Efficacy of intravenous clonazepam for paediatric convulsive status epilepticus
This original article is commented by Mifsud on pages 968–969 of this issue.
Abstract
Aim
To compare the efficacy of intravenous clonazepam (CLZ) for the initial management of convulsive status epilepticus (CSE) in children as a function of the first-line in-hospital dose used.
Method
This monocentric retrospective study included children who received a first dose of CLZ for CSE at Montpellier University Hospital, France, between January 2016 and June 2019. Data from medical records (clinical, treatment, course) were collected and compared as a function of the first CLZ dose used.
Results
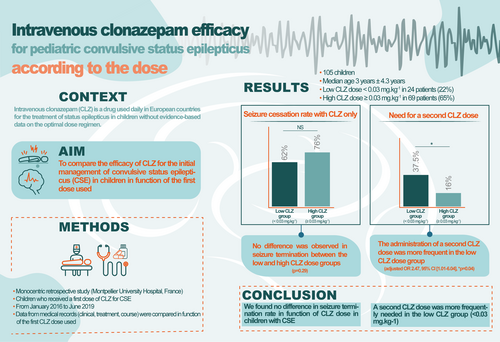
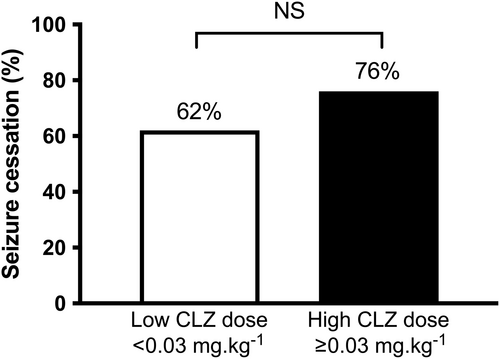
Among the 310 children treated for CSE, 105 received at least one CLZ dose (median age 3 years; quartile 1–quartile 3 [Q1–Q3] = 1 years 2 months–6 years 6 months). Among these 105 patients, 24 (22%) received a dose less than 0.03 mg/kg (low dose) and 69 (65%) received a dose of at least 0.03 mg/kg (high dose). Seizure cessation rate was not different between the low- and high-dose groups (62.5% vs 76%; odds ratio 0.53, 95% confidence interval [CI] 0.19–1.44, p = 0.29). The administration of a second dose of CLZ was more frequent in the low- than the high-dose group (37.5% vs 16%; odds ratio 3.2, 95% CI 1.1–9.1, p = 0.04).
Interpretation
Our study did not find any difference in seizure termination rate as a function of CLZ dose in children with CSE. However, a second CLZ dose was more frequently needed in the group receiving low (less than 0.03 mg/kg) CLZ.
Graphical Abstract
This original article is commented by Mifsud on pages 968–969 of this issue.
Abbreviations
-
- CLZ
-
- clonazepam
-
- CSE
-
- convulsive status epilepticus
-
- GABA
-
- γ-aminobutyric acid
What this paper adds
- There are no evidence-based data on the optimal dose of clonazepam to treat paediatric status epilepticus.
- High and low doses of intravenous clonazepam are efficacious in stopping generalized status epilepticus in children.
- A second infusion was more often needed in the group receiving a low dose (<0.03 mg/kg) of clonazepam.
