4.0 per 100 000 person-years respectively. The global rates are 5.8 and 4.3 per 100 000 person-years for men and women, respectively (Rajabli et al. 2013), which indicate that the ASR of Iranian men is much higher than that of their global counterparts. Recent reports also state high incidence of leukaemia in the southern cities, especially in Zahedan that is located in the southeast of Iran (Mashhadi et al. 2010). After gastric and lung cancer, leukaemia is the third cancer in terms of fatality rate (Farahmandbeigi & Kadivar 2002). A study in the city of Shiraz in southern Iran showed that the place of birth, jobs related to animals/husbandry, genetic factors and cigarette smoking are among the main risk factors for acute leukaemia incidence (Hadi et al. 2008). In an attempt to explain the high incidence of one sub-type of acute myeloid leukaemia (AML) (acute promyelocytic leukaemia) in the northwest of Iran, the presence of carcinogens in the diet as well as environmental factors are suggested (Ziaei 2004).
Deterioration in nutritional status and dietary intake during cancer treatment has been increasingly referred to in recent decades (Arrieta et al. 2010; Creaser 2010) and is reported to affect well-being as well as treatment outcome in cancer patients (Ross et al. 2004; Nourissat et al. 2008). Taking Arrieta et al.'s (2010) study as an example, the nutritional status of lung cancer patients was assessed using Subjective Global Assessment (SGA) and serum albumin, once before chemotherapy and once again after two cycles. They found that 51% of patients were malnourished, and those who were malnourished had higher incidence of chemotherapy-induced toxicity compared with well-nourished patients (Arrieta et al. 2010). Patients suffering from debilitating diseases may also encounter an imbalance in metabolic conditions, such as catabolism (Bosaeus et al. 2002); in an assessment of newly diagnosed oesophageal cancer patients, those in the weight loss group were found hypermetabolic as compared with those in the weight stable group. Chemotherapy treatment may have an influence on the energy requirements of the patients and put them in a catabolic state, on the one hand, and impair their dietary intake as a result of chemotherapy-related side effects such as nausea, vomiting, loss of appetite and taste changes, on the other hand (de Vries et al. 1982). The latter study retrospectively assessed adult AML and acute lymphoblastic leukaemia (ALL) patients during their induction chemotherapy. However, there is still uncertainty about the impact of altered metabolic rate on the nutritional status as there was no difference in the status of oesophageal cancer patients with either normal or hypermetabolic rate, before undergoing chemotherapy treatment (Bosaeus et al. 2002). Interval nutritional assessment of cancer patients attending the chemotherapy unit revealed that although the malnutrition rate was high, variations in nutritional status could be maintained throughout the treatment (Creaser 2010). Nevertheless, most studies have agreed on weight loss as a result of infection, gastrointestinal morbidities or insufficient dietary intake during the chemotherapy treatment (Jager-Wittenaar et al. 2010). A review study on the impact of oral nutrition intervention on cancer patients of all types showed an improvement in nutritional status as a result of increased energy intake and the prevention of weight loss. This further resulted in an improvement in some aspects of quality of life (QOL) and a better response to the treatment as well (Baldwin et al. 2012). Several studies on the dietary intake of children with haematological cancers have been conducted (Sala et al. 2012; Tan et al. 2013; Brinksma et al. 2014), although similar studies on adults are rare.
In this study, the aim of calculating diet diversity score (DDS) and food variety score (FVS) was to test whether there is any change in the quality of the patients' diet during first induction chemotherapy. Assessment of FVS and DDS provides information regarding the adequacy of nutrient intake (Drewnowski & Warren-Mears 2001; Ruel 2003). More specifically, DDS focuses upon the diversity of food group intake and FVS focuses upon the variety of food item consumption. Having an overview of patients' DDS and FVS during chemotherapy treatment can ensure dietitians of patients' nutrients adequacy and subsequently give necessary clues as to what they have to focus on while planning an appropriate dietary regimen. Diet quality of leukaemia patients has been rarely studied, particularly in developing countries such as Iran.
This study was conducted hoping that its results can help to capture the attention of health providers as to the importance of deteriorated dietary intake and the nutritional status in leukaemia patients, which can impose an increased risk of infection and raise the health care costs of the treatment.
