Higher levels of bodily pain in people with long-term type 1 diabetes: associations with quality of life, depressive symptoms, fatigue and glycaemic control – the Dialong study
Abstract
Aims
To compare reported level of bodily pain, overall and health-related quality of life (QoL), depression and fatigue in people with long-term type 1 diabetes vs. a comparison group without diabetes. Further, to examine the associations of total bodily pain with QoL, depression, fatigue and glycaemic control in the diabetes group.
Methods
Cross-sectional study of 104 (76% of eligible) people with type 1 diabetes of ≥ 45 years’ duration attending the Norwegian Diabetes Centre and 75 persons without diabetes who completed questionnaires measuring bodily pain (RAND-36 bodily pain domain), shoulder pain (Shoulder Pain and Disability Index), hand pain (Australian/Canadian Osteoarthritis Hand Index), overall QoL (World Health Organization Quality of Life – BREF), health-related QoL (RAND-36), diabetes-specific QoL (Audit of Diabetes-Dependent Quality of Life; only diabetes group), depression (Patient Health Questionnaire) and fatigue (Fatigue questionnaire). For people with type 1 diabetes, possible associations between the bodily pain domain (lower scores indicate higher levels of bodily pain) and other questionnaire scores, were measured with regression coefficients (B) per 10-unit increase in bodily pain score from linear regression.
Results
The diabetes group reported higher levels of bodily (P = 0.003), shoulder and hand pain (P < 0.001) than the comparison group. In the diabetes group, bodily pain was associated with lower overall and diabetes-specific QoL [B (95% confidence intervals)]: 0.2 (0.1, 0.2) and 0.2 (0.1, 0.3); higher levels of depression −1.0 (−1.3, −0.7) and total fatigue −1.5 (−1.9, −1.2); and worse glycaemic control HbA1c (mmol/mol; %) −0.8 (−1.5, −0.1); −0.1 (−0.1, −0.01).
Conclusions
People with long-term type 1 diabetes experience a high level of bodily pain compared with a comparison group. Total bodily pain was associated with worse QoL and glycaemic control.
What’s new?
- Long-term survivors of type 1 diabetes are burdened by several complications, conditions and medications that may cause pain. However, a description of the level of bodily pain is lacking.
- This study is the first to describe high levels of bodily pain, which are associated with quality of life, in long-term survivors of type 1 diabetes.
- A broader focus on pain is warranted and screening tools should facilitate this.
Introduction
A large proportion of people with long-term type 1 diabetes suffer from vascular complications [1], of which both neuropathy and cardiovascular disease may cause pain. Recent studies have found that painful conditions in the upper extremities affecting daily life are common in people with long-term type 1 diabetes [2-5]. Moreover, like in the general population, these people suffer from other painful conditions, for example musculoskeletal, rheumatic or neurological comorbidities. In addition, medications such as statins may cause pain [6]. However, the level of bodily pain and its influence on daily working capacity in people with long-term type 1 diabetes have not yet been described.
Previous studies on pain and its associations with depression in long-term type 1 diabetes have been limited to neuropathy and neuropathic pain [7] or to pain in the upper extremities [2, 3, 5]. However, the pertinent pain burden for any individual is the total level of pain, regardless of bodily distribution or cause. Moreover, total bodily pain and its association with quality of life (QoL), depression, fatigue and glycaemic control have not yet been studied in people with type 1 diabetes of more than 25 years’ duration [8]. Therefore, we wanted to describe and compare pain, QoL, levels of depressive symptoms and fatigue among people surviving more than 45 years with type 1 diabetes compared with a group of persons without diabetes but of similar age, sex and socio-economic status. Thus, we aimed to: (i) compare reported levels of bodily pain, overall and health-related QoL, depression and fatigue in people with long-term type 1 diabetes vs. a comparison group without diabetes; and (ii) examine the association of reported levels of bodily pain with QoL, depression, fatigue and glycaemic control in the diabetes group.
Participants and methods
This work is part of the Dialong study, a cross-sectional study in which all persons with type 1 diabetes diagnosed before 1970 (n = 136) attending the Norwegian Diabetes Centre (NDC) in Oslo, Norway, in 2015 were invited to participate by mail.