Convulsive status epilepticus (CSE) is a common paediatric neurological emergency, with an incidence of approximately 17 to 23 events per 100 000 children per year.1 In 2015, the Commission on Classification and Terminology and the Commission on Epidemiology of the International League Against Epilepsy revised the definition of CSE as ‘a condition resulting either from the failure of the mechanisms responsible for seizure termination or from the initiation of mechanisms that lead to abnormally, prolonged seizures (after time point t1) [i.e. 5 minutes]. This condition can have long-term consequences (after time point t2) [i.e. 30 minutes], including neuronal death, neuronal injury, and alteration of neuronal networks, depending on the seizure type and duration’.2 Benzodiazepines are used as first-line treatment of CSE. They exert their antiepileptic action by enhancing the inhibitory effect of γ-aminobutyric acid (GABA) type A receptors. This pre- and postsynaptic inhibition occurs through an increase in the neuronal membrane permeability to chloride ions, which results in membrane hyperpolarization.3, 4 Lorazepam, diazepam, midazolam, and clonazepam (CLZ) are the most widely used benzodiazepines for the initial management of paediatric CSE.5, 6 Intravenous CLZ is frequently used in Europe as the first-line in-hospital benzodiazepine for CSE,7 but not in North America because it is not available locally in injectable form.8 In 2018, the new guidelines by the French Intensive Care Society (Société de Réanimation de Langue Française) and the French Society of Emergency Medicine (Société Française de Médecine d'Urgence) for CSE management in adults and children, excluding newborns and infants, stated that ‘Benzodiazepines should be used as first-line-in-hospital therapy. A rapid single dose of clonazepam by the intravenous route (0.015 mg.kg−1; i.e. 1 mg for 70 kg; maximum 1.5 mg) or of midazolam (0.15 mg.kg−1; i.e. 10 mg for 70 kg) by the intramuscular’.9 This was different from the previous guidelines of the French Intensive Care Society in 2008: one intravenous dose of CLZ between 0.02 and 0.04 mg/kg, with a maximum flow rate of 0.2 mg/minute to reduce the risk of respiratory depression.10 In 1995, the 14th Consensus Conference on Resuscitation and Emergency Medicine recommended using one dose of CLZ between 0.02 and 0.05 mg/kg.11 Therefore, between 1995 and 2018, the dose of CLZ for CSE management in children was reduced by a factor of 2 to 3. To our knowledge, this is not based on any dedicated study on CLZ efficacy as a function of the administered dose. Yet, CLZ is used daily in emergency, intensive care, and neurology wards, as well in outpatient settings. Therefore, the aim of our study was to compare the efficacy of CLZ as first-line in-hospital treatment in children with CSE as a function of the dose used.
METHOD
Study design and participants
All children younger than 16 years old who were treated for CSE (defined as a convulsive seizure lasting 5 minutes) at Montpellier University Hospital (a tertiary hospital), France, between January 2016 and June 2019, were included in this retrospective study. Children who did not receive an intravenous single dose of CLZ as first-line in-hospital treatment of CSE were excluded. The dose of CLZ administered varied according to the physician managing the patient, with most using the recommended dose of 0.05 mg/kg before the 2018 recommendations. For each patient, the following data were retrospectively collected from their medical records after anonymization: demographic data (age, sex, weight), medical history (pre-existing neurodevelopmental disorders, history of seizure or epilepsy), seizure characteristics (first or subsequent seizure, seizure duration, aetiology, primary or secondary generalized seizure), anticonvulsants (molecules, dose), hospitalization ward (conventional, intensive care unit), duration of hospitalization, and the need for mechanical ventilation.
Outcomes
The primary outcome was seizure cessation following single-dose CLZ intravenous injection, defined as the absence of clinical signs of seizures assessed by the medical team, as a function of the used CLZ dose: above 0.03 mg/kg (i.e. high dose) or below 0.03 mg/kg (i.e. low dose). This cut-off of 0.03 mg/kg was chosen to obtain a median of 0.0175 mg/kg in the group with the CLZ dose less than 0.03 mg/kg, which is close to the current recommendation to use 0.015 mg/kg. The main aim was to determine whether the two dosing modalities were equally effective in stopping seizures. Secondary outcomes as a function of the used CLZ dose were the need for a second CLZ dose, seizure duration, initial seizure type (focal, generalized), seizure aetiology, prehospital treatment (rectal diazepam, buccal midazolam), CLZ dose greater or less than 1 mg, introduction of a CLZ maintenance dose, the need for a second-line antiseizure medication (phenobarbital, fosphenytoin, thiopental), hospitalization in intensive care unit, and introduction of an antiepileptic treatment after CSE.
Statistical analysis
The collected data were anonymized. The distribution of quantitative variables was described using means and standard deviation, medians, and minimum and maximum values. Qualitative variables were described with frequencies and percentages.