Material and Methods
Newly diagnosed acute lymphoblastic and myelogenous leukaemia patients with the confirmation of the oncologists after being applied with diagnostic procedure, which is composed of bone marrow aspiration or biopsy, flow cytometry and molecular biology or real-time polymerase chain reaction technique, were recruited to be assessed before starting the chemotherapy treatment. Second assessment was carried out after 1 month, before ongoing further treatment. Inclusion criteria were all newly diagnosed ALL or AML patients who were about to undergo induction chemotherapy provided that they had had no previous cancer treatment and were aged 15 years old and above. The study was conducted in three hospitals named Imam Reza, Rasoul Akram and Imam Khomeini, which will be referred to in the text as ‘reference hospitals’. The reference hospitals' protocol for the induction chemotherapy treatment was as follows:
For AML: Cytarabine 100 mg/m2/day 7-day infusion plus either daunorubicin 45 mg/m2/day or idarubicin 10–12 mg/m2/day daily for the first 3 days.
For ALL: Cyclophosphamide 1000 mg/m2 on first day plus daunorubicin 45 mg/m2 for the first three days, vincristine 1.2–2 mg/m2 on day 2,8,15,22, prednisolone either 50–60 mg/m2/day for 28–30 days.
Data collection was carried out through a questionnaire consisting of three sections (socioeconomic-demographic, nutritional status and dietary indices). Patients Subjective Global Assessment (PG-SGA) questionnaire for the assessment of nutritional status (Bauer et al. 2006) and 24-h dietary recall along with a 136-item food frequency questionnaire (FFQ) for dietary intake assessments were applied at two time points.
The first part of the PG-SGA questionnaire, including weight loss history (last month), dietary intake, activities and functions, was administered by the interviewer, while the second part, which concerns the patient's medical history and its relation to the nutritional requirement, the metabolic demand (stress) and the physical examination, were completed with the help of either the oncologists or resident students of the oncology ward in each reference hospital. The rest of the questionnaire, such as dietary intake sections, was all completed by the main researcher. All of the patients were interviewed in person prior to chemotherapy. One month after the start date of the induction treatment, most patients were interviewed in person again, while a few of them who were discharged were interviewed by telephone. Data related to weight at the time of hospitalisation and at the end of the first month were attained from the records found in patients' files. PG-SGA provides both numerical and qualitative assessment. The qualitative scale categorises patients to three categories: well nourished, moderately malnourished and severely malnourished. In terms of scoring, a higher score of PG-SGA indicates a worse nutritional status and, accordingly, the extent of the requirement for the nutrition recommendation is determined.
Dietary Intake Assessment and Analysis
Two 24-h dietary recall questionnaires were completed at each time point, before and after the induction chemotherapy, making a total of four questionnaires. At each time point, patients were asked to recall their previous day intake and a weekend/holiday dietary intake. The mean intake of the two recalls was calculated to obtain patients' current dietary intake. The interview time fell just prior to the start of the induction therapy for the ‘before chemotherapy’ assessment and prior to discharge of the patients for the ‘after induction’ assessment. The questionnaire covered six meals: breakfast, lunch, dinner and three snacks. Household portion sizes such as cups and spoons were used to estimate the amount of food consumed. Intake of energy and macronutrients (protein, fat and carbohydrate) and micronutrients (iron, vitamin B subgroups, vitamin A, calcium and vitamin C) was determined using the Nutritionist IV software, with additional food items that are contained in a normal Iranian diet.
To calculate the energy requirement of patients, first, the basal metabolic rate for men and women was determined using the Harris & Benedict equation, which is specified for age and gender. A correction factor of 1.4 for injury and 1.2 for activity in cancer patients was assumed for calculation of total energy requirement (Bauer et al. 2006). Patients' protein needs were calculated based upon the additional recommended protein intake for cancer patients at 1.5 g/kg body weight (Matarese & Gottschlich 1998).
A patient's validated semi-quantitative FFQ, which had been applied to hospital patients in previous studies in Iran (Amirkalali et al. 2008) comprising 136 food items, was used to calculate DDS and FVS. Two time periods were assessed for frequency and amount of intake. The ‘before assessment’ covered the three months prior to the start of chemotherapy. The ‘after assessment’ covered the first month of the induction chemotherapy. For calculating DDS, a method developed by Kant et al. (1995) and applied by Azadbakht et al. (2005) based upon the five main food groups of the U.S. food pyramid was employed (Kant et al. 1995; Azadbakht et al. 2005). For this purpose, all food items derived from the FFQ were converted to a single scale (serving per day) and were assigned into five main food groups: cereals-grains (bread, rice, macaroni, biscuit, spaghetti); meats (fish, poultry, eggs, red meat), vegetables (all vegetables including potato and legumes); fruits (all seasonal fruits, fruits juice, canned fruits); and dairy products (milk, yogurt, cheese). The minimum serving size for data analysis was ½ serving of each food item per day based upon the food pyramid definition for the serving size. The score for each food group was calculated by dividing the sum of the food items of the group consumed (more than ½ serving per day) by the total number of food items presented in the same group. The number obtained was multiplied by 2 to gain each food group's diversity score. Each food group had a maximum score of 2, so the maximum total DDS was 10. The total DDS was the sum of all the five food groups' diversity scores.
