Feasibility of colorectal endoscopic submucosal dissection (ESD) carried out by endoscopists with no or little experience in gastric ESD
Abstract
Background and Aim
Colorectal endoscopic submucosal dissection (ESD) is recommended to be carried out only by endoscopists with sufficient experience in gastric ESD. However, early gastric carcinoma is less common in Western countries than in Japan, and endoscopic maneuverability differs between the stomach and colorectum. We assessed the feasibility of colorectal ESD carried out by endoscopists with no or little experience in gastric ESD.
Methods
We analyzed en bloc resection, R0 resection and perforation rates in 180 consecutive colorectal ESD carried out by three endoscopists who had no or <5 cases of experience in gastric ESD. We also identified factors associated with R0 resection failure.
Results
Overall en bloc and R0 resection rates were 93.3% (168/180) and 82.2% (148/180), respectively. All 11 cases with perforation were treated endoscopically. Dividing 180 cases into three learning phases (early, middle, or late phases), the en bloc and R0 resection rates increased from 88.3% and 75.0% in the early phase to 98.3% and 88.3% in the late phase, respectively. Perforation rate also improved from 10.0% to 3.3%. Factors associated with R0 resection failure were location at junctions (odds ratio: 6.8, 95% CI: 1.9–27.5), preoperative factors reflecting fibrosis (5.8, 1.9–19.0), and late phase (0.2, 0.1–0.7).
Conclusion
Endoscopists without experience in gastric ESD carried out colorectal ESD safely. In the early and middle phases (≤40 cases), they should treat mainly rectal lesions but may also resect lesions in the colon avoiding flexures. Lesions located at junctions and those with preoperative factors reflecting fibrosis should be resected after completing 40 procedures.
Introduction
Endoscopic mucosal resection (EMR) is the standard endoscopic treatment for colorectal adenomas and early colorectal carcinomas, with which most lesions can be resected en bloc. However, if EMR is used, lesions ≥2 cm in diameter cannot be resected en bloc, resulting in non-curative resection and inaccurate histological diagnosis. Endoscopic submucosal dissection (ESD) is a clinically efficacious technique that is now widely used for the treatment of esophageal and gastric lesions, as it enables en bloc resection even of large lesions. The usefulness of colorectal ESD has also been reported, and it can be used for the en bloc resection not only of tumors ≥2 cm in diameter but also of lesions that are difficult to remove by EMR because of submucosal fibrosis.1-3 As these lesions had previously required surgery, ESD has great advantages in terms of reducing the hospitalization period and preserving postoperative cosmetic appearance. Despite its merits, however, colorectal ESD requires a higher level of endoscopic skill and involves a higher risk of perforation and other adverse events than EMR.4, 5 Therefore, prior to carrying out colorectal ESD, endoscopists are recommended to have experience in 20–50 gastric ESD, which have better endoscopic maneuverability and lower risk of perforation.6-9
However, for endoscopists who want to become proficient in colorectal ESD, there are several problems in starting their training with gastric ESD. First, early gastric carcinoma which should be resected by ESD is less common in Western countries than in Japan.10, 11 This means that it takes a longer time for Western endoscopists to obtain sufficient experience in gastric ESD, making it difficult to step up to colorectal ESD. In fact, the en bloc and R0 resection rates in Western countries were reported to be lower than in Japan; and many reports were limited to treatments of the rectum or distal colon.12-15 The aforementioned studies, particularly those from Japan, were retrospective analyses of the experience of endoscopists who have stepped up to colorectal ESD after gaining sufficient experience in gastric ESD.6-8 It may be difficult to apply these results directly to Western endoscopists. Second, endoscopic maneuverability varies greatly between the stomach and colorectum. Unlike the stomach, the colorectum has a thinner wall and its lumen is flexed and tortuous. These characteristics make it difficult to establish a uniform strategy for lesions of the same location. Therefore, even with some degree of experience in gastric ESD, further experience in colorectal ESD is still necessary to become proficient.
Although gastric and colorectal ESD share commonality regarding endoscopic techniques, colorectal ESD requires endoscopic maneuverability quite different to that of gastric ESD. We have therefore reported the clinical outcomes achieved by endoscopists who have started to carry out colorectal ESD with no or <5 gastric ESD.16, 17 Endoscopists who were proficient in colonoscopies and colorectal EMR, and were therefore well aware of the thin wall and endoscopic maneuverability of the colorectum, could safely start to carry out colorectal ESD even without experience in gastric ESD. However, these reports included only a small number of cases, and further analysis is required.
