A core outcome set for multimorbidity risk in individuals with cerebral palsy
Abstract
enAim
To: (1) investigate the importance of outcome measurement instruments (OMIs) within a core outcome set (COS) for multimorbidity (at least two chronic health conditions) risk in individuals with cerebral palsy (CP); (2) investigate the feasibility of OMIs within the COS in international clinical research settings in adolescents and adults with CP; and (3) describe the associations between the COS data and Gross Motor Function Classification System (GMFCS) levels.
Method
Eighty-three individuals with CP completed a survey on health outcomes: physical behaviour, nutrition, sleep, endurance, body composition, blood pressure, blood lipids, and glucose. A cross-sectional study assessed the feasibility of the COS in 67 adolescents and adults with CP (mean age 30y, SD 15y 1mo, min–max: 14–68y, 52.2% male) at four centres. Prevalence of multimorbidity risk and associations with GMFCS levels are described.
Results
Most participants rated physical behaviour, nutrition, sleep, and endurance as very important. Body composition, blood pressure, nutrition, and sleep were highly feasible since data were collected in 88% or more participants who consented to having the assessments. Physical behaviour, cardiorespiratory endurance, and blood draws were collected in less than 60% of participants. Total time sedentary (ρ=0.53, p<0.01) and endurance (ρ=−0.46, p<0.01) were significantly associated with GMFCS level.
Interpretation
The COS identified that most participants had poor sleep quality and endurance, did not have healthy diets, and showed increased sedentary behaviour. Individuals with CP valued these outcomes as most important, suggesting a need to assess these modifiable behaviours in this population. Objective measures of physical behaviour and cardiorespiratory endurance in the COS required additional personnel, time, and participant burden. We recommend that healthcare providers should perform a simpler first screen using questionnaire-based assessments and then focus the use of the remainder of the COS if required for the patient.
Resumo
ptUm desfecho central definido para risco de multimorbidade em indivíduos com paralisia cerebral
Objetivo
(1) Investigar a importância dos instrumentos de medição de desfechos (IMD) dentro de um conjunto de desfechos principais (CDS) para risco de multimorbidade (pelo menos duas condições crônicas de saúde) em indivíduos com paralisia cerebral (PC); (2) Investigar a viabilidade de IMD dentro do CDS em cenários internacionais de pesquisa clínica em adolescentes e adultos com PC; e (3) Descrever as associações entre os dados do CDS e os níveis do Sistema de Classificação da Função Motora Grossa (GMFCS).
Método
Oitenta e três indivíduos com PC completaram uma pesquisa sobre desfechos em saúde: comportamento físico, nutrição, sono, resistência, composição corporal, pressão arterial, lipídios no sangue e glicose. Um estudo transversal avaliou a viabilidade do COS em 67 adolescentes e adultos com PC (idade média de 30 anos, desvio padrão de 15 anos e 1 mês, min-max: 14-68 anos, 52,2% do sexo masculino) em quatro centros. São descritas a prevalência do risco de multimorbidade e as associações com os níveis de GMFCS.
Resultados
A maioria dos participantes classificou o comportamento físico, nutrição, sono e resistência como muito importantes. Composição corporal, pressão arterial, nutrição e sono foram altamente viáveis, uma vez que os dados foram coletados em 88% ou mais dos participantes que consentiram em realizar as avaliações. Comportamento físico, resistência cardiorrespiratória e coleta de sangue foram coletados em menos de 60% dos participantes. O tempo total de sedentarismo (ρ = 0,53, p < 0,01) e resistência (ρ = −0,46, p < 0,01) foram significativamente associados ao nível de GMFCS.
Interpretação
O CDS identificou que a maioria dos participantes tinha má qualidade e resistência do sono, não tinha dietas saudáveis e apresentava um comportamento sedentário aumentado. Indivíduos com PC valorizaram esses desfechos como mais importantes, sugerindo a necessidade de avaliar esses comportamentos modificáveis nessa população. Medidas objetivas de comportamento físico e resistência cardiorrespiratória no CDS exigiram pessoal adicional, tempo e sobrecarga do participante. Recomendamos que os profissionais de saúde realizem uma primeira triagem mais simples usando avaliações baseadas em questionários e, em seguida, concentrem o uso do restante do CDS, se necessário para o paciente.
Abbreviations
-
- COS
-
- Core outcome set
-
- OMI
-
- Outcome measurement instrument
-
- PSQI
-
- Pittsburgh Sleep Quality Index
-
- SFFFQ
-
- Short Form Food Frequency Questionnaire
-
- WHR
-
- Waist-to-hip ratio
What this paper adds
- Individuals with cerebral palsy (CP) and their caregivers perceived physical activity, nutrition, sleep, and endurance as very important.