Food variety score (FVS) was calculated by summing the total number of food items consumed (more than ½ serving per day) during the previous month (Schebendach et al. 2008; Oldewage-Theron et al. 2010). Thus, two FVS and DDS scores, before and after chemotherapy, were obtained.
This study was approved by the Ethics Committee of the Faculty of Medicine and Health Sciences, Universiti Putra Malaysia and the three reference hospitals. Informed written consent was also obtained from all patients.
Statistical Analysis
Data analysis was performed via SPSS, version 17.0 for Windows (Chicago, IL, USA). Descriptive data were expressed as mean ± SD for changes in weight status, nutritional status, macronutrient and micronutrient intake. For numerical data, changes were analysed with paired t-test, while meal intake changes were analysed through Wilcoxon signed-rank test. General linear regression was applied to see the relationship between nutritional status indicators (which include weight changes and PG-SGA score) and changes in dietary intake indices. Statistical significance was considered at P < 0.05 in all analyses.
Results
A total of 63 patients were recruited for this study comprising 41 men and 22 women, of whom 31 patients (49.2%) were below 30 years old, while 32 (50.8%) were 30 and above. The distribution of patients in the three reference hospitals, Imam Reza, Rasoul Akram and Imam Khomeini, was 35, 17 and 11 patients respectively. Patients' general characteristics are shown in Table 1. Mean daily energy intake were 1396.16 kcal (1379.33 and 1411.46 kcal for AML and ALL, respectively) and 1046.74 kcal (1031.55 and 1060.56 kcal for AML and ALL, respectively), before and after chemotherapy, respectively, which indicate significantly lower total energy intake after chemotherapy in all patients (P < 0.001). Mean protein intake before induction chemotherapy were 63.30 g (AML) and 64.91 g (ALL) and these were reduced to 47.26 g (AML) and 50.44 g (ALL) after the induction. The significance of this reduction was examined by the paired t-test (P < 0.01). Achievement of energy and protein intake as a percentage of Daily Recommended Intake (DRI) before induction was 55% (energy) and 70% (protein) in all of the patients. This decreased to 40% of the optimal energy intake and 46% of the recommended protein intake after the induction.
| Characteristics | ALL (n = 33) number (%) | AML (n = 30) number (%) | Total number (%) | |
|---|---|---|---|---|
| Gender | Male | 22 (34.9) | 19 (30.1) | 41 (65) |
| Female | 11 (17.5) | 11 (17.5) | 22 (35) | |
| Age (years) | Mean ± SD | 26.01 ± 1.3 | 40.73 ± 3.2 | 33.03 ± 15.4 |
| 15–31 | 22 (34.9) | 10 (15.9) | 32 (50.8) | |
| >31 | 11 (17.5) | 20 (31.7) | 31 (49.2) | |
| Marital status | Single | 19 (30.1) | 10 (15.9) | 29 (46) |
| Married | 13 (20.7) | 20 (31.7) | 33 (52.4) | |
| Divorced | 1 (1.6) | 0 | 1 (1.6) | |
| Educational status | No formal education | 2 (3.2) | 3 (4.8) | 5 (8) |
| Primary school | 8 (12.7) | 7 (11.1) | 15 (23.8) | |
| Secondary school | 17 (27) | 14 (22.2) | 31 (49.2) | |
| University educated | 6 (9.5) | 6 (9.5) | 12 (19) | |
| Occupation | Unemployed/Household | 9 (14.3) | 7 (11.1) | 16 (25.4) |
| Low level employee | 8 (12.7) | 8 (12.7) | 16 (25.4) | |
| Medium level employee | 12 (19) | 14 (22.2) | 26 (41.2) | |
| High level employee/or private employment | 4 (6.35) | 1 (1.65) | 5 (8) |
- ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia.
Mean DDS were 5.01 (AML) and 4.70 (ALL) before induction, while after induction, they decreased to 3.64 (AML) and 3.73 (ALL). The mean FVS of AML patients were reduced from 42.30 before induction chemotherapy to 28.80 after induction chemotherapy. Similarly, mean FVS scores of ALL were reduced from 44.60 to 31.12. There was a significant reduction in both DDS and FVS of leukaemia patients after chemotherapy treatment. The results of paired t-test showed a significant change in DDS and FVS (P < 0.001) after induction chemotherapy.
