The histopathology of polymicrogyria: a series of 71 brain autopsy studies
Abstract
Aim
Polymicrogyria (PMG) is one of the most common forms of cortical malformation yet the mechanism of its development remains unknown. This study describes the histopathological aspects of PMG in a large series including a significant proportion of fetal cases.
Method
We have reviewed the neuropathology and medical records of 44 fetuses and 27 children and adults in whom the cortical architecture was focally or diffusely replaced by one or more festooning bands of neurons.
Results
The pial surface of the brain overlying the polymicrogyric cortex was abnormal in almost 90% of cases irrespective of the aetiology. This accords with animal studies indicating the importance of the leptomeninges in cortical development. The aetiology of PMG was highly heterogeneous and there was no correlation between cortical layering patterns and aetiology. PMG was almost always associated with other brain malformations.
Interpretation
The inclusion of many fetal cases has allowed us to examine the early developmental stages of PMG. The study indicates the significance of surface signals responsible for human corticogenesis and the complex interaction between genetic and environmental factors leading to this common endpoint of cortical maldevelopment.
What this paper adds
- Fetal cases allow study of the early developmental stages of polymicrogyria (PMG).
- PMG is commonly associated with pial disruption, regardless of aetiology.
- Abnormal festooning of the cortical neuronal band can occur well before normal cortical folding is expected to start.
- PMG is very often associated with other brain malformations.
This article is commented on by Ten Donkelaar on page 7 of this issue.
Abbreviations
-
- PMG
-
- Polymicrogyria
Polymicrogyria (PMG) is a common and highly heterogeneous malformation of cortical development with variable clinical phenotype, imaging, and histology.1-3 PMG may cause a variety of symptoms including seizures, developmental delay, motor problems, isolated language delay, feeding problems, microcephaly, and multiple congenital anomalies, all to a variable degree of severity. It usually occurs in association with other brain malformations and/or multiple congenital anomalies or intellectual disability.1
During life, diagnosis of PMG is based on neuroimaging. The magnetic resonance imaging (MRI) features of PMG consist of an irregular and bumpy appearance of the cortex, with apparent cortical thickening, and a stippled grey–white matter junction.2, 4 PMG can be focal or diffuse, unilateral or bilateral. Different imaging patterns have been described including bilateral frontal, frontoparietal, perisylvian, lateral parietal, parasagittal parietooccipital, and generalized, as well as unilateral PMG.2, 5-11
Although histology is regarded as the diagnostic criterion standard, post-mortem pathology is rarely obtained. The descriptions in the neuropathological literature are based on patients who died or required surgery for intractable epilepsy and likely represent the most severe end of the spectrum. Detailed examination of fetal brains reveal the cortical malformation in the early developmental stages.
The architectonic features of PMG were first described by Bielschowsky in 191512 and since by others.13-18 Diagnostic criteria for PMG as defined by Friede in 1989 include abnormal arrangement of cell layers, an intracortical fibre plexus, extensive folding of all layers or of the upper layers only, and fusion of gyral surfaces.16 Norman highlighted that cases of PMG are morphologically heterogeneous and that identical morphology does not necessarily imply similar aetiology.18
The association of extrinsic factors such as intrauterine cytomegalovirus infection, fetal cerebral ischemia due to placental perfusion failure, twin-to-twin transfusion syndrome, loss of a twin in utero, or maternal drug ingestion has been well documented in PMG.19-23 Reports of PMG in association with malformation syndromes,24, 25 chromosomal abnormalities,26, 27 and the identification of mutations in genes (e.g. tubulin genes, RTTN, OCLN, KIF5C, KBP and LAMC3)28-37 in patients with PMG, has resulted in an increased acknowledgement of the role of genetic factors in its pathogenesis. It seems likely that PMG is the common endpoint of many different aetiological processes, occurring at particular times in cortical development. How genetic background, environmental factors, and timing interact and result in a specific PMG phenotype is the subject of ongoing research and motivates the present study.
We describe the neuropathological changes seen in PMG as well as the associated brain abnormalities in a series of 71 patients in whom brain autopsy was available. The inclusion of a large proportion of fetal brains in combination with brains of older children and adults allowed us to identify the early, as well as the established, forms of the malformation.
