Cerebral vasculitis in severe Kawasaki disease: early detection by magnetic resonance imaging and good outcome after intensive treatment
Abstract
Kawasaki disease is an acute vasculitis, that has a classic complication of acquired coronary artery aneurysm. Severe forms with multi-organ involvement or neurological dysfunction are rare. Cerebral vascular involvement has been related to large-vessel injury or cardioembolism, leading to focal brain infarction. A 4-year-old female presented with unusual, rapidly catastrophic Kawasaki disease with refractory shock, acute renal failure, and coma, requiring intensive haemodynamic management. The observation of diffuse micro-haemorrhages (T2*-weighted sequence) associated with white matter injury on brain magnetic resonance imaging (MRI) pointed towards lesions of the medium/small blood vessels. Cerebral vasculitis was suspected and the immunosuppressive treatment was increased Subsequently, the patient’s recovery was rapid. On follow-up severe, bilateral vitritis was evident and surgery improved visual outcome. Early recognition of severe or unusual forms of Kawasaki disease could lead to more favourable outcome using appropriate treatment strategies. Diffuse cerebral micro-haemorrhages on T2* brain MRI sequences might be a key sign for the diagnosis of medium or small cerebral vessel involvement.
What this paper adds
- •
T2* weighted brain MRI can demonstrate cerebral vasculitis requiring further immunosuppressive treatment in severe Kawasaki disease.
Kawasaki disease is an acute vasculitis of unknown aetiology, predominantly affecting infants and young children. Criteria for a typical Kawasaki disease diagnosis are well known: fever lasting at least 5 days and the presence of four out of five main clinical features (rash, bilateral non-exudative conjunctivitis, erythema, changes in the extremities, and cervical lymphadenopathy).1 Hyperechogenicity of coronary arteries and coronary aneurysms are classic complications in this condition. Severe Kawasaki disease requiring admission to an intensive care unit is rare and is mainly due to hypotension, altered neurological status, apnoea, or mesenteric infarction.2 Cerebral infarction involving large-sized blood vessels has been reported.3 Pathological analyses led to the diagnosis of cerebral vasculitis, involving small- or medium-sized vessels in some children.4 Here, we report for the first time that brain magnetic resonance imaging (MRI) is a useful tool for the diagnosis of cerebral vasculitis in Kawasaki disease.
Case Report
A previously healthy 4-year-old female presented with a 6-day history of cervical lymphadenopathy, skin rash, conjunctivitis, and hyperthermia. She had hyperleukocytosis, mild anaemia, and thrombocytopenia, associated with marked inflammation. As the diagnostic criteria for Kawasaki disease were met, two courses of intravenous immunoglobulins (1g/kg) and oral acetylsalicylic acid were administered. The patient’s level of consciousness deteriorated, with acute renal failure, and she was transferred to the intensive unit care 2 days after hospital admission. She still had fever, diffuse erythematous rash, conjunctivitis, cervical lymphadenopathy, changes in oral mucosa, hand and perineal oedema, and peeling. Her condition worsened within 24 hours, with the occurrence of oliguria, coma, and severe hypotension. Electroencephalography showed specific diffuse slow waves. Ultrasound of the coronary arteries demonstrated hyperechogenicity with no dilation suggesting coronary artery involvement. Volume resuscitation, high doses of epinephrine, norepinephrine, and terlipressin treatment were necessary to restore the haemodynamic balance. Low-dose norepinephrine had to be maintained for 7 days. In addition, acute renal failure required continuous high-volume haemofiltration during the same period. Four daily doses of methylprednisolone (10mg/kg/d) were given in view of severe multi-organ involvement and the persistence of fever. Analysis of cerebrospinal fluid showed normal cell count, hyperproteinorrachia (1.24g/l), and a mild intrathecal immunoglobulin secretion (IgG index 1.8, normal <0.7). Blood analyses did not show any bacterial or viral agent, antinuclear or anti-cytoplasmic antibody. After withdrawing sedative drugs, accurate neurological assessment became possible on day 15. It showed tetraplegia and incomplete recovery of consciousness. Deep tendon areflexia