Qualitative variables were compared between groups using χ2 or Fisher's exact tests, as a function of the data distribution. Quantitative variables were compared between groups using Student's t- or Wilcoxon tests, as a function of the distribution, or with a Kruskal–Wallis test if there were more than two groups. Associations between the primary outcome and covariates were described using odds ratio (OR) and their 95% confidence intervals (CIs).
The propensity score was used to balance the association between the administered dose and seizure cessation rate, to limit confusion bias. The propensity score assesses the probability of seizure termination based on the observed covariates. This probability was estimated using a logistic regression model with seizure cessation as the dependent variable and the associated covariates as explanatory variables (e.g. seizure aetiology, age at diagnosis, weight, CLZ dose, use of prehospital treatment). A Mantel–Haenszel χ2 test was used to analyse qualitative variables after stratification.
A subgroup analysis of the primary and secondary outcomes was performed after dividing children by their age (cut-off 5 years, which is the usual age of cessation of febrile seizures).
The significance threshold was set at 5% for all tests. Statistical analyses were performed with SAS University Edition (SAS Institute, Cary, NC, USA).
Ethics
The local institutional ethics committee (Institutional Review Board Montpellier University Hospital) approved the study under the accreditation number 198711 and waived patient consent for this retrospective analysis of anonymized data. The trial is registered at ClinicalTrials.gov, number NCT04287361.
RESULTS
Patients
Between January 2016 and June 2019, 310 children were admitted for CSE (Figure S1); 205 were excluded because they did not receive one dose of intravenous CLZ as first-line in-hospital treatment. Among these 205 patients, 79 received a prehospital benzodiazepine (rectal diazepam or buccal midazolam).
The included patients (n = 105) were divided in two groups using the 0.03 mg/kg as cut-off dose of first-line CLZ. Thus, 24 patients (22% of 105) received one CLZ dose less than 0.03 mg/kg (median 0.0175 mg/kg ± 0.005) (low-dose group) and 69 (65%) received one CLZ dose of at least 0.03 mg/kg (median 0.05 mg/kg ± 0.06) (high-dose group). The CLZ dose was not recorded in the medical record of 12 patients (11.4%). Consequently, the study focused on the 93 patients with a known CLZ dose (Figure S1). The patients' characteristics are reported in Table 1. None of the 32 patients with epilepsy were on long-term benzodiazepine therapy or any therapy interacting with benzodiazepines (such as cannabidiol or stiripentol).
| Low CLZ dose <0.03 mg/kg (n = 24) | High CLZ dose ≥0.03 mg/kg (n = 69) | p | |
|---|---|---|---|
| Age (years:months) | <0.001* | ||
| Median (SD) | 10:2 (4:8) | 1:11 (3:1) | |
| <5 years, n (%) | 6 (25) | 53 (76.8) | |
| ≥5 years, n (%) | 18 (75) | 16 (23.2) | |
| Sex, n (%) | 0.68 | ||
| Male | 13 (54.2) | 34 (49.3) | |
| Female | 11 (45.8) | 35 (50.7) | |
| Weight (kg) | <0.001* | ||
| Median (SD) | 40 (23.4) | 12 (9.1) | |
| ≤10 kg | 1 (4.2) | 21 (30.4) | |
| >10 and <30 kg | 8 (33.3) | 44 (63.8) | |
| >30 kg | 14 (58.3) | 4 (5.8) | |
|
Pre-existing neurodevelopmental disorder, n (%) |
7 (29) |
21 (30) |
0.90 |
- * Significant result.
- Abbreviations: CLZ, clonazepam; SD, standard deviation.