- Body composition, blood pressure, nutrition, and sleep were feasible to measure in adolescents and adults with CP.
- Objective measures of physical behaviour and cardiorespiratory endurance were challenging to collect in clinical settings.
- Most participants did not have healthy diets, had poor sleep quality, and engaged in sedentary behaviour or sitting for more than three-quarters of their time.
Cerebral palsy (CP) results in functional limitations and restrictions in activities of daily living, which can lead to increased risk for adverse health issues. CP is a lifelong condition and some studies have identified a secular trend in improved life expectancy over the past several decades, with mixed results depending on the level of disability.1, 2 Recent evidence suggested that aging with CP is associated with increased risk of cardiovascular3 and other non-communicable diseases.4-7 Furthermore, multimorbidity, defined as the presence of at least two chronic health conditions, is highly prevalent in adults with CP8 and occurs at a younger age compared to the general population.9 Not surprisingly, persons with CP have greater healthcare utilization and costs, a greater all-cause mortality risk, and lower life expectancy than the general population and the differences are pronounced in those with greater degrees of motor impairment.1, 2, 10, 11 Thus, prevention and management strategies for multimorbid health conditions in individuals with CP are urgently needed.
To the best of our knowledge, there are no screening programmes in place for cardiometabolic disease and multimorbidity risk in individuals with CP. Developing a feasible and generalizable set of tools to assess multimorbidity risk in this population requires an international approach. CP is complex and heterogeneous and our understanding of health conditions in this population is often limited to few clinical studies with small sample sizes. The CP-Multimorbidity Risk Assessment and Prevention consortium was formed in 2017 with the goal of developing and testing a core outcome set (COS) of outcome measurement instruments (OMIs) for multimorbidity risk, including cardiometabolic disease, in adolescents and adults with CP in clinic and research settings.12 In previous research, clinicians and researchers employed a pragmatic approach to develop the COS,13 and then investigate the importance of the COS from the perspectives of individuals with CP and their families and test the feasibility of data collection of OMIs within the COS in parallel. Therefore, the aims of this study were to: (1) investigate the importance of OMIs within the COS from the perspectives of individuals with CP and their families/caregivers; (2) understand the feasibility of OMIs within the COS in international clinical research settings; and (3) describe associations with Gross Motor Function Classification System (GMFCS)14 levels.
METHOD
This study was conducted as the third phase of the overarching project on the development and feasibility testing of OMIs within a COS for multimorbidity risk in adolescents and adults with CP.12 The COS comprised eight OMIs related to multimorbidity risk, which are summarized in Table 1.13 Details pertaining to the extensive literature search (phase 1) and expert Delphi survey (phase 2) to derive the COS are reported elsewhere.13 Briefly, the experts that contributed to the Delphi survey were from Canada, the Netherlands, and the USA.
| Outcome | OMI | OMI details |
|---|---|---|
| Physical behaviour | Activ8 system |
Activ8 was worn on the right or least affected upper thigh for 7d Minimum wear time of at least 5d of 11h per day was required for the analysis Six distinct body postures and movement classes: (1) lying down; (2) sitting; (3) standing; (4) walking; (5) running; (6) cycling Sedentary behaviour was the time spent lying and sitting Physical activity was the time spent standing, walking, running, and cycling |
| Nutrition | SFFFQ |
Twenty different foods or drinks were consumed in a typical week Diet quality score was calculated from fruits, vegetables, oily fish, fat, and non-milk extrinsic sugar intake A diet that was not healthy was defined as an SFFFQ <12 |
| Sleep | PSQI |
Nineteen items grouped into 7 components that are weighted on a 0–3 scale Component scores were summed to create a global PSQI score (0–21) (higher scores mean worse sleep quality) A global score ≥5 distinguished poor sleep quality |
| Cardiorespiratory endurance |
Continuous incremental protocol; McMaster all-out protocol |
Progressive maximal exercise test on an electronically braked cycle ergometer (GMFCS levels I–III) or arm ergometer (GMFCS levels IV and V) Heart rate was measured using a monitor VO2 and CO2 were measured using a calibrated mobile gas analysis system VO2max was taken as an average value during the final 30sec of the test (ml/kg/min) |
| Body size and composition | Stadiometer or flexible tape measure (height); flexible or anthropometric tape measure (waist and hip circumference); digital standing scale or wheelchair scale (weight) |
Height and weight in standing position for GMFCS levels I and II Height (supine) and weight (seated) for GMFCS levels III–Va BMI=kg/m2 Waist and hip circumference measured supinely after normal expiration Waist circumference measured at the narrowest part of the torso Hip circumference measured at the widest part of the hips |
| Blood pressure | Automated sphygmomanometer |
Seated position after 10-minute rest Two measurements were performed on the least affected side If two values differed by >5mmHg for SBP, a third measurement was taken and the average recorded |
| Blood lipids and glucose | Non-fasting venous blood test |
Total cholesterol, HDL-C, LDL-C (mmol/l) Glucose (mmol/l) |
- Abbreviations: BMI, body mass index; GMFCS, Gross Motor Function Classification System; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PSQI, Pittsburgh Sleep Quality Index; SFFFQ, Short Form Food Frequency Questionnaire. SBP, systolic blood pressure.