suggested that peripheral nerve injury was at least in part responsible for the tetraplegia. Complete ophthalmoplegia and loss of visual reactivity were observed. Nerve conduction studies revealed no response or abnormal motor response, with markedly reduced action-potential amplitudes in compound muscle. Conduction blocks were not observed. Sensory responses were not obtained. Needle electromyography showed massive acute denervation in several muscles. Results of the neurophysiological tests can be found in Table SI (online supporting information). Electroencephalography showed persisting slow waves, also suggesting central involvement. The first brain MRI, performed on day 15, displayed bilateral hyperintensities in the cerebellar cortex and white matter, in pedunculae, caudate nuclei, and in the anterior and posterior periventricular white matter, which were observed on diffusion-weighted and T2 imaging and on fluid-attenuated inversion recovery (FLAIR) sequences. The T2* sequence revealed multiple supra- and infratentorial micro-haemorrhages in the same regions. Magnetic resonance angiography showed normal arteries of the circle of Willis. Such features were suggestive of diffuse brain vasculitis associated with ischaemic damage (Fig. 1). Because of the suspicion of cerebral vasculitis, the immunomodulative treatment was therefore increased. The patient, whose renal function had meanwhile recovered, received six fortnightly doses of cyclophosphamide (500mg/1.73m2) in association with four additional daily doses of methylprednisolone. Oral treatment with prednisone (1mg/kg/d) was subsequently administered and was combined with azathioprine (2mg/kg/d). Complete blindness persisted, and the ophthalmological examination 2 months after the onset of symptoms revealed bilateral vitritis. Thus, the fundi could not be observed, but mode B ultrasound did not show any retinal abnormality. A vitrectomy with epimacular membrane peeling was performed on both eyes, and intraocular steroid infusions were administered. No cardiac dysfunction was observed and coronary artery hyperechogenecity disappeared within 1 month. The patient’s neurological status slowly improved, with non-assisted breathing and a few voluntary movements after 1 month. She was able to sit and started to speak again after 2 months, and walked with aid after 3 months. On follow-up examination 15 months after discharge, the neurological outcome was good as the patient could speak fluently and was attending normal school. She exhibited only a mild left foot drop. Electroneuromyographic study revealed a conduction block on the left peroneal nerve and normal conduction elsewhere. Such segmental and asymmetrical damage retrospectively favoured mononeuropathy multiplex. No relapse occurred. Eye examination showed decreased visual acuity (right, 2/10; left, 6/10) with normal intraocular pressure and without any sign of local inflammation. Examination of the fundi showed slight peripheral retinal fibrosis and normal macula. Oral costicosteroids were tapered off within 6 months and azathioprine within 12 months. The MRI performed 1 year after symptom onset showed dramatic improvement, with few hyperintensities in the white matter on T2 weighted and FLAIR sequences and without any cortical atrophy (Fig. 2).
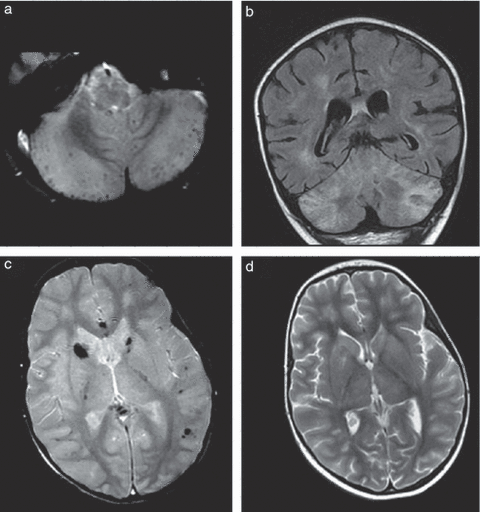
Brain magnetic resonance imaging performed 15 days after the onset of disease. (a) axial T2* image at the level of the cerebellum showing diffuse micro-haemorrhages; (b) coronal fluid-attenuated inversion recovery image showing hyperintensities of the white and grey matter; (c) axial T2* image at the level of the basal ganglia showing diffuse micro-haemorrhages, mainly in the white matter and basal ganglia; and (d) axial T2 image showing hyperintensities on the basal ganglia and white matter.
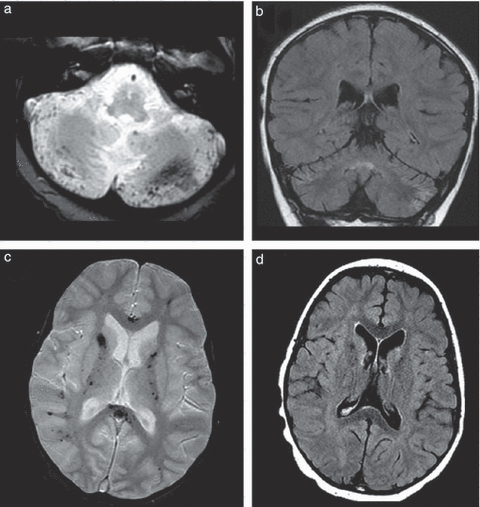
Brain magnetic resonance imaging performed 12 months after the onset of disease. (a) axial T2* image at the level of the cerebellum, showing several sequelar micro-haemorrhages; (b) coronal fluid-attenuated inversion recovery image showing moderate cerebellar atrophy and improvement of hyperintensities of the white matter; (c) axial T2* image at the level of the basal ganglia showing diffuse sequelar micro-haemorrhages; and (d) axial T2 image showing sequelar hyperintensities on the basal ganglia and in the white matter without any cortical atrophy.