Macronutrient intake of patients significantly declined after the induction in terms of total energy, carbohydrate, protein and fat intake (Table 2). According to this table, paired t-test showed a significant change in each of the macronutrient and total energy intake (P < 0.05). However, the intake of macronutrients as a percentage of total energy intake did not change significantly after induction chemotherapy. A comparison of patients' micronutrient intake with DRI before and after induction chemotherapy is illustrated in Table 3. As shown, after induction, the proportion of most micronutrient intake in relation to the recommended amount decreased in ALL patients. This reduction was statistically significant only for vitamin B1, B3, B6 and iron, which saw more than 20% reduction (P < 0.05). As for AML patients, micronutrient intake decreased as a proportion of the recommended amount, but the difference was only significant for vitamin B3 and iron.
| Leukaemia type | Mean ± SD | Mean change | t | Significance | ||
|---|---|---|---|---|---|---|
| Before | After | |||||
| Energy intake (kcal) | AML | 1379.33 ± 393 | 1031.55 ± 335 | −351 | 5.27 | *0.001 |
| ALL | 1411.46 ± 418 | 1060.56 ± 325 | −350 | 5.27 | *0.001 | |
| Both leukaemia | 1396.16 ± 403 | 1046.74 ± 328 | −349.42 | 6.88 | *0.000 | |
| Protein (g) | AML | 63.30 ± 26.7 | 47.26 ± 18.6 | −16.04 | 3.81 | *0.002 |
| ALL | 64.91 ± 27.9 | 50.44 ± 17.5 | −14.46 | 2.87 | *0.007 | |
| Both leukaemia | 64.14 ± 26.74 | 48.92 ± 17.97 | −15.21 | 4.41 | *0.000 | |
| % Protein/Total energy intake | AML | 18.7 ± 7.5 | 19.48 ± 6.71 | 1 | −0.6 | 0.553 |
| ALL | 18.75 ± 7.5 | 19.4 ± 6.4 | −0.72 | −0.45 | 0.653 | |
| Both leukaemia | 18.58 ± 7.01 | 19.44 ± 6.71 | 0.85 | 0.75 | 0.454 | |
| Carbohydrate (g) | AML | 200.5 ± 71.3 | 162.46 ± 70.5 | −38.06 | 1.9 | 0.068 |
| ALL | 184.19 ± 61.4 | 140.6 ± 55.4 | −43.56 | 3.56 | *0.001 | |
| Both leukaemia | 191.97 ± 66.32 | 151.03 ± 80.81 | −40.94 | −3.58 | *0.001 | |
| % of carbohydrate/Total energy intake | AML | 56.1 ± 9.3 | 55.3 ± 15.2 | −0.77 | −0.24 | 0.807 |
| ALL | 51.3 ± 8.83 | 52.6 ± 12.12 | −1.33 | −0.49 | 0.626 | |
| Both leukaemia | 53.91 ± 13.54 | 53.58 ± 9.30 | 6.252 | 3.661 | 0.872 | |
| Fat (g) | AML | 41.95 ± 19.4 | 29.6 ± 17.19 | −12.3 | 2.92 | *0.007 |
| ALL | 50.11 ± 20.3 | 34.5 ± 17.7 | −15.61 | 3.95 | *0.001 | |
| Both leukaemia | 46.23 ± 20.16 | 32.17 ± 17.51 | −14.05 | −4.89 | *0.001 | |
| % of fat/total energy intake | AML | 24.4 ± 8.01 | 23.93 ± 10.45 | −0.53 | 0.22 | 0.826 |
| ALL | 30.6 ± 8.01 | 27.4 ± 10.7 | −3.21 | 1.31 | 0.200 | |
| Both leukaemia | 27.68 ± 8.75 | 25.76 ± 10.66 | −1.93 | 1.12 | 0.263 | |
- *Significant at P < 0.05.
- ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia.