The study group with type 1 diabetes was asked to invite their spouse and/or close friends to participate in the comparison group; exclusion criteria were first-degree relatives, known diabetes or HbA1c > 48 mmol/mol (6.5%). The comparison group participated at the same time and in the same examinations as the diabetes group, except for the diabetes-specific examinations (capillary blood glucose, interview questions about hypoglycaemia, retina photos, and diabetes-specific QoL).
In total, 104 people (77%) with long-term type 1 diabetes and 75 members in the comparison group participated in the study (Table 1). At the NDC, the diabetes participants’ charts were reviewed by two physicians who registered data on complications, comorbidities and medications. Participants first visited the NDC to answer questionnaires and undergo interviews and clinical examinations. Within three weeks, they attended Oslo University Hospital for fasting blood tests (eGFR, HbA1c), urine sample (albuminuria), retina photos and joint questionnaires. Details of the data collection methods have been published elsewhere [9]. The questionnaires assessed participants’ reports of pain, QoL, depression and fatigue.
The Regional Committee for Medical and Health Research Ethics South-East, Norway, approved the study (project no. 2014/851). Written informed consent was obtained from each participant.
Patient-reported measures
We used Norwegian translated versions of all instruments (Table 2 and Table S1), and these were scored according to published algorithms [10-17].
Pain
The main study variable is the participant’s own rating of his/her total bodily pain assessed by the bodily pain domain of the widely used RAND-36, which includes two items: ‘How much bodily pain have you had during the past 4 weeks?’, and ‘During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?’. We also analysed the two pain items as separate variables. Higher scores indicate lower levels of bodily pain and its interference with work [16].
Upper extremity pain severity and degree of difficulty with activities of daily living requiring upper extremity use were measured by the Shoulder Pain and Disability Index (SPADI) [14]. Hand pain and functioning was measured by the Australian/Canadian Osteoarthritis Hand Index (AUSCAN) [15]. For both measures, higher scores indicate more and worse symptoms. Participants were also asked: ‘Do you have burning or aching pain in your feet or legs?’ (yes/no).
Quality of Life
Overall QoL was assessed by the first overall item of the World Health Organization Quality of Life – BREF (WHOQoL-BREF): during the previous two weeks: ‘How would you rate your quality of life?’ [17]. Health-related QoL was assessed by the RAND-36 physical and mental component scores (PCS and MCS) [16]. Diabetes-specific QoL was measured by the Audit of Diabetes-Dependent Quality of Life (ADDQoL-18), which assesses how diabetes affects QoL [10]. For all instruments, higher scores represent better QoL.
Depression
The symptoms of depression were measured by the Patient Health Questionnaire (PHQ-9) [13]; higher scores represent higher symptom levels.
Fatigue
Extent and severity of fatigue was measured by the Fatigue Questionnaire (FQ); higher scores represent higher levels of fatigue. Chronic fatigue was identified as a duration of ≥ 6 months [12, 11
].
Comorbidities and medications
Glucose was measured using a capillary blood glucose meter right after completing the questionnaires at the first visit at the NDC. HbA1c, haemoglobin and thyroid-stimulating hormone were measured by standard methods when attending Oslo University Hospital for fasting blood tests (second visit) [9]. Hypoglycaemia (capillary blood glucose < 4 mmol/l) and severe hypoglycaemia were defined as number of self-reported episodes per month, and severe hypoglycaemia as a hypoglycaemic episode requiring assistance from another person for treatment.
Retinopathy, neuropathy, persistent albuminuria and past cardiovascular event have been defined previously [9]. In short, retinopathy was categorized as none, background or proliferative. Neuropathy was defined based on the presence of both typical symptoms in the lower extremities and symmetrical signs in both lower extremities using standard monofilament and vibration tests. Persistent albuminuria was defined as a urinary albumin to creatinine ratio of > 2.9 mg/mmol on two consecutive samples for the diabetes group and one for the comparison group. Past cardiovascular event included a diagnosis of coronary heart disease, cerebrovascular disease or peripheral vascular disease. Hypothyroidism was based on a recorded diagnosis of hypothyroidism or the use of levothyroxine. Erectile dysfunction in men was defined based on a self-reported ‘yes/no/unsure’ on whether they had been unable to perform sexual intercourse or maintain erection without aids/medications for the previous months/years. Other painful chronic conditions included conditions associated with pain, like intermittent claudication, angina pectoris, rheumatic diseases, neurological comorbidity, inflammatory bowel disease and lumbar disc herniation.