Outcomes
There was no significant difference in seizure termination rate (primary outcome) between the low-dose (62.5%) and high-dose (76%) groups (OR 0.53, 95% CI 0.19–1.44, p = 0.29) (Figure 1). Secondary outcomes are reported in Table 2. The administration of a second CLZ dose was more frequent in the low-dose (37.5%) than the high-dose (16%) group (unadjusted OR 2.26, 95% CI 1.06–4.79; adjusted OR 2.47, 95% CI 1.01–6.04, p = 0.04). No difference was observed in second-line treatments between the two groups (41.6% vs 24.6%; OR 1.76, 95% CI 0.78–5.45, p = 0.19). Prehospital administration of rectal diazepam was more frequent in the high-dose group (37.5% vs 68.1%; OR 0.53, 95% CI 0.31–0.91, p = 0.016).
| Low CLZ dose <0.03 mg/kg (n = 24) | High CLZ dose ≥0.03 mg/kg (n = 69) | Unadjusted odds ratio (95% CI) | p | |
|---|---|---|---|---|
| Second CLZ dose | 9 (37.5) | 11 (16) | 2.26 (1.06–4.79) | 0.04* |
| Seizure duration | ||||
| ≤15 minutes | 6 (25) | 15 (21.7) | 1.31 (0.61–2.86) | 0.74 |
| 15–30 minutes | 1 (4.1) | 6 (8.7) | 0.10 (0.06–4.66) | 0.47 |
| ≥30 minutes | 10 (41.7) | 35 (50.7) | 0.95 (0.28–2.59) | 0.44 |
| Missing data | 7 (29.2) | 13 (18.9) | ||
| First seizure | 9 (37.5) | 49 (71) | 0.59 (0.36–0.96) | 0.01* |
| Start mode | ||||
| Focal | 14 (58.3) | 28 (40.6) | 1.38 (0.88–2.16) | 0.13 |
| Tonic–clonic | 10 (41.7) | 41 (59.4) | 0.74 (0.46–1.20) | |
| Aetiology | ||||
| Febrile seizures | 5 (20.8) | 36 (52.2) | 0.38 (0.17–0.87) | 0.009* |
| Epilepsy | 15 (62.5) | 17 (24.6) | 2.60 (1.56–4.31) | 0.012* |
| Neuromeningeal infection | 1 (4.2) | 6 (8.7) | 0.46 (0.06–3.63) | |
| Other | 2 (8.3) | 6 (8.7) | ||
| Missing data | 1 (4.2) | 4 (5.8) | ||
| Prehospital treatment | ||||
| Rectal diazepam | 9 (37.5) | 47 (68.1) | 0.53 (0.31–0.91) | 0.016* |
| Buccal midazolam | 4 (16.7) | 5 (7.2) | 2.21 (0.64–7.57) | 0.18 |
| None | 11 (45.8) | 18 (26) | ||
| CLZ dose ≥1 mg | 14 (58.3) | 36 (52.1) | 1.3 (0.52–3.37) | 0.60 |
| Maintenance dose | 14 (58.3) | 52 (75.4) | 0.79 (0.56–1.13) | 0.11 |
| Median, mg/kg/day (SD) | 0.09 (0.04) | 0.2 (0.11) | ||
| Second-line treatment | 10 (41.6) | 17 (24.6) | 1.76 (0.78–5.45) | 0.11 |
| Fosphenytoin | 7 (29.1) | 15 (21.7) | 1.29 (0.59–2.79) | 0.46 |
| Phenobarbital | 2 (8.3) | 6 (8.7) | 1.38 (0.37–5.10) | 0.96 |
| Thiopental | 2 (8.3) | 1 (1.5) | 5.44 (0.37–57.40) | 0.1 |
| Hospitalization | ||||
| Intensive care unit | 15 (62.5) | 31 (44.9) | 1.42 (0.96–2.11) | 0.14 |
| Median, days (SD) | 2.0 (1.3) | 2.0 (3.0) | ||
| Intubation | 3 (12.5) | 6 (8.7) | 1.38 (0.37–5.10) | 0.59 |
| Conventional ward | 23 (95.8) | 69 (100) | 0.08 | |
| Median, days (SD) | 3.5 (5.4) | 4.0 (7.7) |
- * Significant result. Data are n (%) unless otherwise stated.
- Abbreviations: CLZ, clonazepam; SD, standard deviation.