- a In case of contractures that impeded straight line measurements, height was measured segmentally.
Study design
The first two aims in the current study employed cross-sectional study designs. For the first aim, an internet survey including individuals with CP or their families/caregivers across North America was conducted to investigate the importance of OMIs within the COS to this population. For the second aim, eight OMIs were assessed in adolescents and adults with CP at four clinical research centres in Canada, the Netherlands, and the USA.
Participants
Internet survey
Participants were recruited online through a non-profit organization, the CP NOW Foundation (https://cpnowfoundation.org). They included individuals (adolescents or adults) with CP or their parent, guardian, or caregiver. Inclusion criteria for individuals with CP were a diagnosis of CP, minimum age of 14 years, and ability to respond to online questions with or without support. Responses were anonymous. By virtue of completing the survey, participants provided implied consent to use the information for the purpose of this study. Approval from the Hamilton Integrated Research Ethics Board was obtained for the survey (no. 5116).
Feasibility of outcome measurement instruments
Four clinical research centres in three countries recruited participants as a convenience sample. Participants were introduced to the study by their healthcare professional, at which time a clinical researcher provided detailed study information and obtained their consent to participate in the study. Participants were recruited and tested on the COS between 2017 and 2020. Inclusion criteria consisted of a diagnosis of CP, minimum age of 14 years, and ability to respond to questionnaires independently or with minimal assistance. Participants were eligible for a cardiorespiratory endurance assessment provided that they were physically capable of exercising on an upright seated ergometer, hand ergometer, or recumbent bike. Participants aged 18 years and older provided signed informed consent before enrolling in the study. Adolescents 14 years and older but younger than 18 years provided written or verbal assent, while their parent/guardian provided written informed consent. Formal research ethics were received at each of the four clinical research centres before study commencement.
Procedures
Internet survey
Information pertaining to the online survey was posted on the website of the CP NOW Foundation. Eligible participants were sent a link to complete an anonymous online survey hosted on the LimeSurvey platform, distributed through the McMaster’s Research Ethics Board free access to the survey service. All surveys were completed between December 2018 and August 2019.
Feasibility of outcome measurement instruments
Participants visited a clinical research centre to be assessed using the COS. Two sites (McMaster Children’s Hospital [Canada] and the University of Michigan [USA]) recruited participants during clinical appointments. The other two sites (Rotterdam and Utrecht [the Netherlands]) recruited participants at baseline entry into a lifestyle intervention programme. During the study visit, participant characteristics (age, sex, and GMFCS level) were recorded. Regarding the OMIs, height, weight, body mass index (BMI), and waist and hip circumference measurements were taken for body size and composition; blood pressure measurements and cardiorespiratory endurance tests were performed and questionnaires were administered by the clinician or clinical researcher. Participants were fitted with an Activ8 accelerometer to wear on their thigh for 7 consecutive days. Participants were instructed to keep an activity diary, where waking hours, bedtime, and periods of non-wear time were recorded.
Internet survey: the importance of outcome measures
The internet survey consisted of eight close-ended questions and one open-ended question. Seven of the eight close-ended questions asked the participant to rate the importance of seven health outcomes as something they would like their family healthcare professional to measure and discuss. The eighth question asked if the participant was an adolescent or adult with CP, parent or guardian of an adolescent or adult with CP, or other. The open-ended question asked for any additional comments. Only responses related to the importance of the seven health outcomes and category of the participant were included in this analysis. Health outcomes included: (1) physical behaviour (physical activity and sedentary [sitting] behaviour); (2) nutrition; (3) sleep; (4) endurance; (5) body size and composition; (6) blood pressure; (7) blood lipids and glucose. Participants responded to the importance of each outcome using a 7-item Likert-type scale, ranging from ‘very unimportant’ to ‘very important’. This method followed a modified Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach for selecting patient-important outcomes.15
Feasibility of outcome measurement instruments
The feasibility of collecting data for the OMIs in adolescents and adults with CP was determined as a percentage of those who had a measure successfully performed relative to the number of participants recruited for the study. Additionally, brief interviews with the site clinicians and researchers were conducted to understand the practical feasibility of OMIs within the COS and any issues or challenges encountered.