Method
The databases of the Departments of Neuropathology of the Sainte-Justine Hospital in Montreal; the Montreal Neurological Hospital and Institute, Kings College Hospital in London; and the John Radcliffe Hospital in Oxford were reviewed for cases labelled as ‘polymicrogyria’. Medical records, general autopsy reports, and genetic studies were reviewed by ACJ. Medical records were unavailable for two cases; general autopsy reports were unavailable in seven. Sections of brains retained at autopsy and submitted with original stains were studied. Tissue blocks from the Oxford archive were available for additional staining where required. WS and ACJ reviewed all brain sections together. Cases submitted by other pathologists were reviewed with them.
Cases were included when regions of the cortical plate consisted of one or more festooning bands of neurons replacing the normal cortical architecture. Fusion of the molecular layers was not obligatory for inclusion. Cases with PMG as part of typical cobblestone lissencephaly were excluded.
The polymicrogyric cortex and its boundaries, the overlying leptomeninges, the white matter, hippocampus, deep grey nuclei, brainstem, and cerebellum were systematically assessed.
Patterns of abnormal cortical folding were classified on the basis of the scheme described by Norman18 where possible:
- Unlayered cortex: a molecular layer and a single band of unlayered neurons looping back and forth, an appearance Crome described as ‘festooned’.13
- Four-layered cortex: a molecular layer, then a layer of unlaminated neurons arranged in a sinuous band, then a straight horizontal cell-poor layer, which in older individuals usually contains myelin, beneath which there is another straight horizontal layer of unlaminated neurons.
- Parallel four-layered cortex: this has the same four layers as in (2) with all layers parallel.
- Miniature gyri: miniature gyri with fusion of the molecular layers of adjacent gyri. This can be seen in combination with unlayered or four-layered cortex and can result in complex patterns.
- Poorly laminated: four layers may not always be evident. In some instances, there may be a molecular layer, with a distinct festooned layer of fairly densely packed neurons and a less dense layer of neurons beneath.
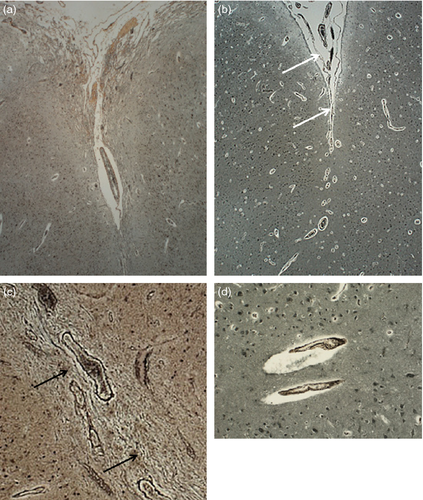
We paid particular attention to abnormalities of the pial surface of the brain and to the presence and characteristics of cortical fusion. ‘True fusion’ was designated as Type 1 and characterized by leptomeningeal remnants, visualized as blood vessels and bundles of collagen fibres with reticulin stain, observed within the molecular layer between loops of the festooning neuronal band. ‘No definite fusion’ was designated as Type 2 and characterised by prominent blood vessels but no leptomeningeal remnants within a smooth molecular layer between the loops of a festooning neuronal band.
Cases were designated to one of four aetiological groups: (presumably) genetic, early hypoxic–ischemic injury, prenatal infection, or ‘unknown’. A hypoxic–ischemic aetiology was determined when focal arterial territory infarction was identified and was assumed when focal necrosis or gliosis and calcification were identified in multiple areas of the brain together with failure to identify infection by histological, immunocytochemical, or microbiological criteria. Genetic aetiology was determined when a pathogenic mutation in a gene related to PMG or a significant chromosomal rearrangement was identified, and was assumed in cases of familial recurrence of PMG or in the presence of multiple congenital anomalies. Infections were diagnosed by immunocytochemistry or positive viral serology at autopsy.