Subgroup analysis
Because the two groups as a function of CLZ dose were heterogeneous, particularly in terms of age and weight (Table 1), a subgroup analysis was performed in children older or younger than 5 years (Table 3). There was no significant difference in seizure termination rate and the need for a second CLZ dose in the two age groups. Children younger than 5 years who received a low dose seemed to be more often hospitalized in an intensive care unit (83%) than those who received a high dose (43%) (p = 0.06) and required more mechanical ventilation (33% vs 7.5%, p < 0.05).
| Age <5 years (n = 59) | Age ≥5 years (n = 34) | |||||
|---|---|---|---|---|---|---|
| Low CLZ dose <0.03 mg/kg (n = 6) | High CLZ dose ≥0.03 mg/kg (n = 53) | p | Low CLZ dose <0.03 mg/kg (n = 18) | High CLZ dose ≥0.03 mg/kg (n = 16) | p | |
| Clinical seizure cessation | 4 (66) | 40 (75) | 0.63 | 11 (61) | 13 (81) | 0.20 |
| Second CLZ dose | 2 (33) | 9 (17) | 0.33 | 7 (39) | 2 (13) | 0.08 |
| Seizure duration | ||||||
| ≤15 minutes | 1 (16) | 14 (26) | 0.60 | 5 (38) | 1 (7.7) | 0.1 |
| 15–30 minutes | 1 (16) | 3 (5) | 0.30 | 0 | 0 | — |
| ≥30 minutes | 2 (33) | 26 (49) | 0.46 | 8 (62) | 9 (69) | 0.49 |
| Missing data | 2 | 10 | ||||
| First seizure | 3 (50) | 39 (74) | 0.23 | 6 (35) | 10 (63) | 0.09 |
| Start mode | ||||||
| Focal | 3 (50) | 17 (32) | 0.38 | 11 (61) | 11 (69) | 0.64 |
| Generalized | 3 (50) | 36 (68) | 0.38 | 7 (39) | 5 (31) | 0.64 |
| Aetiology | ||||||
| Febrile seizures | 3 (50) | 35 (66) | 0.44 | 2 (11) | 1 (6.3) | 0.62 |
| Epilepsy | 2 (33) | 11 (21) | 0.48 | 13 (72) | 6 (38) | 0.04* |
| Neuromeningeal infection | 0 (0) | 2 (3.8) | 0.63 | 1 (5.6) | 4 (25) | 0.11 |
| Other | 1 (16) | 5 (9.4) | 0.54 | 1 (5.6) | 1 (6.3) | 0.93 |
| Prehospital treatment | ||||||
| Rectal diazepam | 4 (66) | 39 (74) | 0.72 | 5 (28) | 8 (50) | 0.18 |
| Buccal midazolam | 2 (33) | 2 (3.8) | <0.01* | 2 (11) | 3 (19) | 0.53 |
| CLZ dose ≥1 mg | 1 (16.6) | 21 (39.6) | 0.27 | 13 (72.2) | 15 (93.7) | 0.10 |
| Maintenance dose | 4 (66) | 39 (74) | 0.72 | 11 (61) | 13 (81) | 0.20 |
| Second-line treatment | 2 (33) | 13 (25) | 0.64 | 8 (44) | 4 (25) | 0.69 |
| Fosphenytoin | 0 (0) | 12 (23) | 0.19 | 7 (39) | 3 (19) | 0.20 |
| Phenobarbital | 0 (0) | 5 (9.4) | 0.43 | 2 (11) | 1 (6.3) | 0.53 |
| Thiopental | 2 (33) | 1 (1.9) | <0.01* | 0 | 0 | — |
| Hospitalization | ||||||
| Intensive care unit | 5 (83) | 23 (43) | 0.06 | 10 (56) | 8 (50) | 0.75 |
| Intubation | 2 (33) | 4 (7.5) | <0.05* | 1 (5.6) | 2 (13) | 0.48 |
| Conventional ward | 6 (100) | 53 (100) | — | 17 (94) | 16 (100) | 0.34 |
- * Significant result. Data are n (%) unless otherwise stated.
- Abbreviation: CLZ, clonazepam.