Statistical analyses
Statistical analyses were performed using Stata v13.1 (StatCorp, College Station, TX, USA). COS data from each clinical research centre were combined into a pooled database. Summary statistics were reported for the importance of each OMI within the COS from online surveys. Descriptive summary statistics for the outcome data from the COS were calculated as means, standard deviations, minimum, median, maximum, and lower and upper quartiles for continuous variables and as percentages for categorical data. We describe the associations between each OMI and GMFCS levels using non-parametric correlations (Spearman’s ρ). Since this was a cross-sectional feasibility study, no formal sample size calculation was performed. Through convenience sampling at clinical research centres, we strived to include representation from all five GMFCS levels.
RESULTS
Internet survey
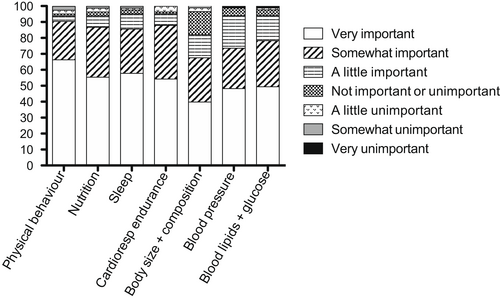
A link to the survey was sent to 123 participants. Eighty-three participants (67.5%) completed the online survey. Of these, just over half were adolescents or adults with CP (n=42, 50.6%); the remaining participants were a parent, guardian, or caregiver of an individual with CP (n=38, 45.8%); three participants identified as ‘other’. All 83 participants rated the importance of each of the seven health outcomes. Notably, 66% and 24% of participants rated physical behaviour as very important or somewhat important respectively, 55% and 31% of participants rated nutrition as very important or somewhat important respectively, 58% and 28% of participants rated sleep as very important or somewhat important respectively, 54% and 34% of participants rated endurance as very important or somewhat important respectively, 40% and 28% of participants rated body composition as very important or somewhat important respectively, 48% and 25% of participants rated blood pressure as very important or somewhat important respectively, and 49% and 29% of participants rated cholesterol and blood sugar as very important or somewhat important respectively. A breakdown of participants’ responses for each health outcome is provided in Figure 1.
Feasibility of outcome measurement instruments
Sixty-seven participants (mean age 30y, SD 15y 1mo; minimum–maximum: 14–68y, 52.2% male) with CP participated in the feasibility study across the four clinical research centres. Twenty-five (37.3%) participants were assessed at McMaster Children’s Hospital, seven (10.4%) in Rotterdam, 25 (37.3%) in Utrecht, and 10 (14.9%) at the University of Michigan. Most participants were ambulatory (classified in GMFCS level I or II; n=39, 58.2%). Participant characteristics are reported in Table 2.
| Characteristic | Total (n=67) | McMaster University (Canada) | Erasmus Medical Center Rotterdam (the Netherlands) | Utrecht University Medical Center (the Netherlands) | University of Michigan (USA) |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Males | 35 (52) | 18 (72) | 3 (43) | 8 (32) | 6 (60) |
| Females | 32 (48) | 7 (28) | 4 (57) | 17 (68) | 4 (40) |
| GMFCS level, n (%) | |||||
| I | 16 (24) | 4 (16) | 2 (29) | 7 (28) | 3 (30) |
| II | 23 (34) | 3 (12) | 1 (14) | 14 (56) | 5 (50) |
| III | 12 (18) | 3 (12) | 4 (57) | 4 (16) | 1 (10) |
| IV | 7 (11) | 7 (28) | |||
| V | 8 (12) | 8 (32) | |||
| Unknown | 1 (1) | 1 (10) | |||
| Age, y:mo, mean (SD) | 30:0 (15:1) | 16:0 (1:0) | 29:5 (10:1) | 33:8 (8:6) | 54:6 (10:11) |
| Height, cm, mean (SD) | 165.0 (9.4) | 159.5 (7.6) | 166.6 (9.3) | 169.9 (8.2) | 163.3 (9.9) |
| Weight, kg, mean (SD) | 67.1 (19.0) | 56.2 (16.3) | 73.5 (27.9) | 72.7 (15.4) | 74.8 (16.4) |
| Waist circumference, cm, mean (SD) | 81.6 (18.3) | 68.9 (21.2) | 85.1 (21.2) | 84.8 (11.2) | 92.6 (15.9) |
| Hip circumference, cm, mean (SD) | 95.1 (17.4) | 79.6 (21.0) | 102.4 (19.0) | 102.3 (8.2) | 98.0 (10.4) |
- Abbreviations: GMFCS, Gross Motor Function Classification System.