The study was approved by the Ethics Committee of the UZ Brussel, Belgium (B.U.N. B14320096137) and the Local Research Ethics Committee (10/H0604/83) and the local NHS research governance regulations (Ref 6321) Oxford.
Results
Sample characteristics
Based on the database review, 92 cases were identified, 71 of which fulfilled the selection criteria and were included in the study. Cases came from the Departments of Pathology at the Montreal Neurological Hospital and Institute (two adult cases), Kings College Hospital in London (one child and four adult cases), the Sainte-Justine Hospital in Montreal (15 fetuses, three neonates, four infants, one child), and the John Radcliffe Hospital in Oxford (29 fetuses, three neonates, three infants, five children, one adult). Data were collected on 21 fetuses up to 28 weeks’ gestation, 23 between 29 weeks’ gestation and term, six neonates (term–1mo), seven infants (1mo–1y), seven children (1–17y), and seven adults (18y and older). A schematic overview of the clinical findings is provided in Table SI (online supporting information).
Thirty-six patients presented with abnormal fetal ultrasound, of which brain anomalies were reported in 17. These included structural abnormalities, calcification, or haemorrhage. Encephalocoele was found in four, posterior fossa abnormalities, including mega cisterna magna and Dandy-Walker variant, were suspected in four. Ultrasound scans showed ventriculomegaly in 17 patients, which was isolated in three. In one patient with isolated ventriculomegaly, ultrasound scan was followed by fetal MRI, which revealed additional brain abnormalities. Extracranial abnormalities consisted of osteoarticular malformations such as clubfeet (n=9) or scoliosis (n=2), intrauterine growth restriction (n=7), polyhydramnios (n=6), oligohydramnios (n=5), and congenital malformations of kidney (n=6), heart (n=3), or liver (n=1). In the infants, children, and adult patients, presenting features were highly variable, and included neonatal seizures, later onset epilepsy, dysmorphic features, developmental delay, or cerebral palsy.
Forty-four cases died before 40 weeks’ gestation, 24 from a termination of pregnancy. Of these, fetal ultrasound showed abnormalities in the brain in nine, both in and outside of the brain in 12, only outside of the brain in two. In one case, ultrasound data not were available. Five fetuses were stillborn, five were not resuscitated or failed resuscitation, seven died of various causes, and for three data were not available. Cause of death was related to seizures in two adults and six neonates/infants, to infections in six, and cancer in two.
Head circumference was available for 35 out of 71 patients. Microcephaly was present in 34% and macrocephaly in 20%.
Aetiology
Information on aetiology was available in 68% of all cases, of which more than 50% were of genetic or presumed genetic origin. Four patients had a confirmed genetic diagnosis (one mutation in GPR56, one mutation in OCLN, one microdeletion in chromosomal region 22q11 [del22q11], and one chromosomal rearrangement involving chromosomes 11 and 12). Twenty-three patients had a ‘presumed genetic cause’ because of the presence of multiple congenital anomalies in 16 (see associated brain malformation section below), a positive family history of PMG (four patients from two families) or of other malformations in three. There was a localized middle cerebral artery infarction in five patients, which was due to maternal gestational trauma in two. In 10 other patients, hypoxic–ischemic injury was determined to be the primary cause of the observed brain malformations. Congenital cytomegalovirus infection was found in five, and toxoplasma in one. There were no findings to indicate aetiology in 23. Overall, the aetiologies found in our patients were genetic and presumed genetic (38%), early hypoxic/ischemic injury (21%), prenatal infection (9%), or ‘unknown’ (32%).
There were five sets of twins. The first were bichorionic biamniotic, resulting from a pregnancy which was complicated by bleeding in the first trimester, arterial hypertension in the second trimester, and pre-eclampsia leading to premature birth at 32 weeks’ gestation. Both twins showed intra-uterine growth restriction and arthrogryposis, one had a cleft lip and absent larynx, and both had PMG. The second set of twins consisted of one child with PMG and one stillbirth with no evidence of brain malformation. The third set of twins consisted of one child with PMG and one with hydrops. The two other twins each had a healthy co-twin.
