Exacerbation of subacute cutaneous lupus erythematosus following vaccination with BNT162b2 mRNA vaccine
Unspecific inflammatory cutaneous reactions are an expected finding in course of vaccinations as a broad cytokine release orchestrates the activation of both cellular and humoral components of the immune system.1 Due to an interferon-γ driven pathogenesis, exacerbation and new onset of cutaneous lupus erythematosus (CLE) have been described in course of influenza- and DPT (diphtheria, pertussis, and tetanus) combination vaccines.2, 3 Most clinical trials of different coronavirus disease 2019 (covid-19) vaccines did not include rheumatic patients; disease worsening was expected in interferonopathies and systemic lupus erythematosus from a pathophysiological point of view.4 Very recently, an Italian group published first experiences with cutaneous adverse reactions due to a messenger ribonucleic acid (mRNA)-based covid-19 vaccine, which included erythema, swelling and generalized erythematous rash including urticaria.5 We here present a case of a subacute CLE (SCLE) patient, who developed a disease exacerbation after receiving the first dose of BNT162b2 (Comirnaty, BioNTech/Pfizer), but tolerated the second dose without symptoms.
A 73-year-old female patient was diagnosed with SCLE in 2005 and remained under our care thereafter. Apart from typical skin lesions, which partly resulted in hypopigmentation, she recurrently suffered from Raynaud's syndrome with digital ulcerations, diffuse hair loss and joint pain. Her antibody profile revealed SCLE-typical findings with high titers of anti-Sjögren's-syndrome-related antigen A (anti-SSA)-autoantibodies. In the past, she intermittently required systemic treatment with hydroxychloroquine and corticosteroids, however, since 01/2020 she had been in full remission without systemic disease-modifying drugs. She continued to use rigorous protection measures against ultraviolet light. The patient received the first dose of BNT162b2 mRNA vaccine on 27th March 2021 in accordance with local prioritization conventions. Ten days later she suffered from disseminated burning erythematous patches (Figure 1) and fatigue in absence of fever. A therapeutic attempt with prednisolone 10 mg p.o. failed to achieve control, hence a pulse therapy starting with 60 mg prednisolone p.o. tapered over 3 weeks in combination with topical mometasone furoate ointment lead to amelioration. She received her second dose as scheduled after 6 weeks and did not experience any adverse reaction. Informed consent was obtained from the individual participant to be included in this report. We thank the patient for granting permission to publish this information.
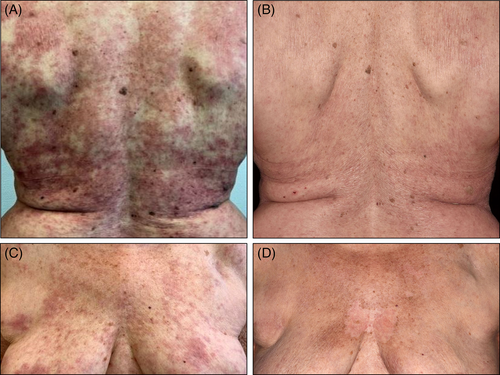
Various external factors may result in disease flares in CLE, including drugs and vaccines, even in patients who achieve long-standing remission.6 All types of vaccines inevitably evoke immune responses reflecting a mounting protection against specific pathogens; a robust immune response may ultimately result in exacerbation of preexisting autoimmune disease and even triggering off autoimmune diseases de novo in particular individuals.7 Available data remains scant as the mRNA group of vaccines has only recently been used in large populations in light of the covid-19 pandemic. While still under ongoing investigation, reactions to these agents might differ from classical vaccine types (live-attenuated-, toxoid-, subunit-, component-, viral-vector-based vaccines). We are convinced that there is a direct link between the vaccine and the symptoms experienced by our patient. It remains ambiguous however, why the second dose was well-tolerated in turn. Most probably, lupus-specific inflammatory pathways had been effectively inhibited by the preceding pulse of prednisolone to reduce the individual susceptibility for another flare.
In spite of the potential of vaccines to elicit flares in CLE patients, the individual benefit of protection against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outlies the risk by far. In this case, disease control could readily be achieved by a short pulse of prednisolone combined with potent topical corticosteroid ointments. Hence, CLE patients should be encouraged to claim covid-19 vaccines once available in their country.8 Whether vector-based or mRNA-based covid-19 vaccines employ a larger risk to patients with preexisting autoimmune diseases must be addressed in further studies.
CONFLICT OF INTEREST
The authors have been an advisor and/or received speakers' honoraria or travel expense reimbursements and/or received grants and/or participated in clinical trials of the following companies/organizations: Dennis Niebel: BMS, Novartis, GSK, Celgene, L'Oreal, MSD, Kiowa Kyrin. Christine Braegelmann: Novartis, L'Oreal, GSK. Thomas Bieber: was speaker and/or consultant and/or Investigator for AbbVie, Allmiral, AnaptysBio, Arena, Asana Biosciences, Bayer Health, BioVerSys, Böhringer-Ingelheim, BMS, Celgene, Daichi-Sankyo, Dermavant/Roivant, DermTreat, Domain Therapeutics, DS Pharma, RAPT/FLX Bio, Galapagos/MorphoSys, Galderma, Glenmark, GSK, Incyte, IQVIA, Janssen, Kirin, Kymab, LEO, LG Chem, Lilly, L'Oréal, MenloTx, Novartis, OMPharma/Vifor, Pfizer, Pierre Fabre, Sanofi/Regeneron, UCB. Thomas Bieber is founder of the non-profit biotech company “Davos Biosciences” within the International Kühne-Foundation. Joerg Wenzel: GSK, Novartis, Medac, Merck/Serono, Roche, Actelion, Pfizer, Spirig, ArrayBio, Biogen. The remaning authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Dennis Niebel wrote the first draft of the manuscript. Dennis Niebel and Christine Braegelmann prepared the figure. Veronika Ralser-Isselstein, Kristel Jaschke, and Joerg Wenzel were directly involved in the treatment of the patient. All authors added critical intellectual content and read and approved the final version of the manuscript.
ETHICS STATEMENT
The patient gave her written consent about publication of the case and the pictures.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