Self-reported daily use of painkillers (yes/no) included non-steroidal anti-inflammatory drugs (NSAIDs) or other forms of painkillers. We chose to define amitriptyline as a painkiller because it is prescribed for neuropathic pain in diabetes and is rarely used for depression in Norway. Anxiolytics was defined as any use of benzodiazepines. Hypnotics were generated from any use of zopiclone, zolpidem or alimemazine, and antidepressants from any use of paroxetine, sertraline, venlafaxine, citalopram, escitalopram or mianserin.
Statistical analysis
Data are displayed as mean ± sd, median (quartiles) or n (%). We used t-tests with correction for unequal variances between groups (or Wilcoxon rank-sum test if skewed data) and chi-square tests to compare the clinical characteristics and questionnaire scores between the study and comparison groups.
In the type 1 diabetes group, we examined associations between the primary variable of interest, the bodily pain domain score of RAND-36, and the outcomes of overall QoL (WHOQOL-BREF), diabetes-specific QoL (ADDQoL-18), depression (PHQ-9), fatigue (FQ) and glycaemic control (HbA1c), using bivariate and adjusted linear regression analyses. The pain domain score of RAND-36 was divided by 10 before inclusion in the regression models to obtain regression coefficients per 10-unit change in bodily pain. Higher scores on the pain domain score reflect less pain. To examine if pain alone yielded different results, we repeated the analysis with only item 7 of the RAND-36 bodily pain domain (‘How much bodily pain have you had during the past 4 weeks?’). We applied three different levels of adjustment of known, possible confounders. First, we adjusted for age, sex and education. We then added use of painkillers daily as an additional adjustment variable and lastly, we added glycaemic control (HbA1c). Successive addition of adjustment variables did not change the regression coefficient for bodily pain substantially and we therefore only reported the final adjusted model. To make it possible to compare the strength of the associations between different outcomes and different measures of pain we also reported standardized regression coefficients.
A P-value < 0.05 was considered statistically significant, no adjustments were made for multiplicity of statistical tests. All analyses were performed using STATA/SE 15.0.
Results
Of 136 eligible persons with long-term type 1 diabetes, 105 (77%) agreed to participate, of whom 104 (99%) completed the questionnaires. All 75 persons (number of persons invited unknown) agreeing to participate in the comparison group completed the questionnaires. There were no statistically significant differences between the two groups in age, sex, education level, smoking status, or daily use of painkillers, anxiolytics, hypnotics or antidepressants (Table 1). A statistically significant higher percentage of the diabetes group were living alone, not working and physically inactive. Furthermore, relative to the comparison group they reported more comorbidities, and more use of levothyroxine, statins, aspirin and anti-hypertensive medication. They also had lower haemoglobin and higher thyroid-stimulating hormone levels.
| Diabetes group | n | Comparison group | n | P-value | |
|---|---|---|---|---|---|
| Sociodemographic | |||||
| Age (years) | 61 ± 7.3 | 104 | 61 ± 6.9 | 75 | 0.485 |
| Sex male | 52 (50) | 104 | 34 (45) | 75 | 0.538 |
| Education college | 64 (62) | 104 | 55 (73) | 75 | 0.099 |