Feasibility of outcomes in the COS are reported in Table 3. All participants invited to participate in the study agreed to do so and provided written informed consent. However, not all participants completed all aspects of the COS. Thirty-nine (58.2%) participants agreed to wear an Activ8 device to measure physical behaviour. However, 11 participants did not meet the minimum wear time criteria of at least 5 days and 11 hours per day. Therefore, physical behaviour was feasible in 28 (42%) participants. Refusal to wear the device was largely related to the inconvenience of keeping it on for 7 days or the need to return the device either in person or through the mail. Participants from Utrecht and Rotterdam (n=32) did not complete the Short Form Food Frequency Questionnaire (SFFFQ) because it was not available in Dutch. Therefore, 33 (94%) participants from McMaster University and the University of Michigan completed the SFFFQ. Two adult participants (classified in GMFCS levels I and II) did not complete the SFFFQ for unreported reasons. Sixty-five (97%) participants completed the Pittsburgh Sleep Quality Index (PSQI). The same two adult participants who did not complete the SFFFQ did not complete the PSQI. Thirty-seven (55%) participants completed a cardiorespiratory endurance assessment on either a cycle ergometer (arm or seated) or treadmill. All 37 participants were classified in GMFCS levels I, II, or III. No participants were excluded from the cardiorespiratory endurance assessment. Twenty-six participants declined the cardiorespiratory endurance assessment due to the inconvenience of having to attend the clinical research centre on a different day for the assessment, while an additional four participants declined for unknown reasons. Sixty-three (94%) participants had their height, weight, and BMI measured. One participant in GMFCS level I had no time, while three participants were classified in GMFCS level IV and chose not to leave their wheelchairs to have a supine height measurement. Fifty-nine (88%) participants had waist and hip circumference measurements performed. Six participants without these measures were classified in GMFCS level IV or V, had previously been out of their wheelchairs for height and weight assessments, and chose not to leave their wheelchairs again. The other two participants were classified in GMFCS level I or II and had finished their clinical appointment before researchers could perform the assessments. Sixty (90%) participants had blood pressure assessments performed. Reasons for missing assessments included muscular contractures impeding proper automated blood pressure cuff placement (n=5) or insufficient time during clinical encounter (n=2). Lastly, two of the four clinical research centres (McMaster University and the University of Michigan) asked to perform blood draws. Of the 35 eligible participants, 19 agreed to have a blood draw performed but only 16 completed the assessment. Reasons for foregoing this assessment were participant refusal (n=16) or participants did not proceed to have their blood drawn after receiving a request (n=3). Nine participants had to attend two separate visits for cardiorespiratory endurance measurements (n=6) or blood draws (n=3). Feedback during interviews with clinicians and researchers at each site aligned with the feasibility results; at McMaster University and the University of Michigan, having to refer participants to a different clinical setting for both blood draw and cardiorespiratory endurance assessment, and the uncertainty of participants completing these assessments, affected practical feasibility. Also, added personnel to distribute, collect, curate, and analyse accelerometer data were challenges encountered at each site.