| Micronutrients | ALL | t | Z | P | AML | t | Z | P | Micronutrients (as % of DRI) | Mean ± SD/Median Before | Mean ± SD/Mean After | t | Z | P | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD as % of DRI/Median | Mean ± SD as % of DRI/Median | |||||||||||||||
| Before | After | Before | After | |||||||||||||
| Vitamin B1 | 71.50% | 57.16% | −2.87 | 0.004* | 79.18% | 59.93% | −1.53 | 0.125 | Vitamin B1 | 77.72 | 59.16 | −3.25 | 0.001 | |||
| Vitamin B2 | 85.2 ± 37.1% | 76.8 ± 32.5% | 1.08 | 0.284 | 98 ± 51.4% | 81.5 ± 33.8% | 1.63 | 0.114 | Vitamin B2 | 91.31 ± 45.70 | 79.08 ± 32.97 | 1.95 | 0.055 | |||
| Vitamin B3 | 125.5 ± 70% | 90.1 ± 46.5% | 2.41 | 0.021* | 119.7 ± 70.1% | 81.9 ± 47.2% | 2.6 | 0.014* | Vitamin B3 | 122.77 ± 70.99 | 86.22 ± 46.69 | 3.57 | 0.001 | |||
| Vitamin B12 | 95.08% | 99.70% | −0.31 | 0.714 | 99.58% | 94.37% | −1.67 | 0.094 | Vitamin B12 | 97.83 | 96.29 | −0.76 | 0.440 | |||
| Vitamin B6 | 95.53% | 73.15% | −2.17 | 0.030* | 90.80% | 63.15% | −0.99 | 0.318 | Vitamin B6 | 94.38 | 70.15 | −2.15 | 0.031 | |||
| Folate | 40.8 ± 20.5% | 35.2 ± 12.7% | 1.16 | 0.252 | 46.9 ± 27.3% | 38.8 ± 13.7% | 1.4 | 0.175 | Folate | 43.77 ± 24.05 | 36.93 ± 21.85 | 1.82 | 0.073 | |||
| Vitamin A | 42.81% | 32.84% | −1.81 | 0.070 | 31.70% | 29.95% | −0.40 | 0.688 | Vitamin A | 34.87 | 31.12 | −1.60 | 0.109 | |||
| Vitamin D | 0.50% | 4.08% | −1.08 | 0.277 | 0.70% | 0.77% | −0.55 | 0.580 | Vitamin D | 0.70 | 0.84 | −1.16 | 0.244 | |||
| Vitamin C | 132.15% | 159.11% | −0.93 | 0.350 | 160.41% | 126.05% | −0.40 | 0.688 | Vitamin C | 148.44 | 140.44 | −0.923 | 0.353 | |||
| Calcium | 53.4 ± 23.5% | 55.1 ± 21.3% | −0.49 | 0.621 | 57.7 ± 32.2% | 55.5 ± 27.3% | 0.54 | 0.591 | Calcium | 55.94 ± 32.83 | 56.33 ± 29.24 | −0.08 | 0.934 | |||
| Iron | 80.3 ± 38.4% | 59.2 ± 27.4% | 4.98 | 0.001* | 83.7 ± 25.3% | 63.3 ± 30.7% | 3.38 | 0.002* | Iron | 81.93 ± 40.19 | 61.29 ± 28.90 | 5.75 | 0.001 | |||
- *Significant at 0.05.
- DRI, Dietary Reference Index, USDA, NAL, Food and Nutrition Information Center.
Meal frequency of the patients was also assessed by the number of meals that they consumed before the induction chemotherapy and the number of meals they had during the first month of chemotherapy (Fig. 1). Based upon this figure, 81.1% of ALL patients tended to take 3–5 meals/day after the induction chemotherapy compared to 63.6% before the induction chemotherapy. In a similar trend, 66.7% of AML patients took 3–5 meals/day after chemotherapy as compared to only 50% before chemotherapy. However, these changes were not statistically significant (P > 0.05).
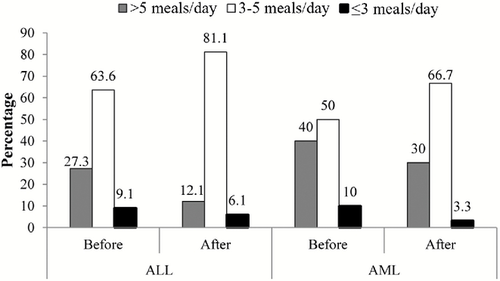
Daily meal frequency prior to and after chemotherapy. ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia.
There are three meals served in a day for hospitalised patients of the reference hospitals. Patients were recommended to intake extra meals on their own budget. They were recommended to have cooked vegetables, but if raw, were advised to properly wash them and to peel the fruits. The staple food in the Iranian diet is rice, so the main meals prepared in these hospitals were cooked rice plus an animal protein, usually beef or chicken. A cup of yogurt and some cooked vegetables as a side dish were often available. Patients were generally recommended to increase their food energy intake by having snacks between meals. Individual recommendations were given to patients who were referred by their physician for dietary consultation. In one of the hospitals, Rasoul Akram, patients were recommended to follow a Neutropenic diet with an emphasis on restricting their intake of raw vegetables and simple sugars. The type of meat for haematology cancer patients in the aforementioned hospital was mostly chicken rather than beef or lamb.