The polymicrogyric cortex
A schematic overview of the histopathological findings is provided in Table SII, online supporting information. The cortex was defined as polymicrogyric when one or more undulating or festooning neuronal layers replaced the normal six-layered cortical architecture.
The precise number of cortical layers was often difficult to assess and the microscopic appearance of the cortex frequently varied from place to place in an individual patient. Six fetuses were too young to assess cortical layering. Despite these difficulties, it was estimated that the polymicrogyric cortex was unlayered in 40%, four-layered in 20% and combined both two- and four-layers in the remaining 40%. No correlation was found between the two-layered or four-layered patterns of PMG and aetiology or age.
The histological variants of the polymicrogyric cortex which we identified were very similar to those reported by Norman18 in 1995 (see Figs 1-5).
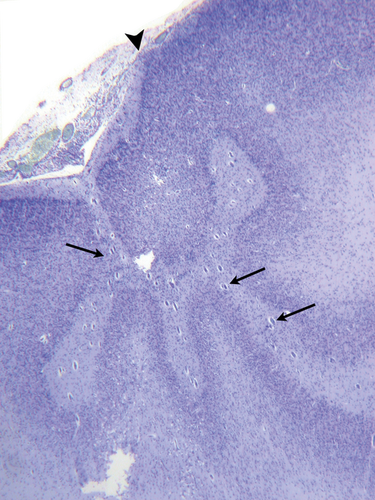
Two types of molecular layer ‘fusion’ were identified (Fig. 6). True fusion with remnants of the leptomeninges (Type 1) was observed in 20 cases, and Type 2 in 29 cases.
The brain surface
The brain surface overlying the polymicrogyric cortex was abnormal in 59 cases (87%). In three fetal patients the leptomeninges could not be assessed, as they were lost during processing. Leptomeninges were abnormal in 52 (76%) patients, 36 of which showed neural or glial invasion. In the group of 16 patients (23%) in whom the leptomeninges were normal, the pia was normal in four but was disrupted, thickened, or showed subpial gliosis in seven. Data regarding the pia were unavailable in five.
Of the 68 cases in which the leptomeninges and pia could be studied, 25 had a proven or presumed genetic origin, and 21 were acquired (hypoxic–ischemic injury or congenital infection). In the group with a genetic origin, the brain surface was normal in 8 (32%) whereas in the acquired group the surface was normal only in 2 (10%).
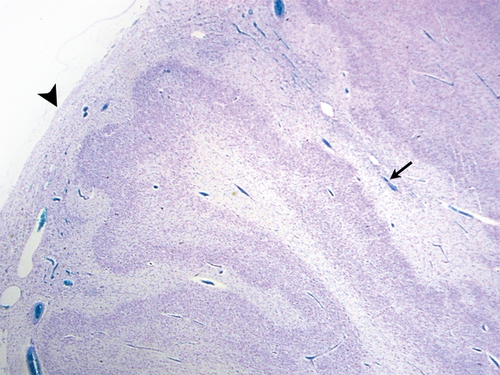
The transition from normal to thickened meninges coincided with the transition from normal to polymicrogyric cortex (Figs S1 and S2, online supporting information). The meninges became thicker and adherent to the brain surface at the point at which the cortical folding pattern became abnormal. The pial collagen fibres were reduplicated and often showed focal breaks with migration of heterotopic neural or glial cells into the thickened leptomeninges.
Normal to abnormal cortex
Transition from normal to abnormal cortex was abrupt in most cases but more gradual transitions were also seen (Figs S2 and S3, online supporting information).
Grey-white matter junction
Information on the grey–white matter junction was recorded for 62 patients. The junction between the polymicrogyric cortex and the underlying white matter was blurred in 63%, sharp in 13%, and both blurred and sharp borders were observed underlying the PMG in the same patient in 24% (Fig. S3). In the group with blurred grey–white matter junction the median age of the patients was 32 weeks' gestation, in the group with both blurred and sharp grey-white matter junction 37 weeks’ gestation, and in the group with sharp grey–white matter junction 15 weeks of postnatal life.