| Living alone | 26 (25) | 104 | 8 (11) | 75 | 0.016 |
| Working | 44 (42) | 104 | 45 (60) | 75 | 0.019 |
| Lifestyle factors | |||||
| Physical inactivity* | 10 (9.9) | 101 | 1 (1.4) | 74 | 0.021 |
| Currently smoking | 5 (4.8) | 104 | 7 (9.3) | 75 | 0.232 |
| Diabetes-related factors | |||||
| Glucose tested at visit 1, mmol/l | 9.3 ± 3.6 | 103 | — | — | |
| HbA1c, mmol/mol | 58 ± 8.6 | 102 | 36 ± 3.1 | 71 | < 0.001 |
| HbA1c, % | 7.4 ± 0.8 | 5.5 ± 0.3 | |||
| Diabetes duration, years | 49 (45-54) | 104 | — | — | |
| Hypoglycaemia, number last month | 12 (5.5-20) | 104 | — | — | |
| Severe hypoglycaemia last year (requiring assistance), yes | 16 (15) | 104 | |||
| Number of times, median (min-max) | 2 (1-10) | 16 | |||
| Morbidity | |||||
| Retinopathy† | |||||
| None | 5 (4.8) | 104 | — | — | |
| Background | 54 (52) | 104 | — | — | |
| Proliferative | 45 (43) | 104 | — | — | |
| Neuropathy‡ | 66 (64) | 104 | 16 (21) | 75 | < 0.001 |
| Persistent albuminuria§ | 17 (16) | 104 | 4 (5.5)¶ | 73 | 0.028 |
| eGFR < 60 | 9 (8.7) | 104 | 2 (2.7) | 75 | 0.100 |
| Hypothyroidism‖ | 36 (35) | 104 | 9 (12) | 75 | 0.001 |
| Erectile dysfunction in men | 24 (46) | 52 | 6 (18) | 34 | 0.019 |
| Past cardiovascular event** | 21 (20) | 104 | 6 (8.0) | 75 | 0.025 |
| Other painful chronic conditions†† | 52 (50) | 104 | 33 (44) | 75 | 0.428 |
| Medication | |||||
| Painkillers, daily | 10 (9.6) | 104 | 6 (8.0) | 75 | 0.924 |
| Anxiolytics | 2 (1.9) | 104 | 2 (2.7) | 75 | 0.740 |
| Hypnotics | 6 (5.8) | 104 | 1 (1.3) | 75 | 0.131 |
| Antidepressants | 6 (5.8) | 104 | 1 (1.3) | 75 | 0.131 |
| Sleep medication/anxiolytics and hypnotics | 7 (6.7) | 104 | 3 (4.0) | 75 | 0.433 |
| Anxiolytics or antidepressants | 8 (7.7) | 104 | 3 (4.0) | 75 | 0.310 |
| Levothyroxine | 23 (22) | 104 | 3 (4.0) | 75 | 0.001 |
| Statins | 56 (54) | 104 | 12 (16) | 75 | < 0.001 |
| Acetylsalicylic acid (ASA) | 33 (32) | 104 | 9 (12) | 75 | 0.002 |
| Antihypertensives | 56 (54) | 104 | 18 (24) | 75 | < 0.001 |
| Laboratory variables | |||||
| Haemoglobin g/dl | 14.0 ± 1.3 | 102 | 14.5 ± 1.0 | 73 | 0.013 |
| Thyroid-stimulating hormone (TSH) mU/l | 2.6 ± 2.0 | 99 | 1.9 ± 1.0 | 71 | 0.014 |
- Data are mean ± sd, median (quartiles) or n (%) unless otherwise stated.
- * Physical inactivity was defined as < 30 min of fast walking 1–2 times a week.
- † Retinopathy was categorized as either none, background or proliferative (pan-retinal photocoagulation scars or proliferative findings) retinopathy based on retina photos.
- ‡ Neuropathy was defined based on the presence of both typical symptoms in the lower extremities (numbness, unsteadiness, aching or burning pain or pins and needles) and symmetrical signs in both lower extremities using standard monofilament and vibration tests.
- § Persistent albuminuria was defined as an albumin/creatinine ratio of > 2.9 mg/mmol on two consecutive samples.
- ¶ Based on one urine sample measuring albumin/creatinine ratio.
- ‖ Based on hypothyroidism diagnosis or use of thyroxin.
- ** Past cardiovascular event included a diagnosis of coronary heart disease (previous verified angina, acute coronary syndrome or revascularization), cerebrovascular disease or peripheral vascular disease.
- †† Other painful chronic conditions including conditions associated with pain, like intermittent claudication, angina pectoris, rheumatic diseases, neurological comorbidity excluding peripheral diabetic neuropathy, inflammatory bowel disease and lumbar disc herniation.