| Participants | GMFCS level | Age | Sex | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=67) | I (n=6) | II (n=3) | III (n=12) | IV (n=7) | V (n=8) | <18y (n=25) | ≥18y (n=32) | Male (n=35) | Female (n=32) | |
| Physical behaviour | 28 (42) | 8 (50) | 12 (35) | 8 (67) | 0 (0) | 0 (0) | 0 (0) | 28 (100) | 9 (32) | 19 (68) |
| Sleep (PSQI) | 65 (97) | 15 (94) | 22 (96) | 12 (100) | 7 (100) | 8 (100) | 25 (38) | 40 (62) | 34 (52) | 31 (48) |
| Cardiorespiratory endurance | 37 (55) | 12 (75) | 16 (70) | 8 (67) | 0 (0) | 0 (0) | 0 (0) | 37 (100) | 15 (41) | 22 (59) |
| Body size (BMI) | 63 (94) | 15 (94) | 23 (100) | 12 (100) | 4 (57) | 8 (100) | 21 (33) | 42 (67) | 31 (49) | 32 (51) |
| Body composition (WHR) | 59 (88) | 15 (94) | 22 (96) | 12 (100) | 2 (29) | 7 (88) | 17 (29) | 42 (71) | 29 (49) | 30 (51) |
| Blood pressure | 60 (90) | 15 (94) | 21 (91) | 11 (92) | 4 (57) | 8 (100) | 19 (32) | 41 (68) | 32 (53) | 28 (47) |
| Participants | GMFCS level | Age | Sex | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=35) | I (n=7) | II (n=8) | III (n=4) | IV (n=7) | V (n=8) | <18y (n=25) | ≥18y (n=10) | Male (n=24) | Female (n=11) | |
| Nutrition (SFFFQ) | 33 (94) | 6 (86) | 7 (88) | 4 (100) | 7 (100) | 8 (100) | 25 (76) | 8 (24) | 23 (70) | 10 (30) |
| Blood draw | 16 (46) | 5 (71) | 5 (63) | 1 (25) | 1 (14) | 4 (50) | 9 (56) | 7 (44) | 11 (69) | 5 (31) |
Note
- All values are reported as n (%).
- Abbreviations: BMI, body mass index; GMFCS, Gross Motor Function Classification System; PSQI, Pittsburgh Sleep Quality Index; SFFFQ, Short Form Food Frequency Questionnaire; WHR, waist-to-hip ratio.
Table 4 displays the summary statistics for the values for each OMI. Notably, 28 participants were considered as not having a healthy diet with a total SFFFQ score less than 12. Sleep quality was poor in 45 participants (PSQI total score ≥5). Seventeen adolescents and 29 adults achieved the minimum recommended hours of sleep per night (8 hours for adolescents and 7 hours for adults). Seventeen participants had poor cardiorespiratory endurance based on age and sex cut-offs. Thirty-one participants had a BMI of 25 or greater (overweight) and 17 participants had a BMI of 30 or greater (obese). Nine (n=5 in GMFCS level V) participants were underweight with a BMI of less than 18.5. Fourteen females had a waist-to-hip ratio (WHR) of 0.83 or greater, while 13 males had a WHR of 0.90 or greater, indicative of increased risk for cardiovascular disease. Nine had a systolic blood pressure of 140mmHg or higher and/or a diastolic blood pressure of 90mmHg or higher, indicative of grade 1 hypertension. Four participants were at risk for cardiovascular disease with total cholesterol values greater than 5.20mmol/l. Three participants were at risk for hyperglycaemia (>5.4mmol/l). Ten participants had two or more cardiometabolic risk factors (overweight or obesity, hypertension, hyperglycaemia, or dyslipidaemia).
| Outcome | Mean | SD | Minimum | Lower quartile | Median | Upper quartile | Maximum |
|---|---|---|---|---|---|---|---|
| Physical behaviour, n=28 | |||||||
| Sedentary time (min) | 712.4 | 129.2 | 484.9 | 616.2 | 702.2 | 817.1 | 948.7 |
| Sedentary time (%) | 76.6 | 12.5 | 56.1 | 66.5 | 76.3 | 87.9 | 97.8 |
| Physical activity timea (min) | 213.8 | 113.7 | 18.6 | 113.1 | 220.2 | 318.0 | 408.0 |
| Physical activity time (%) | 23.1 | 12.5 | 2.2 | 12.1 | 23.7 | 32.5 | 43.9 |
| SFFFQ, n=33 | 9.9 | 1.5 | 6.0 | 9.0 | 10.0 | 11.0 | 12.0 |
| PSQI total score, n=64 | 6.6 | 3.4 | 0 | 4.0 | 6.0 | 9.0 | 13.0 |
| Cardiorespiratory endurance, n=37 | 30.5 | 8.7 | 11.6 | 24.0 | 31.0 | 38.0 | 46.0 |
| BMI, n=63 | 24.7 | 6.4 | 11.5 | 21.0 | 24.3 | 29.0 | 45.0 |
| WHR, n=59 | 0.86 | 0.09 | 0.70 | 0.80 | 0.83 | 0.91 | 1.13 |
| Systolic blood pressure, n=60 | 119.2 | 12.6 | 78.0 | 111.0 | 119.0 | 127.5 | 146.0 |
| Diastolic blood pressure, n=60 | 75.1 | 12.7 | 40.0 | 67.5 | 74.0 | 84.0 | 107.0 |
| Blood draw | |||||||
| Total cholesterol, n=15 | 4.4 | 1.2 | 2.9 | 3.6 | 4.3 | 5.4 | 7.3 |
| HDL-C, n=15 | 1.4 | 0.3 | 1.0 | 1.2 | 1.4 | 1.5 | 2.1 |
| LDL-C, n=15 | 2.6 | 1.1 | 1.1 | 1.9 | 2.4 | 3.3 | 5.6 |
| Glucose, n=9 | 5.4 | 0.5 | 4.9 | 5.0 | 5.2 | 5.6 | 6.4 |
- Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PSQI, Pittsburgh Sleep Quality Index; SFFFQ, Short Form Food Frequency Questionnaire; WHR, waist-to-hip ratio.