The mean PG-SGA scores of AML and ALL patients before chemotherapy were 7.30 ± 2.50 and 8.18 ± 2.60, respectively, which rose to 14.02 ± 3.0 and 14 ± 8.80 after chemotherapy. The paired t-test revealed a significant change in nutritional status after chemotherapy (P < 0.001). Based upon the nutritional recommendation guidelines for the PG-SGA questionnaire, there were considerably more patients in need of a type of nutrition intervention such as counselling or nutrient supplementation after induction chemotherapy compared to that of before induction chemotherapy (Table 4). Mean weight losses of patients during the month prior to the start of chemotherapy were 3.40 kg (AML) and 3.37 kg (ALL). These weight losses during the first induction chemotherapy were 3.68 kg (AML) and 3.27 kg (ALL). For all patients, the mean weight losses of the previous month were 3.38 and 3.46 kg before and after the induction therapy. Mean weight losses before and after induction chemotherapy were not significantly different (P > 0.05) neither between the leukaemia types nor between the two time points. No significant difference was also found in dietary intake indices between the leukaemia types as measured by Mann–Whitney U-test (P > 0.05).
| Score/Nutrition recommendation | ALL (n = 33) | AML (n = 30) | Total (n = 63) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| 0–1: No intervention required | – | – | – | – | – | – |
| 2–3: Patient or family education required | 16 (48.5) | 0 | 16 (53.3) | 0 | 32 (50.8) | 0 |
| 4–8: dietitian intervention required | 17 (51.5) | 1 (3) | 14 (46.7) | 1 (3.4) | 31 (49.2) | 2 (3.2) |
| ≥9: Critical need for nutrition intervention | 0 | 32 (97) | 0 | 29 (96.6) | 0 | 61 (96.8) |
There were inverse correlations between PG-SGA score and dietary indices (energy, protein intake, fruits diversity and dairy products diversity score) after induction chemotherapy (Table 5). This effect was later attenuated in regression analysis. After chemotherapy, changes in total energy intake, protein intake and DDS along with FVS were dietary indicators in the regression equation, and weight loss and a change in PG-SGA score were nutritional status variables. There was no significant relationship between either weight loss or PG-SGA score and changes in dietary intake indices analysed by linear regression (F = 1.57, P > 0.05).
| Total energy | Protein | CHO | Fat | DDS | FVS | Bread DS* | Vegetable DS | Fruit DS | Dairies DS | Meat DS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PG-SGA Before | r | −0.054 | −0.117 | −0.158 | −0.116 | −0.06 | −0.16 | −0.05 | −0.124 | −0.16 | −0.167 | −0.2 |
| Significance | 0.67 | 0.36 | 0.21 | 0.36 | 0.62 | 0.2 | 0.67 | 0.33 | 0.21 | 0.19 | 0.42 | |
| PG-SGA After | r | −0.338 | −0.326 | −0.28 | −0.2 | −0.08 | −0.081 | −0.115 | −0.15 | −0.311 | −0.286 | −0.15 |
| Significance | 0.007* | 0.009* | 0.83 | 0.1 | 0.53 | 0.52 | 0.37 | 0.25 | 0.013* | 0.023* | 0.23 |
- *Significant at P < 0.05.
- DDS, diet diversity score; DS, diversity score; FVS, food variety score; PG-SGA, Patients Subjective Global Assessment.
- Pearson's correlation test.
Discussion
In general, we assessed patients' dietary intake changes and its relation to their nutritional status attained by PG-SGA questionnaire. Habitual and current dietary intake of patients, which were assessed by 24-h recall and a 136-item FFQ, showed a significant reduction in their intake. A change in dietary intake and diet quality as attained through meal intake, DDS and FVS assessment, predicted a high probability of nutrient inadequacy in patients during the first month of induction chemotherapy. The previous month weight history of patients showed a 3.81-kg weight loss before the start of the treatment as well as a 3.47-kg weight loss during the first month of induction chemotherapy. The PG-SGA scores also showed a deteriorated nutritional status in both leukaemia types with a mean score of 7.30 prior to the treatment versus a 14.02 post-treatment score.
In terms of changes in dietary intake of patients, a reduction in macronutrient and micronutrient intake may predict future depletion in supplies of serum and tissue if this deteriorated status continues after the first month of chemotherapy. As mentioned in the previous investigations, diminished nutrient intake may be attributed to anti-cancer therapy or other factors related to host and tumour site interaction (Cutsem & Arends 2005). In this study, mean protein intake as a component of total energy intake decreased by approximately 23% after the first month of commencing chemotherapy treatment. Lower protein and energy intake is well proven to be in relation to lower micronutrient intake (Shenkin 2006). A significant negative nitrogen balance was seen in lung, breast and colon cancer patients under chemotherapy despite their normal energy and protein intake (Geirsdottir & Thorsdottir 2008). The researchers concluded that these cancer patients may have a deteriorated status even if they were putting on weight in further stages. However, this assumption might be proven only after comprehensive laboratory assessment of patients in future studies.