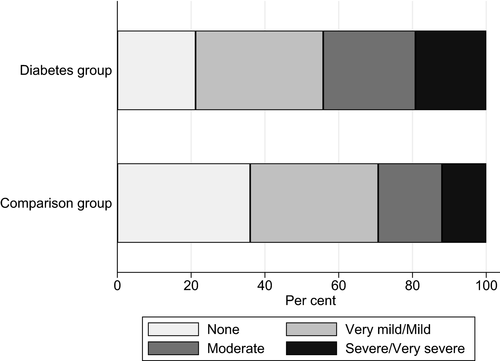
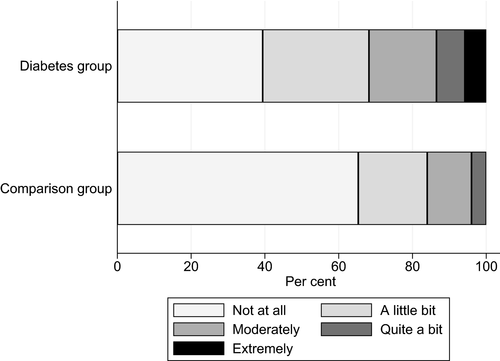
Table 2 outlines the results from the questionnaires. Mean bodily pain as measured by RAND-36 indicated higher levels of bodily pain in the long-term type 1 diabetes group than in the comparison group (66 ± 28.2 vs. 78 ± 24.1; P = 0.003), lower scores indicate higher levels of bodily pain. Based on RAND-36 item 7, ‘How much bodily pain have you had during the past 4 weeks?’, the diabetes group was more likely to experience moderate to very severe pain (44% vs. 29%; P = 0.043) (Fig. 1). Among those experiencing moderate to very severe pain, 17% in the diabetes group and 27% in comparison group used painkillers daily. Relative to the comparison group, the diabetes group reported more shoulder pain (SPADI Pain subscale score) (median 8.0 vs. 0.0; P < 0.001), hand pain (AUSCAN Pain subscale) (mean ± sd score: 4.1 ± 4.8 vs. 1.7 ± 2.8; P < 0.001), and more pain in their feet or legs (interview question) (26% vs. 5.3%; P < 0.001). Other painful chronic conditions were similar in the diabetes and comparison groups (50% vs. 44%) (Table 1). Based on RAND-36 item 8, ‘During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?’, the diabetes group reported more interference than the comparison group (32% vs. 16%; P = 0.017) (Fig. 2).
| Diabetes group | n * | Comparison group | n * | P-value | |
|---|---|---|---|---|---|
| Pain | |||||
| RAND-36 | |||||
| Bodily pain domain score [0–100]† | 66 ± 28.2 | 104 | 78 ± 24.1 | 75 | 0.003 |
|
Item 7: ‘How much bodily pain have you had during the past 4 weeks?’ Moderate/severe/very severe pain |
46 (44) | 104 | 22 (29) | 75 | 0.043 |
|
Item 8: ‘During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?’ Moderate/quite a bit/extreme interference |
33 (32) | 104 | 12 (16) | 75 | 0.017 |
| Upper and lower extremities | |||||
| SPADI Index score [0–100]†, ‡ | 8.1 (1.6–37.0) | 102 | 0.6 (0.0–7.1) | 72 | < 0.001 |
| SPADI Pain subscale [0–100]†, ‡ | 8.0 (0.0–38.0) | 102 | 0.0 (0.0–11.0) | 72 | < 0.001 |
| AUSCAN Index score [0–60]† | 13.4 ± 13.0 | 101 | 5.0 ± 7.4 | 73 | < 0.001 |
| AUSCAN Pain subscale [0–20]† | 4.1 ± 4.8 | 101 | 1.7 ± 2.8 | 73 | < 0.001 |
| Interview question: ‘Do you have burning or aching pain in your feet or legs?’ ‘Yes’ | 27 (26) | 104 | 4 (5.3) | 75 | < 0.001 |
| Quality of Life (QoL) | |||||
| Overall QoL | |||||
| WHOQoL-BREF [1–5]† | 3.4 ± 1.1 | 102 | 4.0 ± 0.8 | 74 | < 0.001 |
| Health-related QoL | |||||
| RAND-36 Component Scores | |||||
| Physical Component Score (PCS) | 49.9 ± 11.6 | 99 | 57.6 ± 8.1 | 73 | < 0.001 |
| Mental Component Score (MCS) | 38.6 ± 7.0 | 99 | 39.4 ± 4.9 | 73 | 0.399 |
| Diabetes-specific QoL | |||||
| ADDQoL-18 [−9 to +9]† | −1.3 (−2.3, 0.7) | 100 | NA | NA | NA |
| Depression | |||||
| PHQ-9 [0–27] † | 6.3 ± 4.9 | 104 | 4.0 ± 3.6 | 75 | < 0.001 |
| PHQ-9 Clinically significant depression (score ≥10) | 20 (19) | 104 | 6 (8.0) | 75 | 0.035 |
| Fatigue | |||||
| FQ Total fatigue [0–33]† | 15.2 ± 6.1 | 104 | 11.6 ± 3.0 | 75 | < 0.001 |
| FQ Physical fatigue [0–21]† | 10.3 ± 4.5 | 104 | 7.5 ± 2.6 | 75 | < 0.001 |
| FQ Mental fatigue [0–12]† | 4.9 ± 2.1 | 104 | 4.1 ± 1.0 | 75 | 0.003 |
| FQ Chronic fatigue (total) (score >4) | 36 (35) | 104 | 5 (6.7) | 75 | < 0.001 |
| FQ Chronic physical fatigue (score >4) | 32 (31) | 104 | 4 (5.3) | 75 | < 0.001 |
| FQ Chronic mental fatigue (score >4) | 0 | 104 | 0 | 75 | |
- Unless otherwise stated, numbers presented are mean ± sd, median (quartiles) or n (%).