Dose greater than 1 mg and overdosing
More than half the patients received a CLZ dose of at least 1 mg: 58.3% in the low-dose group and 52.1% in the high-dose group (OR 1.3, 95% CI 0.52–3.37, p = 0.64). Eight patients in the high-dose group received a CLZ dose above the maximum recommended dose (i.e. 1.5 mg; median 1.96 mg [1.7–6.5]), but none in the low-dose group. Overdosing might increase the risk of undesirable effects, particularly respiratory depression. However, none of the patients was intubated, and only one patient required ventilatory support by high-flow nasal cannula for 4 hours. There was no significant difference in the hospitalization ward, hospitalization duration, and the need for mechanical ventilation between groups.
Inappropriate treatment
According to recommendations, a second line of treatment should be administered after two doses of benzodiazepines.9 Among the 105 patients admitted for CSE who received one dose of intravenous CLZ as first-line in-hospital treatment, 31 (29%) received at least three doses of benzodiazepines: before (rectal diazepam/buccal midazolam) and after hospital admission (intravenous CLZ).
DISCUSSION
The aim of our study was to compare the efficacy of first-line in-hospital CLZ in children treated for CSE according to the dose used. We found that a low dose (less than 0.03 mg/kg, median 0.0175 mg/kg) was as effective as a high dose more or equal to 0.03 mg/kg, median 0.05 mg/kg), but increased the need for a second dose. These results are in line with the 2018 guidelines of the French Intensive Care Society and the French Society of Emergency Medicine: ‘It is probably necessary to repeat the benzodiazepine injection, with the exception of midazolam, in the event of clinical persistence of the generalized tonic-clonic status epilepticus … five minutes after the first injection. If the respiratory state worsens, it is prudent to administer only half dose’.9
Lorazepam, midazolam, and CLZ are the most used benzodiazepines as first-line in-hospital treatment of paediatric CSE worldwide.12 A meta-analysis that included six studies and 774 patients concluded that midazolam was superior to diazepam, regardless of the administration route (buccal, nasal, intramuscular) (relative risk 1.52; 95% CI 1.27–1.82).13 On the other hand, a prehospital study showed that midazolam by intramuscular injection was at least as effective as intravenous lorazepam.14 Lorazepam is rarely used in Europe because the injectable form is not routinely available. Lorazepam dose usually prescribed in patients with seizures is 0.1 mg/kg, with a maximum dose of 4 mg.8 Conversely, CLZ is used in several European countries, particularly Germany and Spain, but rarely in the USA because of the unavailability of the injectable form.15-18 The 2012 German guidelines (Deutsche Gesellschaft für Neurologie) recommended using 0.015 mg/kg of CLZ, to be repeated once after 5 minutes, with a maximum dose of 3 mg.16 The Spanish recommendations of 2012 (Guia oficial de practica clinica en epilepsia) also proposed first-line CLZ for CSE management (1–2 mg, with a maximum dose of 4 mg).17
To our knowledge, no study has compared CLZ with other benzodiazepines for CSE management in children, only in adults. Alvarez et al. performed a prospective observational cohort study in Switzerland and the USA in which they compared CLZ, lorazepam, and midazolam for managing CSE and non-CSE in adults. The following doses were considered sufficient: 0.1 mg/kg for lorazepam, 0.15 mg/kg for midazolam, and 0.015 mg/kg for CLZ. Lorazepam was significantly associated with a higher risk of refractory status epilepticus compared with CLZ (OR = 6.4; 95% CI = 2.66–15.5). However, lorazepam doses were considered insufficient and there was probably a centre effect in this study.19
Navarro et al. performed a prehospital, controlled, randomized, double-blind study to compare the effectiveness of adding intravenous levetiracetam (2.5 g) to CLZ (1 mg) for treating generalized tonic–clonic status epilepticus in adults. The CLZ dose used was 1 mg, regardless of the patients' weight. No difference was observed between the CLZ with levetiracetam and CLZ with placebo (control) groups. CSE was controlled in 57% of patients after the first CLZ injection and in 84% after administration of a second dose. This suggests a dose-dependent efficacy. Moreover, the administration of two antiseizure medications as first-line treatment to control CSE might not bring any additional benefit.20 In our study, no patient received two antiseizure medications at treatment initiation.