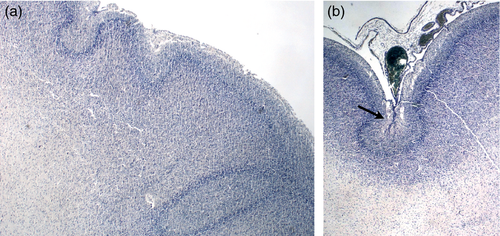
Premature cortical folding
The observation of cortical festooning in fetal cases dying before 28 weeks’ gestation indicates that the polymicrogyric cortex may start to fold before the time at which normal gyration is expected to start (Fig. S4, online supporting information).
Associated brain malformation
Clusters of heterotopic neurons in the white matter were found in approximately 60% of cases. Heterotopia were located in the deep white matter in six, in the subcortical white matter in 15, in the periventricular white matter in five, and showed combined patterns in 15 (Fig. S5, online supporting information). The proportion of heterotopic neurons in the white matter was higher in the group with abnormal leptomeninges (74%) than in the group with normal leptomeninges (62%). There was a partial or complete agenesis of the corpus callosum in seven out of 38 patients. The corpus callosum was thin in nine and thick in two. The hippocampus was abnormal in 21 out of 37 patients in whom data were available (57%) and included poor folding, abnormal orientation, or dysplasia. The deep grey nuclei were normal in the majority of patients, but were hypoplastic and/or malformed in 14 out of 52 patients on whom data were available. Cerebral peduncles were hypoplastic in 26 out of 44 patients on whom data were available (59%). Pyramidal tracts were hypoplastic or absent in 42 out of 62 patients on whom data were available (68%). The olivary nuclei were malformed in 24 out of 55 patients on whom information was available. This was associated with a malformation of the dentate nucleus of the cerebellum in 10.
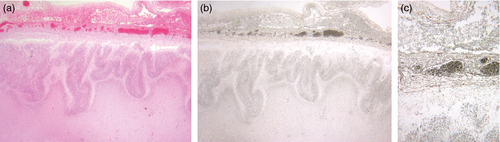
Information on the cerebellum was available in 63 out of 71 cases (89%). Cerebellar hypoplasia was found in 21 out of 44 and vermis hypoplasia in five out of 29 cases where it could be evaluated. Malformations of the cerebellar cortex are seen in association with congenital cytomegalovirus infection (see panel a, Fig. S6, online supporting information) but are otherwise exceptional in combination with PMG, outside of the spectrum of the cobblestone lissencephalies. There were only two non-infectious cases with clear cerebellar abnormalities. In the first case, which was associated with a mutation in GPR56, the leptomeninges overlying the cerebellar cortex were thickened, dysplastic, and contained heterotopic neurons and striated muscle. The cerebellar cortex contained focal heterotopic neurons but was otherwise normal. The vermis was highly dysplastic; cerebellar hemispheres were hypoplastic. The dentate nucleus and cerebellar white matter were normal (see panels c–f, Fig. S6). In the second non-infectious case, where the aetiology was unknown, the leptomeninges overlying the brainstem and cerebellum were thickened containing heterotopic neurons. The cerebellar cortex was clearly abnormal with nodules of molecular layer in the internal granular layer (see panel b, Fig. S6). The dentate was malformed. The leptomeninges overlying the cerebellum were abnormal in two additional cases, one of which had an encephalocoele and the other had thickened vascular leptomeninges throughout, with macrophages in the cortex at the grey–white matter junction, in combination with hepatosplenomegaly, tricuspid regurgitation, and a cleft lip. An infectious cause could not be proven.
Seven patients showed evidence of gliosis in the cerebellar cortex, with or without Purkinje cell loss. There was evidence of infection in one, hypoxic–ischemic injury in two, and refractory epilepsy with longstanding use of phenytoin in one.