- NA, not applicable.
- * Total population (n) varies some for each characteristic reliant on the actual completion of the diverse questionnaires (missing range diabetes group: 0–5; comparison group: 0–3).
- † Scoring scales. For the Shoulder Pain and Disability Index score (SPADI), Australian/Canadian Osteoarthritis Hand (AUSCAN), Patient Health Questionnaire (PHQ-9) and Fatigue Questionnaire (FQ) a higher score indicates more symptoms and is worse. For the RAND-36, World Health Organization Quality of Life-BREF (WHOQOL-BREF) and Audit of Diabetes-Dependent Quality of Life (ADDQoL-18) a higher score indicates better quality of life.
- ‡ Wilcoxon rank-sum test (due to skewed data).
The diabetes group reported lower overall QoL (WHOQoL-BREF) than the comparison group (mean ± sd score: 3.4 ± 1.1 vs. 4.0 ± 0.8; P < 0.001), lower health-related QoL measured by the RAND-36 PCS (mean ± sd: 49.9 ± 11.6 vs. 57.6 ± 8.1; P < 0.001) but not in the MCS (mean ± sd score: 38.6 ± 7.0 vs. 39.4 ± 4.9; P = 0.399). The diabetes group reported higher levels of depressive symptoms (PHQ-9) (mean ± sd score: 6.3 ± 4.9 vs. 4.0 ± 3.6; P < 0.001), and a higher prevalence (19% vs. 8.0%; P = 0.035) of clinically significant depression. They also reported more chronic fatigue (35% vs. 6.7%; P < 0.001) as measured by the FQ (score > 4) (Table 2).
In regression analysis of the type 1 diabetes group, bodily pain (RAND-36) was associated with QoL, depression, fatigue and glycaemic control (HbA1c), with lower scores on bodily pain, indicating higher influence of bodily pain (Table 3). Bodily pain was associated with overall QoL (WHOQOL-BREF) [adjusted regression coefficients (B) (95% confidence intervals) and standardized regression coefficients: 0.2 (0.1, 0.2) and 0.4] and diabetes-specific QoL (ADDQoL-18) [0.2 (0.1, 0.3) and 0.5], indicating that more pain was associated with lower general and diabetes-specific QoL. Further, more bodily pain was associated with higher levels of depressive symptoms (PHQ-9) [−1.0 (−1.3, −0.7) and −0.6], fatigue (FQ) [total −1.5 (−1.9, −1.2) and −0.7; physical −1.1 (−1.4, −0.9) and −0.7; mental −0.4 (−0.6, −0.3) and −0.6], and worse glycaemic control (HbA1c mmol/mol; %) [−0.8 (−1.5, −0.1); −0.1 (−0.1, −0.01) and −0.3]. Adjustments for age, sex, education, use of painkillers daily and HbA1c did not change results markedly.