It was found in previous studies that the efficacy of protein intake can be improved by physical exercise through stimulating the protein anabolism in haematological cancer patients in months following the intensive chemotherapy (Guadagni & Biolo 2009). In terms of nutritional intervention, there is not sufficient evidence indicating the efficiency of counselling on improvement of dietary intake in cancer patients (Ovesen et al. 1993). However, there were studies on cancer patients undergoing chemotherapy with promising results of a positive change in patients who received diet counselling and supplementation (Ollenschläger et al. 1992; Baldwin et al. 2012).
Reduction seen in B1, B2, B3 and B6 consumption can be attributed to the decreased total energy intake, as the resources of B vitamins are mainly whole grains, which were reduced in patients' dietary intake. This could be due to several reasons including an increase in prevalence of gastrointestinal symptoms such as nausea and vomiting, appetite loss or other oral intake difficulties (Davidson et al. 2012). Moreover, lower intake of dark green vegetables, nuts and dairy products which are rarely part of a hospital-based diet, could also result in the poor quality of diet. Another point that became apparent to the research group was doctors' recommendation regarding the restriction of fresh vegetable intake during the first induction. Patients were told the reason is because of their vulnerability to the infection due to a general weakened immune system during induction therapy. Physicians had microbial safety concerns in case these vegetables had not been properly washed. This is a common advice for haematological patients undergoing chemotherapy treatment and is a part of neutropenic diet that was recommended for these patients in at least two out of the three reference hospitals. Nevertheless, no inference can be made based upon this observation as no relationship was found between the dietary intake and nutritional status after the first induction chemotherapy.
In some of the epidemiological studies, DDS and FVS are commonly assessed to observe their relationship with the risk of cancer incidence or any other diseases. An inverse relationship between DDS and FVS with cancer incidence has been reported (La Vecchia et al. 1997), which suggests the importance of consuming diverse food ingredients.
In the current study, there was a reduction of 30% in mean DDS and 31% in mean FVS after chemotherapy. This reduction can predict an inadequate intake of micronutrients, which, in turn, can have a deleterious effect upon the nutritional and health status of patients. A variety of studies on the relationship between diversity of dietary intake and nutrient adequacy have been conducted on healthy populations which have shown a positive relationship between higher score of variety of food intake and nutrient adequacy (Ruel 2002; Rathnayake et al. 2012). The average DVS and DDS in preoperative gastric cancer patients in Korea showed a more varied dietary intake among women than men. In addition, total calcium intake was much lower than the recommended amount (Lim et al. 2012). In our study, no significant difference was found between the dietary intakes of men and women. Patients' micronutrient intake was significantly reduced in terms of B vitamins and iron, which is consistent with the reduced intake of total energy and protein intake respectively. Similar to the Korean study, calcium intake of patients was very low in both assessment points. According to a study on healthy individuals, a direct correlation between variety of food intake and mean probability of adequacy of intake for 13 main micronutrients of healthy individuals was found (Foote et al. 2004). However, such studies in cancer patients are rare. Nevertheless, no relationship between this impaired intake and PG-SGA score was found in the current study.
As for the number of meals, raising the number of meal intakes for patients whose tolerance to bulky food intakes was low is promising and shows an adaptation to the high incidence of symptoms they experienced during the treatment, as indicated in the study of the same patients (Malihi et al. 2013). This increase in meal pattern of the patients could be, to some extent, affected by dietitians' recommendation in these reference hospitals.
Weight loss before and during cancer treatment is an important indicator to identify malnourished cancer patients (Capuano et al. 2008). This weight loss during chemotherapy in particular may have an impact on treatment outcome (Renshaw et al. 2008). It is reported that remission rate time may be increasingly affected by weight loss in leukaemia patients (Cederholm et al. 2002). Mean weight loss in the latter study was 5 ± 4.9 kg, with approximately 20% of the patients having >10-kg weight loss after completion of their first remission, over 71 ± 51days. In our study, completion of remission was not included in the objectives and has not been assessed. Nevertheless, weight loss during the first month of chemotherapy (28–30 days) was significant and both types of leukaemia patients lost weight at approximately the same rate. However, mean weight loss of 1 month prior to the chemotherapy (4.22 kg in ALL and 3.40 kg in AML) was also evident. This may imply that deteriorated nutritional status is greatly affected by the disease itself rather than the side effects of the treatment. This should however be interpreted cautiously through clinical trials before any confirmation can be made. The mean weight loss of the first month of chemotherapy seen in the current study (3.47 kg) was similar to that of Slaviero et al. (2003) in advanced cancer patients receiving palliative chemotherapy in their first 3 months (Slaviero et al. 2003).