Discussion
Clinical findings
In the cases diagnosed postnatally, presenting symptoms included epilepsy, developmental delay, cerebral palsy, and microcephaly, which is consistent with the findings in imaging based series.2 Death was related to epilepsy in six neonates/infants and two adults, reflecting the severity and refractory nature of the epilepsy in some cases of PMG.2, 38
Diagnosis in fetal cases was more complex. Although whole body fetal ultrasound was abnormal in 36 cases, in the majority (n=19) no brain abnormalities were identified by ultrasound but PMG was present on post-mortem examination. The 17 patients who did have brain abnormalities on fetal ultrasound all had ventriculomegaly, which was isolated in only two cases. This confirms that patients with ventriculomegaly in combination with other brain abnormalities have a high risk of cortical abnormalities.39 These observations highlight the need for careful examination of the brain, preferably by three-dimensional ultrasound or fetal MRI40 when extra-central nervous system lesions or ventriculomegaly are observed.
Although head circumference might not always be accurate in young fetuses, it was within the normal range in approximately half of the cases, which is in line with neuroimaging-based series.2 PMG associated with macrocephaly has been reported in mutations of the PI3-Akt pathway41 and with microcephaly in mutations in WDR62.33, 42
Aetiology
Only a minority of the cases in this series had clearly defined causal factors, including proven infections, hypoxic–ischemic injury, maternal trauma, or a proven genetic defect. Findings which have been attributed to acquired aetiologies, such as calcifications and porencephalic cysts, may also have resulted from genetic causes, such as mutations in OCLN or COL4A1,34, 43 further increasing possible genetic contributions to the aetiology of PMG. It is anticipated that further study of the histopathological and imaging patterns associated with mutations in specific genes will facilitate the recognition of causative mechanisms in the future. This has been illustrated for mutations in GPR56 and the tubulin genes.44, 45 However, despite advances in imaging and genetic techniques, defining aetiology for PMG may still represent a major challenge in clinical practice.
The polymicrogyric cortex
Two features deserve special mention, as they have not been considered in previous pathological studies of PMG; these are the almost universal abnormality of the pial surface of the polymicrogyric cortex and the observation of cortical folding in fetuses before the age at which normal gyration is expected to occur.
The brain surface
The brain surface was abnormal in 87% of cases. Adherent, thickened leptomeninges invaded by neural or glial cells overlying the abnormal cortex were most frequent in acquired cases (90%) but were also very common in proven or presumed genetic origin (68%). They were not specific to any of the aetiological categories.
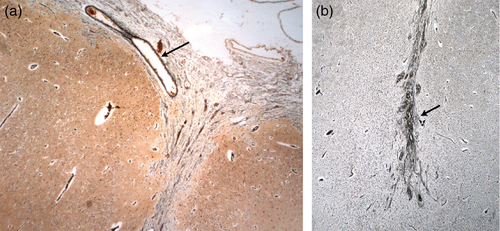
All of the elements of the cortical surface (arachnoid trabeculae, pial cells, basement membrane, radial glial/astrocytic end feet, and Cajal–Retzius cells) have been implicated in cortical malformation. The fetal meninges regulate cortical neuronogenesis, cell migration and positioning, and maintain the pial basement membrane, which is critical for attachment of radial glial end feet.46 The importance of the integrity of the brain surface for normal corticogenesis has been demonstrated using both lesional and genetic animal models. Damage to the upper cortical layers or meninges in newborn rats caused abnormalities in the neuronal band including PMG, while the same insults after day 4 of life caused necrosis.47, 48 Surgical removal of the meninges and their associated blood vessels led to apoptosis of radial glial cells and reduction in cortical size.49 Studies in Gpr56−/− mice have shown that, the basement membrane develops normally but later breaches occur through which neurons migrate into the leptomeninges.50 This has been observed in two human fetuses with mutations in GPR56, a fetus at 27 weeks’ gestation included in our series (Fig. S7, online supporting information), and a fetus at 35 weeks’ gestation reported by Bahi-Buisson et al.44 Mutations in GPR56 seem to result in a specific histopathological phenotype of PMG, in combination with features of cobblestone lissencephaly, (see panels c–f, Fig. S6), and patchy dysmyelination.44 Overmigration of neurons through breaches in the pial basement membrane has also been observed in a fetus at 27 weeks’ gestation with a mutation in TUBB2B resulting in a more ‘classical’ form of PMG. Disorganized radial glial fibers were identified beyond the pial basement membrane.30 However, despite these studies, it remains to be clarified whether leptomeningeal pathology participates in the genesis of PMG or represents a secondary change.
