Complete response of locally advanced cutaneous squamous cell carcinoma of the eyelid to topical imiquimod 3.75%
An 80-year-old man with a history of fibromyalgia, arthralgias, and arterial hypertension presented for the recurrence of a skin lesion on the right infraorbital region. Three years earlier, an incisional biopsy reported actinic keratosis and the lesion was treated with cryotherapy and topical diclofenac 3% gel, with only partial improvement.
On physical examination, an erythematous, scaly, well-demarcated plaque of 5.5 cm in diameter was present on the lower eyelid, extending to the medial canthus of the right eye. Ectropion was also evident (Figure 1A). Histological examination of a new incisional biopsy revealed the presence of cutaneous squamous cell carcinoma in situ with a focal invasion of the dermis (Figure 1B).
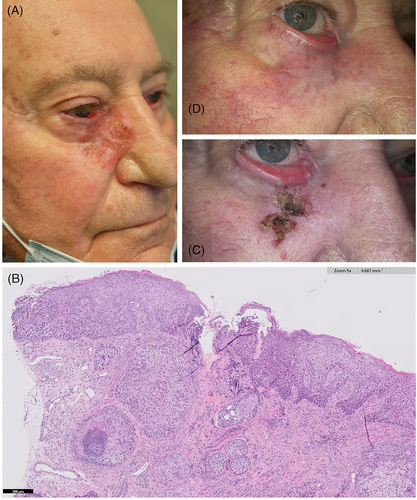
Following multidisciplinary tumor board discussion, surgical and radiation therapy were discouraged due to the high risk of potentially irreversible functional impairment and scarring. The patient was thus started on cemiplimab 350 mg i.v. every 3 weeks. Despite initial clinical improvement, subsequent severe worsening of arthralgias warranted the interruption of the treatment after seven courses. Therefore, after written informed consent, off-label treatment with topical 3.75% imiquimod daily for two consecutive weeks was prescribed. After two courses of two consecutive weeks, we observed a marked reduction in lesional size (Figure 1C). The treatment was well tolerated. Because of partial response, the patient was advised to apply two more courses of topical 3.75% imiquimod. After 6 months, complete response was evident (Figure 1D). In addition, the patient did not report any local or systemic adverse effects from the application of the topical drug.
Imiquimod (1-(2-2methylpropyl)-1H-imidazo [4, 5-c] quinolin-4-amine) is a non-nucleoside heterocyclic amine that activates toll-like receptor (TLR)-7 and -8 inducing activation of the innate and adaptive immune systems. Imiquimod exerts its action through host immunity rather than as a direct antiviral agent.1
Topical 5% imiquimod is licensed by the European Medicines Agency for the management of superficial basal cell carcinomas (sBCCs), actinic keratoses, and external genital warts in adult patients, while the 3.75% formulation is only approved for the topical management of typical, non-hyperkeratotic and non-hypertrophic actinic keratoses on the face or balding scalp in immunocompetent adults. Nevertheless, since its approval, numerous off-label uses for imiquimod have been studied, including squamous cell carcinomas (SCCs) in situ.2
Locally advanced squamous cell carcinoma is defined as nonmetastatic cSCC who cannot undergo surgery or radiation therapy, because of invasion, infiltration recurrences, large size, or when complete resection would cause intolerable complications.3
In our patient, cemiplimab was administered as first-line treatment for a locally advanced SCC of the eyelid. However, poor clinical response in association with worsening of arthralgias was observed. We thus chose the 3.75% concentration of imiquimod due to its better tolerability and anatomical location.
Dealing specifically with the periocular region, a recent literature review showed that almost half of the patients treated with topical imiquimod in this area developed ocular side effects, typically conjunctivitis, blink discomfort, and keratitis. Most of these issues were however generally mild and resolved a few days after withdrawal of the drug.4
To our knowledge, only three cases reporting application of topical imiquimod 3.75% for SCC have been published to now, one localized in the penis, one in the scalp, and one in the cheek.5 However, no data are available for the treatment of locally advanced SCC in the periocular region with topical 3.75% imiquimod.
In conclusion, application of topical 3.75% imiquimod daily for seven courses led to complete regression of the tumor, while no severe side effects requiring withdrawal of the therapy were detected. This immune response modifier drug, at a lower concentration, could be a useful alternative therapeutic option in patients with minimally invasive squamous cell carcinomas in difficult-to-treat areas who cannot undergo surgery, radiation, or systemic therapy. Further studies would nonetheless be required to confirm the effectiveness of topical 3.75% imiquimod in a larger cohort of patients, evaluate the long-term response, and establish the occurrence of relapses.
AUTHOR CONTRIBUTIONS
Francesco Toso: Data collection, writing—original draft preparation, writing—review & editing. M. Chiara Tronconi, Andrea Cortese, Giovanni Fiorillo, Alessandra Bressan: Conceptualization, writing—original draft preparation, writing—review & editing. Antonio Costanzo, Riccardo G. Borroni: Conceptualization, writing—original draft preparation, writing—review & editing. All authors reviewed the results and approved the final version of the manuscript.
ACKNOWLEDGMENT
Open access funding provided by BIBLIOSAN.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