PG-SGA, which is considered to assess nutritional status, is based upon the score which mainly highlights the influence of nutrition impact symptoms on nutritional status (Kubrak et al. 2010). In this study, statistically significant change in this score after the first month conveyed a message of immediate action to dietitians and other health care providers. Similar evidence was found in a cross-sectional study involving several patients of whom patients in their first 2 months of treatment were identified as being in critical need for nutrition intervention (Marques de Oliveira et al. 2010). However, a better estimation of the nutritional status of the patients could be attained by the application of some objective and biochemical measurements.
There was a correlation between weight loss and total energy intake as well as FVS in the first month of chemotherapy, which suggests that patients with higher energy intake lost less body weight than those with lower energy intake. However, in the linear regression, there was no significant relationship between changes in dietary intake and deteriorated nutritional status. Similarly, in a study of cancer patients receiving chemotherapy treatment, 40% of patients were malnourished as a result of unintentional weight loss but their energy intake did not significantly change. This change in nutritional status was found to be unrelated to patients' dietary intake (Geirsdottir & Thorsdottir 2008). There is also another study on head and neck cancer patients undergoing radio- and chemotherapy that found no significant change or even an increase in the energy intake of the patients (Ravasco et al. 2005). In the latter study, patients whose energy intake decreased lost more weight compared to patients with sufficient intake. Nevertheless, the relationship between nutritional status and dietary intake remained unchanged.
A review by Ströhle et al. (2010) of cancer patients has found a deteriorated dietary intake in terms of macro- and micronutrients due to the side effects of treatment-induced nutrition impact symptoms such as anorexia, nausea and vomiting (Ströhle et al. 2010). In this latter study, they concluded that if the decreased dietary intake resulting in malnutrition is not compensated by early intervention, further failure in QOL and treatment outcome can be expected. The same conclusion was also derived elsewhere (Santarpia et al. 2011). In our study, a further reduction in macro- and micronutrient intake was found, which can, to some extent, be attributed to the drug side effects.
In summary, in the current study, no direct relationship between deteriorated dietary intake and diminished nutritional status was found. This might be because of the fact that PG-SGA is a subjective tool, which is highly sensitive to symptoms and assesses different important factors such as metabolic stress and physical activity level rather than details of patients' intake. Although it has a brief section regarding the possible impaired food intake of the patients, its focus is more on the impact of symptoms on nutritional intake and thus may not solely be a sensitive indicator of malnutrition, particularly in the first month of treatment when muscle-wasting is less likely to occur. PG-SGA is particularly useful for dietitians in screening of malnutrition in patients with the aim of planning an early nutritional intervention before and during any treatment.
Application of some objective tools such as anthropometric measurements could have been of utmost help to determine the nutritional status more efficiently and perhaps find some relationship between dietary intake and nutritional status. Further biochemical assessment of vitamins and minerals could have also been very much beneficial to better detect the status of micronutrients and compare it with their intakes. These are some of the limitations of this study as a result of a lack of budget and resources. As an important factor in patient weight status, proper assessment of the fluid retention should also be taken into account in future studies.
To sum up, decreased DDS and FVS, which corresponded to a decline in energy, protein and micronutrients' intake of patients during this period, are worth noting for future investigations. Furthermore, based upon PG-SGA records, the majority of patients required a form of nutritional intervention. This highlights the importance of the role of dietitians along with physicians, nurses and other health providers to improve patients' nutritional status over the periods of chemotherapy treatment.
Recommendations
Comprehensive symptom management by physician or nurse should be considered during the treatment to prevent reduction in dietary intake and subsequent weight loss. Considerable deterioration in dietary intake, which was observed in the first induction of chemotherapy treatment, may be attenuated in the following cycles after complete remission induction due to the usage of less intensive drugs. Thus, assessment of patients in further cycles seems of utmost importance.
Acknowledgements
We wish to thank all the nurses and physicians in the three study hospitals for their kind assistance. Special thanks are extended to Mr R.A. Kalahroudi from Imam Reza hospital and Ms G. Sameie for their kind cooperation. The authors are also grateful to all of the leukaemia patients and their family members for their participation and patience during the study. Special thanks are extended to dear Timothy Morley for his kind effort and great contribution for editing and proof-reading of this article.