A French multicentre, randomized, prospective, prehospital study (Clinical Trial: NCT01870024) is currently under way with the aim of demonstrating the superiority of lorazepam (0.1 mg/kg) compared with CLZ (0.015 mg/kg), and the equivalent efficacy of lorazepam compared with the combination of CLZ (0.015 mg/kg) and fosphenytoin (20 mg/kg) for CSE in adults. The results of this trial have not been published yet.21
These studies in adult populations show some degree of equivalence between lorazepam and CLZ. This is explained by their similar pharmacokinetic properties; however, no study has compared their efficacy as a function of the dose used.
In a preclinical study in rats, Goodkin et al. showed that the same dose of diazepam prevents brief seizures, but not prolonged seizures. They found an inversely proportional relation between diazepam effectiveness and seizure duration, with a decrease in effectiveness after 15 minutes of seizure.22 It is hypothesized that, during status epilepticus, GABA type A receptors are internalized from the synaptic membrane to the cytoplasm. This leads to a reduction in the number of receptors available for binding to benzodiazepines, which might explain the observed resistance to antiepileptic molecules and may enhance the inhibition induced by GABA type A receptors.23 These studies indicate that, after a certain seizure duration, the interest in repeating or increasing the benzodiazepine dose is limited. Therefore, it is recommended using a second-line treatment (not a benzodiazepine) to prevent the progression to refractory status epilepticus.4
With the exception of overdose cases, CLZ is well tolerated in paediatric populations. Our study showed that in more than half of the children with CSE, the prescribed CLZ dose was at least 1 mg, whatever the patient's weight, which corresponds to one vial.20 The main side effects of CLZ in this indication are respiratory depression and impaired alertness. In our study, it was difficult to specify the duration and severity of impaired alertness, which are linked to CLZ sedative properties and to the post-critical phase after CSE. Nevertheless, we did not find any difference between groups (including in the overdose subgroups) in terms of length of hospital stay (conventional ward or intensive care unit) or the need for mechanical ventilation. A future prospective study could specifically investigate the duration and severity of consciousness disorders, by systematically performing a simple clinical neurological monitoring based on the Alert Voice Pain Unresponsive scale.24
Even within the same drug class (i.e. benzodiazepines in this study), some specific properties of each molecule may explain differences in efficacy and tolerance. The speed of diffusion in the central nervous system is evaluated by calculating the drug distribution half-life: 30 minutes for CLZ, 4 to 19 minutes for midazolam, and less than 11 minutes for lorazepam.25 CLZ is less sedative than midazolam and has a faster onset of action than lorazepam (less than 3 minutes for CLZ and 15 minutes for lorazepam).26 The duration of action for CLZ is inversely proportional to the time of action onset: approximately 24 hours compared with 8 to 72 hours for lorazepam. The duration of drug effectiveness is assessed by calculating the elimination half-life. For CLZ, it is intermediate (10–24 hours) between the half-lives of lorazepam and midazolam (less than 10 hours) and diazepam (more than24 hours).25 Therefore, owing to its pharmacokinetic profile, which explains its immediate effect and prolonged duration of action, CLZ is an excellent antiepileptic molecule in emergency situations.26 However, these data were obtained in adults and cannot be completely transferred to paediatric populations. Indeed, benzodiazepines are metabolized mainly in the liver through the action of cytochrome P450. Their metabolism tends to be faster at birth, then longer than in adults at the age of 2 to 3 years, before gradually decreasing until puberty.27, 28
In the treatment of different epilepsy types (focal, generalized), a good correlation between plasma level and daily dose of CLZ has been observed, but not between the plasma level and clinical efficacy.29, 30 To our knowledge, no study has assessed the therapeutic dose–effect and the concentration–therapeutic effect correlations for intravenous CLZ in CSE (children and adults). One paediatric study measured the plasma levels of CLZ after rectal administration of a single dose ranging from 0.05 mg/kg to 0.1 mg/kg, and found that rectal CLZ is rapidly absorbed, but the plasma levels varied from patient to patient.31 Difficulties in demonstrating the dose–effect relation of CLZ could be explained by inter-individual differences in its metabolism, as shown by a study that compared CLZ administered by the intravenous, intramuscular, or oral routes in healthy volunteers.32