The leptomeninges were normal only in a small minority of our cases (<15%). In the case of OCLN mutation for example, PMG was likely to result from disordered signalling between cells of the neurovascular unit and altered permeability of the blood–brain barrier.34
Premature cortical folding
Normal gyration begins at 21 weeks’ gestation, is well defined by 28 weeks, and by term is almost complete. The potential influence of underlying mechanisms (genetic, epigenetic, mechanical, or environmental) on normal cortical folding is still poorly understood. Sulcal emergence has been studied noninvasively in preterm newborn infants, by applying dedicated post-processing tools to MRI acquired shortly after birth ranging from 26 to 36 weeks of age.51-53 However, the mechanisms involved in the early folding in PMG remain completely unknown. Abnormal festooning of the entire cortical neuronal band was seen in a few fetuses younger than 21 weeks, well before the time at which normal cortical folding is expected (Fig. S4). This observation indicates that PMG is not the result of fusion of already formed cortical gyri, but may in some cases result from an abnormally early signal to commence gyration.
Cortical layers
Although many authors refer to the patterns cortical layering in PMG, our results show that this clearly is not the most relevant aspect of the malformation and we agree with Judkins et al.17 who observed co-existence of two- and four-layered patterns in 40% of patients, a further illustrating that the definition of PMG by layer patterns is artificial. We were unable to confirm anecdotal reports of a correlation between laminar patterns and aetiology16 or the age at which cortical formation was disrupted.54
Fusion
More than two out of three of our cases had no definite evidence of cortical fusion and ‘true fusion’ of adjacent gyral surfaces was seen in only 20 cases, consistent with Norman's classification with fusion described in only one of five subtypes of PMG.18 Our findings differ from those of Judkins et al.17 who considered surface fusion as part of the definition of PMG. One case had a unique pattern of fusion, which appeared to be due to ‘zipping up’ of the molecular layers of adjacent gyri (see panel b, Fig. S5).
Normal to abnormal cortex
A relatively abrupt transition from normal cortex to PMG has been reported previously17, 18 and was confirmed by our study. As noted above, the transition is often accompanied by a change in the overlying leptomeninges, as illustrated in Fig. S2.
Grey-white matter junction
Unlike the general concept that the grey-white matter junction underlying PMG is relatively sharp,17 we have shown that it was blurred in the majority of cases, and both blurred and sharp borders co-exist (Fig. S3). A sharp border was most frequently seen in postnatal cases, suggesting a function of age and maturation on the junction of the cortex with the white matter, as has been suggested by imaging.4
Associated malformations
PMG is often considered as an isolated brain malformation but associated malformations including ventriculomegaly and abnormalities of the corpus callosum, brainstem, and cerebellum have been reported in imaging-based studies.28, 55 The identification of PMG on brain autopsy or imaging should therefore prompt the clinician for a careful evaluation of the other brain structures.
Periventricular, subcortical, or deep white matter heterotopia were found in up to 60% of patients, considerably more than the 25% reported by Judkins et al.17 and the 11% reported in the largest neuroimaging series.2 This may reflect the limitations of neuroimaging in detecting subtle malformations. The high number of white matter heterotopia highlights once more that normal migration and the integrity of radial unit, from the ventricular zone throughout the white matter and including the cortical surface, are key to normal cortical development.
It has been reported previously that the medial hemispheric cortex and the hippocampus are usually spared.56 We observed that poor folding or abnormal orientation of the hippocampus was not uncommon, but PMG was unusual in the medial temporal lobe. The olivary nuclei were abnormal in 24 patients, associated with malformations of the dentate nucleus in 10. These findings have been reported in Ohtahara syndrome but remain non-specific.57 Pyramidal tracts were hypoplastic or absent in 68%, in line with the 51% in the imaging-based series and the significant unilateral or bilateral motor deficits encountered in patients with PMG.2 The cerebellar cortex was spared in the large majority of cases, the most important exceptions being congenital cytomegalovirus infection and mutation in GPR56 (Fig. S6). Cerebellar hypoplasia was present in 21 cases of various aetiologies. The vermis and corpus callosum could not always be evaluated in detail.