The parents of the patient gave written consent to the publication of this report.
Discussion
In Kawasaki disease, vascular involvement is attributed to two main concurrent mechanisms: a non-specific inflammation burst that influences large-vessel homeostasis (parietal inflammation) and promotes a prothrombotic inflammatory state, and a diffuse vasculitis predominantly affecting medium- and small-sized vessels. This vascular wall inflammation disrupts the endothelial destruction/regeneration balance. Disregulated endothelial proliferation leads to the constitution of transient or permanent stenoses. The endothelial destruction leads to increased wall vulnerability with blood leaks (micro-haemorrhages) and/or artery dilation (aneurysm in coronary arteries). These different lesion types may occur at the same time, in various organs.5
Central nervous system involvement occurs in less than 1% of children with Kawasaki disease, including cranial nerve palsy and cerebral infarction.3 In previously published cases, cerebral infarction was either attributed to (1) inflammatory stenosis or occlusion of cervical or intracranial large-sized vessels, or (2) thromboembolism from hypokinetic myocardium. Thus, classic parenchymal territories corresponding to the arteries of the circle of Willis and internal carotid arteries were involved.6–8 Autopsies of children with Kawasaki disease revealed cerebral vasculitis affecting medium- and small-sized vessels, with endoarteritis, periarteritis, and perivascular cuffing.4,9 Moreover, as a single-photon emission computed tomography study showed, asymptomatic focal hypoperfusion areas in 29% of patients with Kawasaki disease, similar to other inflammatory conditions, suggests a vasculitis mechanism.10 However, MRI features suggestive of this mechanism have not been reported so far. In our patient, the brain MRI pattern was quite different from a classic large-artery infarction. Diffuse micro-haemorrhages associated with multifocal white matter injury were suggestive of a small-vessel cerebral vasculitis. Diffuse white matter injury is not a classic brain MRI feature in Kawasaki disease. In our patient, it could be related to ischaemic–hypoxic diffuse injury due to prolonged shock, or to diffuse small-vessel vasculitis. The presence of diffuse micro-haemorrhages was a very specific radiological sign that strengthened the vasculitis hypothesis. Diffuse micro-haemorrhages are observed in traumatic diffuse axonal injury, which obviously was not the case in our patient. Micro-haemorrhages are also common in small-vessel conditions such as amyloid angiopathy in elderly people,11 severe chronic hypertension, and genetic cerebral small or medium-vessel conditions.12 They reflect blood leaks through disrupted walls. Considering the similarity of the images, we assumed a pathophysiological similarity. Thus early brain MRI including a T2* weighted sequence may be a very useful and non-invasive tool for the diagnosis of cerebral vasculitis.
Tetraplegia in Kawasaki disease is not mentioned in the literature. Owing to massive and diffuse alterations of the first electroneuromyographic studies, it was not possible to differentiate critical illness polyneuropathy (generalized damage) from mononeuropathy multiplex (segmental, multifocal damage) in the acute phase. The second electroneuromyographic findings favoured mononeuropathy multiplex. Thus, Kawasaki disease multi-organ vasculitis should be considered as a new cause of mononeuropathy multiplex in addition to other vasculitides such as polyarteritis nodosa.13
In this patient with fulminant Kawasaki disease, severe central and peripheral neurological involvement was associated with bilateral vitritis, resulting in blindness. Surgery improved the visual function. Bilateral non-exudative conjunctivitis and mild to moderate anterior uveitis are the most common early ocular complications in Kawasaki disease. Reports of more severe ischaemic or inflammatory lesions are scarce.14 Our case highlights the need for early and repeated ophthalmological evaluations in unconscious patients with severe systemic vasculitis in an attempt to detect and promptly treat such eye involvement. Good neurological outcome was observed despite the initially high degree of clinical severity. In other severe brain inflammatory and vasculitis conditions, such as systemic lupus, therapeutic management should combine cyclophosphamide with glucocorticoids.15 Similarly, we added cyclophosphamide in combination with glucocorticoids to treat the severe, persistent cerebral vasculitis in our patient. We assume that this treatment might have contributed to the favourable outcome. We suggest that severe, life-threatening forms of Kawasaki disease should be recognized and treated early, with potent drugs, to achieve a more favourable outcome.
Cerebral vasculitis can be identified by specific T2* weighted brain MRI diffuse micro-haemorrhages and may require intensive immunosuppressive treatment. Therefore, early brain MRI, including T2* weighted sequencing, may be useful for the therapeutic management of patients with severe Kawasaki disease.