- a Time spent standing, walking, running, and cycling. Cardiorespiratory endurance was reported as relative VO2 (ml/kg/min). Sedentary time (min) was defined as the time spent lying and sitting; sedentary time (%) is the percentage of time spent sedentary relative to total wear time.
Exploratory Spearman’s ρ correlations are presented in Table 5. We observed significant inverse associations between GMFCS level and cardiorespiratory endurance (ρ=−0.46, p=0.005), GMFCS level and total time active (ρ=−0.42, p=0.03), and GMFCS level and percentage time active (ρ=−0.47, p<0.01). Significant positive relationships were observed between GMFCS level and total time sedentary (ρ=0.53, p<0.01) and GMFCS level and percentage time sedentary (ρ=0.43, p<0.01).
| Variable | n | ρ | p |
|---|---|---|---|
| Time sedentary (total) | 28 | 0.53 | <0.01a |
| Time sedentary (%) | 28 | 0.43 | 0.02a |
| Time active (total) | 28 | −0.42 | 0.03a |
| Time active (%) | 28 | −0.47 | 0.01a |
| SFFFQ | 32 | 0.14 | 0.44 |
| PSQI total score | 64 | −0.07 | 0.61 |
| Sleep, hours per night | 64 | 0.10 | 0.43 |
| VO2max | 36 | −0.46 | <0.01a |
| BMI | 62 | −0.20 | 0.12 |
| WHR | 58 | 0.05 | 0.70 |
| SBP | 59 | −0.12 | 0.37 |
| DBP | 59 | −0.19 | 0.15 |
| Total cholesterol | 15 | 0.17 | 0.55 |
| HDL cholesterol | 15 | 0.05 | 0.86 |
| LDL cholesterol | 15 | 0.15 | 0.59 |
| Glucose | 9 | −0.03 | 0.94 |
- Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; GMFCS, Gross Motor Function Classification System; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PSQI, Pittsburgh Sleep Quality Index; SBP, systolic blood pressure; SFFFQ, Short Form Food Frequency Questionnaire; VO2max, maximum rate of oxygen consumption; WHR, waist-to-hip ratio.
- a p<0.05.
DISCUSSION
Our study was unique because it investigated the importance of multimorbidity health outcomes in individuals with CP and their families and assessed the feasibility of measuring these outcomes using an expert-developed COS across international clinical research centres. Survey results identified modifiable behaviours, specifically physical behaviour, nutrition, sleep, and cardiorespiratory endurance, as most important to measure and discuss with their family healthcare professional, suggesting a need to screen and manage these health-related outcomes in this population. Our COS identified blood pressure assessment, BMI, and WHR as highly feasible OMIs (≥88%) to perform in clinic and research settings associated with cardiometabolic disease. On the other hand, blood draws were more challenging to collect.
Although participants viewed cardiorespiratory endurance and physical behaviour as two important outcomes to have assessed by their family healthcare professional, these outcomes proved challenging to collect in clinical research settings. As a team of clinical and research experts, we previously agreed on objective-based physical behaviour assessment (i.e. accelerometry) due to the quality of the evidence.13 However, the technical and analytical requirements to collect and interpret physical behaviour, combined with participant burden of wearing a device for 7 days and then returning the device, had implications on overall feasibility. Feedback from participating clinical centres noted that appointments took longer than before with these extra measures, but were expected to be considered worthwhile by many patients as noted from the participant survey. Additionally, clinical research centres identified endurance and physical behaviour outcomes to require more personnel, expertise (e.g. referring the participant to an additional clinic or centre to have the outcome assessment performed [cardiorespiratory endurance]), and time to retrieve, upload, and analyse physical behaviours. Going forward, it is suggested that a simpler first screen using questionnaire-based measures should precede the COS. For example, members of our team recently published on the pilot testing of a 24-hour activity checklist for children with CP.16 The checklist includes questions about physical activity, screen time, and sleep that can be completed by the patient and family before their clinical appointment. We suggest clinicians should include a short-form physical activity questionnaire alongside the PSQI and SFFFQ questionnaires as a first screen and then proceed to the remainder of the COS or certain aspects of the COS that might be most relevant to the patient (e.g. cardiorespiratory endurance if physical activity and tiredness were concerns). Although recent research found the short-form version of the International Physical Activity Questionnaire to have poor concurrent validity in young people with CP,17 it can be considered a first-line screening for activity in those healthcare settings where accelerometry may not be feasible or practical. Using a simpler first screen before the COS would alleviate some of the feasibility concerns from clinicians and patients but provide valuable information to further clinical screening when required. Also, it is important to consider the clinical utility of measures in the COS. For example, previous research from members of our group found WHR to be independently associated with various indices of cardiometabolic risk in adults with CP, while BMI was not,18 suggesting that clinicians should incorporate WHR as a prognostic marker in this population.