| Unadjusted | Adjusted† | |||||
|---|---|---|---|---|---|---|
| Regression coefficient B* (95% CI) | Standardized regression coefficient | n | Regression coefficient B* (95% CI) | Standardized regression coefficient | n | |
| Model 1: RAND-36 Bodily pain domain | ||||||
| Overall QoL (WHOQoL-BREF) | 0.2 (0.1, 0.2) | 0.5 | 102 | 0.2 (0.1, 0.2) | 0.4 | 100 |
| Diabetes-specific QoL (ADDQoL-18) | 0.2 (0.1, 0.3) | 0.5 | 100 | 0.2 (0.1, 0.3) | 0.5 | 99 |
| Depression (PHQ-9) | −1.1 (−1.4, −0.8) | −0.6 | 104 | −1.0 (−1.3, −0.7) | −0.6 | 102 |
| Fatigue | ||||||
| FQ Total | −1.5 (−1.8, −1.2) | −0.7 | 104 | −1.5 (−1.9, −1.2) | −0.7 | 102 |
| FQ Physical | −1.1 (−1.4, −0.9) | −0.7 | 104 | −1.1 (−1.4, −0.9) | −0.7 | 102 |
| FQ Mental | −0.4 (−0.5, −0.3) | −0.5 | 104 | −0.4 (−0.6, −0.3) | −0.6 | 102 |
| Glycaemic control | ||||||
| HbA1c, mmol/mol | −0.9 (−1.5, −0.3) | −0.3 | 102 | −0.8 (−1.5, −0.1) | −0.3 | 102 |
| HbA1c, % | −0.1 (−0.1, −0.03) | −0.3 | 102 | −0.1 (−0.1, −0.01) | −0.3 | 102 |
| Model 2: RAND-36, Item 7 ‘How much bodily pain have you had during the past 4 weeks?’ | ||||||
| ‘How much bodily pain have you had during the past 4 weeks?’ | ||||||
| Overall QoL (WHOQoL-BREF) | 0.3 (0.1, 0.4) | 0.4 | 102 | 0.2 (0.1, 0.4) | 0.3 | 100 |
| Diabetes-specific QoL (ADDQoL-18) | 0.4 (0.2, 0.5) | 0.4 | 100 | 0.3 (0.2, 0.5) | 0.4 | 99 |
| Depression (PHQ-9) | −1.9 (−2.4, −1.4) | −0.6 | 104 | −1.6 (−2.2, −1.0) | −0.5 | 102 |
| Fatigue | ||||||
| FQ Total | −2.7 (−3.3, −2.1) | −0.7 | 104 | −2.5 (−3.2, −1.8) | −0.6 | 102 |
| FQ Physical | −2.0 (−2.4, −1.6) | −0.7 | 104 | −1.9 (−2.4, −1.4) | −0.7 | 102 |
| FQ Mental | −0.7 (−0.9, −0.4) | −0.5 | 104 | −0.6 (−0.9, −0.3) | −0.5 | 102 |
| Glycaemic control | ||||||
| HbA1c, mmol/mol | −1.6 (−2.7, −0.5) | −0.3 | 102 | −1.5 (−2.7, −0.2) | −0.3 | 102 |
| HbA1c, % | −0.1 (−0.2, −0.04) | −0.3 | 102 | −0.1 (−0.3, −0.02) | −0.3 | 102 |
- Respondents with missing values on any of the explanatory variables were excluded from the regression analyses, giving a sample size ranging from 99 to 104.
- For RAND-36 bodily pain domain, the higher the score, the less influence of bodily pain on quality of life. For the World Health Organization Quality of Life-BREF (WHOQOL-BREF) and Audit of Diabetes-Dependent Quality of Life (ADDQoL-18), a higher score is better. For the Patient Health Questionnaire (PHQ-9) and Fatigue Questionnaire (FQ) a higher score indicates more depressive symptoms or fatigue.
- * The unstandardized regression coefficients measure change in the outcome per 10-unit change in RAND-36 bodily pain domain and per 1-unit change for RAND-36 item 7.
- † Adjusted for age, sex, education, painkillers daily and HbA1c.
When using RAND-36 Item 7, ‘How much bodily pain have you had during the past 4 weeks?’, as the indicator of pain there were only small differences in the standardized regression coefficients (model 2 compared with model 1). Adjustments for age, sex, education, painkillers daily and HbA1c did not change results markedly.
Discussion
People with long-term type 1 diabetes experienced statistically significantly higher levels of bodily pain, worse QoL, worse physical health, higher levels of depressive symptoms and fatigue compared with a group similar in age, sex and socio-economic status without diabetes. In the diabetes group, bodily pain was associated with worse QoL, higher levels of depressive symptoms and fatigue, and worse glycaemic control.