Strengths and weaknesses
The strengths of this study are the large number of cases examined and the inclusion of a high percentage of fetal cases, which allowed us to examine the brain while cortical developmental processes are still ongoing, illustrating the reactions to insults at specific times in human brain development; something that cannot be reproduced in animal models. A key finding in this regard is that abnormal folding of at least some forms of PMG occurs before normal cortical folding.
Use of fetal tissue requires careful interpretation of the findings, taking into account the normal structural differences between the fetal and the postnatal brain. In particular, as we were specifically interested in meningeal reactions, the fetal leptomeninges are more vascular and relatively thicker than in the mature brain but they are easily stripped from the brain surface during post-mortem handling and section preparation. For this reason the leptomeninges were not available to study in some fetal cases. Abnormal fetal leptomeninges are thick and appear to be more adherent, especially in the depths of sulci. This may provide a bias in the analysis if abnormal leptomeninges are more likely to have been preserved and available for study than normal ones.
Examining the fetal brain also allows visualization of the reactions to insults close to the time of their occurrence. Vascular and cellular reactions are more active soon after an insult than weeks or years later. Further, secondary scarring and gliotic processes, which follow tissue damage, may mask the original pathogenetic processes in older brains.
A limitation of this study is that many of the cases were archival and we only had limited sections and were not always able to study all brain areas of interest. Further, we were limited in the range of tissue stains available in the referred cases and in this subgroup we were unable to use additional staining methods in elucidating the pathological processes. Because data were collected retrospectively, case details were not always comprehensive and genetic studies have not been undertaken in the large majority of cases. Diagnostic work-up was not performed according to the same standard algorithm in all cases.
In every case the standard haematoxylin and eosin stain was available. In some cases where tissue blocks were available we were able to undertake further stains and immunocytochemistry to determine specific cell types. A strength of this study is the use of the reticulin stain, which is technically successful in retrospective studies of tissue even after prolonged storage of wax blocks. Reticulin stains type III collagen fibres found in basement membranes and restricted to the vascular and pial basement membranes in normal cortical tissue. It allowed precise definition of abnormal collagen in the malformed cortex, indicating remnants of trapped leptomeninges after abnormal cortical fusion. It also clearly demonstrates disruption and subtle degrees of reduplication and thickening of the pial basement membrane.
A problem inherent in any autopsy study is that only a snapshot at a single point in time may be examined and it is not possible to study the developmental history of a malformation process unless a number of cases at different ages are available with a common inheritance within a single family.58 While radiology cannot provide the resolution to assist in precise description of cortical malformations, serial brain imaging can teach us a great deal about their natural history and evolution.59, 60 Since imaging facilitates the diagnosis of PMG during life, autopsy diagnosis of PMG is rarely sought in older patients. The inclusion of 14 children and adults in this series has enabled the study of the entire spectrum of PMG histopathology, from as early as 18 weeks’ gestation to after myelination and maturation have been completed.
Conclusion
A feature of cortical development which has received little attention by neurologists and neuroradiologists, is the importance of the leptomeninges for normal cortical development, long recognized by neuropathologists and the most striking finding in this study. The high prevalence of abnormalities of the brain surface overlying the polymicrogyric cortex is not surprising as it is here that we find the nexus between the radial glial endfeet, Cajal–Retzius cells, the pial basement membrane, and leptomeningeal cells, all recognized to play a crucial role in cortical formation. Many recent animal studies have highlighted the frequency and importance of pial basement membrane and leptomeningeal abnormalities in a range of malformations of the cortex. We believe that future studies should be directed towards examining the relationship between the cortex and the components of the overlying meninges in normal and abnormal human cortical development. Further determination of the causes and mechanisms of PMG might also shed light on the normal processes of brain gyration.
Acknowledgements
We are grateful to the parents who have allowed us to examine and study their infants’ brains. ACJ has received funding from the Scientific Fund Willy Gepts. The authors have stated they had no interests that might be perceived as posing a conflict or bias.