Given the heightened risk for non-communicable diseases and multimorbidity in individuals with CP compared to the general population,4, 19 our COS of OMIs has clinical implications for screening and managing cardiometabolic and multimorbidity risk factors. Recently, Whitney and Kamdar20 developed a new comorbidity index (the Whitney Comorbidity Index), which identified 27 conditions associated with a 2-year mortality in adults with CP. Cardiometabolic-related comorbidities included hypertension, arrhythmias, cerebrovascular disease, diabetes, and heart failure. Since participation in our cross-sectional study was voluntary, participants could refuse certain or all aspects of the protocol. It was likely that the invasive nature of a blood draw deterred participants from partaking in this assessment; this was observed in previous research in this population.21 Nonetheless, clinical cut-offs for cardiovascular disease risk based on glucose and lipid panels exist, and clinicians should consider requesting these assessments in individuals with CP if they present with overweight, obesity, and/or prehypertension and hypertension, particularly in light of the Whitney Comorbidity Index20 and other multimorbidity risk research in this population.8 Going forward, and at a minimum, we recommend that healthcare providers of adolescents or adults with CP should perform a simpler first screen of modifiable behaviours (i.e. physical activity, sleep, and nutrition) yearly and proceed with the remainder of the COS if there is greater concern, which might require referrals to specialists (e.g. exercise physiologist, somnologist). Additionally, the recent literature suggests that it is difficult for individuals with CP to find specialist care, especially during the transition from adolescence to adulthood and beyond,22 emphasizing the importance of equipping healthcare providers with OMIs to measure multimorbidity risk in this population.
Limitations of the study should be acknowledged. In our convenience sample, the number of individuals classified in GMFCS levels IV and V was low and the feasibility and clinical utility of the COS for these individuals requires further investigation in a larger sample size. The feasibility data of the blood draw may have limited generalizability to other places and countries because it was only assessed in two out of four clinical research sites. For example, in the Netherlands a general practitioner typically performs a blood draw in adults with CP. Another limitation is that the data from the survey and feasibility studies were from two different samples of individuals with CP, making the results not directly relatable to each other. Also, the survey was limited to respondents in North America due to pragmatic reasons, including the English language. Future research should consider the importance of the COS and its OMIs in individuals with CP in other countries. Although we included participants who responded to questionnaires with assistance, we did not gather information on intellectual disability. Therefore, the presence of intellectual disability might affect the feasibility of the COS. Finally, internet surveys are susceptible to (non)response bias23 and these results should be interpreted with caution.
Performing a COS that includes measures that often are not part of routine clinical care can help screen for cardiometabolic and multimorbidity risk, and lead to referral to clinical specialists if required. Healthcare providers that care for adolescents and adults with CP should consider assessing these outcomes as part of routine follow-up to track risk factors for multimorbidity health longitudinally, using questionnaire-based measures first and then the remainder of the COS, while at the same time promoting healthy behaviours.
ACKNOWLEDGEMENTS
This clinical research project was funded by an American Academy for Cerebral Palsy and Developmental Medicine Pedal with Pete grant. Dr McPhee is funded by a Canadian Institutes of Health Research Fellowship (no. FRN 164649). Dr Gorter holds the Scotiabank Chair in Child Health Research. We thank Dr Ronit Mesterman, McMaster University, and Dr Heidi Happala and Dr Mary Schmidt from the University of Michigan for their assistance with participant recruitment. Finally, we thank all the participants, including the CP NOW Foundation and its President, Michele Shusterman, for their involvement in this study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.