The percentage of people with long-term type 1 diabetes reporting moderate to very severe bodily pain was more than twice as high as the percentage using painkillers daily (44% vs. 17%). There is a well-known association between diabetes and pain [18], and recent studies have found increased levels of pain among people with long-term type 1 diabetes [2-5]. There are several possible underlying aetiologies. The prevalence of painful conditions in the upper extremities is relatively high (38–64%) in participants with a shorter diabetes duration [2, 4]. We have previously reported a lifetime prevalence of one or more hand or shoulder diagnoses (frozen shoulder, Dupuytren’s disease, trigger finger, carpal tunnel syndrome) as high as 94% [9] and a large percentage experienced pain (and disability) in their upper extremities, as reflected by the pain subscale scores in SPADI and AUSCAN, reflecting increasing levels of pain with increasing diabetes duration. Moreover, 26% reported burning or aching pain in their feet or legs, indicating peripheral diabetic neuropathic pain. In a Canadian study of longevity in type 1 diabetes, the prevalence of neuropathic pain was 36%; only 8.9% of these used neuropathic pain medications and 16% used other pain medications [19]. Total bodily pain in the present study was associated with worse QoL in people with long-term type 1 diabetes. Thus, there is a need for more attention on pain in clinical consultations.
Higher levels of bodily pain were associated with higher levels of depressive symptoms. This finding is in contrast to the only other study on pain and depression in long-term type 1 diabetes we identified [7]. In the Canadian study of longevity, neuropathy was associated with depression, independent of neuropathic pain, and other vascular complications that might cause pain, like cardiovascular disease, were not associated with depression [7]. By contrast, in a longitudinal study in which most participants had type 2 diabetes, neuropathy was a risk factor for depressive symptoms because it generated pain and unsteadiness [20]. As we studied the level of total bodily pain, we did not control for complications that might mediate the effect of diabetes. The association between bodily pain and depression in our study might reflect supplementary or different mechanisms. Prospective studies of long-term type 1 diabetes should explore such mechanisms more closely.
We found a weak association between more bodily pain and worse glycaemic control. Prior studies show conflicting results regarding pain and glycaemic control. Menting et al. [8] found no association in a group of severely fatigued people with type 1 diabetes duration of 25 years. An association of hand and shoulder pain with worse long-term HbA1c was shown previously in our study population [3, 5], and in a Swedish study of current HbA1c and shoulder impairment [2].
The study participants had several conditions associated with bodily pain, including a past cardiovascular event (20%) and other painful chronic conditions (50%), in addition to the use of statins (54%). These conditions are associated with different pain patterns and require different medical management plans. This emphasizes the need for a systematic focus on pain by including patient-reported outcome measures and organ/disease-specific examination to be able to diagnose and treat pain sufficiently in the routine care for people with long-term type 1 diabetes.
Strengths of this study include having a population surviving more than 45 years with type 1 diabetes who are phenotypically well characterized by extensive examinations and with a high response rate on the questionnaires. Moreover, there were no differences in background data between fully included participants and participants allowing a chart review [21]. Our sample should give a reasonable representation of those with diabetes who were invited to participate. Further, the mean HbA1c among our long-term type 1 diabetes group was similar to that for the Norwegian type 1 diabetes population of more than 45 years duration [9]. However, our study has limitations. The cross-sectional design cannot assess causal relations. In addition, because members of the comparison group had a close relationship to a member of the study group, they were not independent observations. However, the groups were well matched in age and education. Further, the regression coefficients were relatively small and may not be clinically significant, due to type 2 error. Finally, due to the exploratory analysis with multiple comparisons we cannot rule out that some of the significant P-values are due to chance.
In conclusion, people with long-term type 1 diabetes experience high levels of total bodily pain, worse quality of life, and worse physical health, as well as higher levels of depressive symptoms and fatigue compared with a comparison group of similar age, sex and socio-economic status. Bodily pain was associated with worse quality of life, higher levels of depressive symptoms and fatigue, and worse glycaemic control. Pain is a condition with multiple possible underlying aetiologies, but one for which treatment is available, and more effective treatment of these conditions has potential to improve QoL in this group. Clinical guidelines for diabetes refer only to painful neuropathy [22], but a broader focus is warranted. To promote a systematic focus on total bodily pain, participant-reported outcome measures such as questionnaires could be useful as screening tools that hopefully will lead to more pain recognition and treatment, resulting in better QoL.
Funding sources
The Western Norway Regional Health Authority and the Norwegian Diabetes Centre research fund supported this work. The funders have not been involved in the study design, data collection, data analyses, manuscript preparation and/or publication decisions.
Competing interests
None declared.
Acknowledgements
The Western Norway Regional Health Authority and the Norwegian Diabetes Centre research fund supported this work. We thank the staff at the Norwegian Diabetes Centre, the participants in the study and Professor Jon Håvard Loge.