The meta-analysis by Neligan et al. (n = 61 studies published between 1990 and 2007) did not find any difference in CSE mortality, including in children, despite changes in CSE definition and earlier treatments.33 In paediatric studies, the mortality rate was 3.6% (95% CI 2.0–5.2%). In 2018, Gaínza-Lein et al. performed a prospective, multicentre, observational cohort study that included 218 paediatric patients with refractory status epilepticus: 74 (33.9%) received first-line treatment with benzodiazepines in the first 10 minutes, and 144 (66.1%) after 10 minutes of seizures. The mortality rate was higher in patients treated after 10 minutes of seizure (adjusted OR 11.0; 95% CI 1.43 to ∞; p = 0.02).34 Therefore, early benzodiazepine administration is a key prognostic element. The management principle ‘time is brain’ also applies to CSE and stroke. In our study, owing to its retrospective nature, the time from seizure to the first CLZ dose could not be evaluated because it was not systematically recorded in the medical files.
Ours is the first study to compare CLZ efficacy in controlling status epilepticus in children as a function of the dose. It has several limitations due to its monocentric and retrospective design. The populations were dissimilar in the two groups: children treated with CLZ at low dose were older, heavier, and with previous epileptic events; conversely, children who received a high CLZ dose were younger, had more frequently febrile seizures, and rectal diazepam administration before CLZ. Therefore, the response of the children who received a high CLZ dose could have been influenced by the long half-life (more than 24 hours) of diazepam. This may have led to over- or underestimation of the efficacy of CLZ in this group. The absence of any significant difference in efficacy between the low-dose and high-dose groups may be explained by lack of statistical power or by the absence of significant difference. The reported efficacy of benzodiazepines in CSE, all molecules combined, is close to 70%, in agreement with our results.4 No other study has compared the efficacy of CLZ as a function of the administered dose; therefore, comparison of our results with those of similar studies is not possible.
Owing to the retrospective nature of our study, we were unable to collect the time to rescue treatment, which is an important element in the control of CSE. In addition, 48% of patients had CSE lasting longer than 30 minutes, which might have influenced their response to benzodiazepines. Our data nevertheless reflect CSE real-life management, irrespective of these factors.
We performed a subgroup analysis in children older and younger than 5 years to obtain a homogeneous population concerning, for instance, the seizure aetiology (febrile seizures, epilepsy duration) and prehospital treatment (buccal midazolam, rectal diazepam). This analysis did not highlight any significant difference in seizure control or the need for a second CLZ dose. Children younger than 5 years who received a low dose seemed to be hospitalized in an intensive care unit more often (83% vs 43%, p = 0.06) and required more intubation and use of thiopental (Table 3); however, because of the small sample size, these results must be considered with caution.
CONCLUSION
For the first-line in-hospital treatment of CSE in children, a CLZ dose less than 0.03 mg/kg was as effective as a dose of at least 0.03 mg/kg, despite the differences (e.g. age, weight, history of epilepsy) in these two groups. However, clinicians must be vigilant because a second dose may be necessary more often to control CSE rapidly, which is essential from a prognostic point of view. These findings are in accordance with the guidelines of the French Intensive Care Society and the French Society of Emergency Medicine: using a first dose of 0.015 mg/kg of CLZ (maximum 1.5 mg) that can be renewed once after 5 minutes, if not effective, before switching to a second-line antiseizure medication.9 This dose is particularly suited to the paediatric population, and helps to avoid the undesirable effects of overdose, in particular respiratory depression. The results of this monocentric retrospective study need to be confirmed and extended in a randomized controlled clinical trial.
CONFLICT OF INTEREST STATEMENT
The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.