In the present study, we analyzed the performance of colorectal ESD carried out by endoscopists with no or little experience in gastric ESD, assessing its feasibility. More specifically, we evaluated the en bloc resection rate, R0 resection rate, dissection speed, and incidence of adverse events. This was a re-evaluation study involving more endoscopists and procedures than our previous reports. We also investigated factors associated with R0 resection failure. Based on these results, we attempted to suggest an appropriate program for starting colorectal ESD safely without experience in gastric ESD.
Methods
In the present study, three endoscopists with no or little experience in gastric ESD were included. A total of 180 colorectal ESD cases, composed of each endoscopist's first 60 consecutive cases, were retrospectively reviewed. Details of the indication criteria, ESD procedure, pre-ESD requirements of the endoscopist, study design, and statistics are described below.
Indication criteria for colorectal ESD
Indication criteria for colorectal ESD are as follows. Lesions for which endoscopic en bloc resection is required: (i) lesions for which en bloc resection with snare EMR is difficult to apply, such as non-granular laterally spreading tumor (LST-NG), particularly LST-NG pseudo-depressed type (PD), lesions showing a VI-type pit pattern, carcinoma with shallow T1 (SM) invasion, large depressed-type tumors, and large protruded-type lesions suspected to be carcinoma; (ii) mucosal tumors with submucosal fibrosis; (iii) sporadic localized tumors in conditions of chronic inflammation such as ulcerative colitis; and (iv) local residual or recurrent early carcinomas after endoscopic resection.18 In general, (ii), (iii), and (iv) have a high rate of fibrosis in the submucosal layer and a high possibility of perforation, particularly when resected by ESD novices. In the present study, endoscopists carried out all cases of colorectal ESD after the first 20 cases avoiding (ii), (iii), and (iv).
Colorectal ESD procedure and histological analysis
With a water-jet system and a disposable attachment (Elastic touch F-030 or F-025; Top Co., Tokyo, Japan), ultraslim endoscopes were used: PCF-Q260JI (outer diameter: 10.5 mm; Olympus Optical Co., Tokyo, Japan) for lesions in the proximal colon (from the cecum to the descending colon) and GIF-Q260J (outer diameter: 9.8 mm; Olympus Optical Co., Tokyo, Japan) for lesions of the sigmoid colon or rectum. For successful mucosal incision and submucosal dissection, we injected a mixture of a solution of sodium hyaluronate (MucoUp; SEIKAGAKU Co., Tokyo, Japan) and a small volume of epinephrine and indigocarmine in the submucosal layer. A tip-type knife (Dual Knife; Olympus Optical Co., Tokyo, Japan) was mainly used for mucosal incision and submucosal dissection; however, a scissors-type knife (SB Knife Jr; Sumitomo Bakelite Co., Tokyo, Japan) was also used. The selection of knife was left to the judgment of the endoscopist. We used the ICC 200 high-frequency generator (ERBE, Tübingen, Germany). Bowel preparation, details of colorectal ESD procedure, and fixing of resected specimens were described in more detail in our previous report.16
After colorectal ESD procedure, the case in which the tumor was resected in one single piece was judged as endoscopic en bloc resection. If the tumor was resected en bloc endoscopically and the lateral and basal margins were free of tumor cells in the histological analysis, it was defined as R0 (complete) resection. Other cases were defined as R1 (incomplete) or Rx (not evaluable) resection. In cases with submucosal invasive adenocarcinomas accompanied by massive submucosal invasion of more than 1000 μm and/or lymphatic invasion and/or venous invasion, surgical resection with lymph node dissection was recommended because of a high risk of lymph node metastasis.
Pre-ESD requirements of endoscopists
Each endoscopist in the present study carried out more than 2000 colonoscopies and more than 300 colorectal EMR. In addition to the number of colonoscopies and colorectal EMR, we set the condition to carry out colorectal ESD as having sufficient knowledge of the ESD procedure, serving as an assistant to senior endoscopists in more than 20 ESD, and having experience in animal model training. During the pre-ESD period, two endoscopists carried out fewer than five gastric ESD.
With only verbal control, senior endoscopists with experience of more than 50 ESD supervised from the beginning of colorectal ESD to the end. However, in cases with perforation, senior endoscopists carried out consecutive ESD procedures. Therefore, the self-completion rate was almost equal to the rate without perforation.
Study design
Colorectal ESD were carried out by three endoscopists between July 2009 and May 2014. The first 60 consecutive ESD of each endoscopist were evaluated retrospectively. Written informed consent for the ESD procedure was obtained from all participants based on a protocol approved by the ethics committee of Tohoku University Hospital.
We included adenocarcinomas and adenomas more than 20 mm in diameter. The site of tumors was divided into four groups: the rectum, left colon (sigmoid colon and descending colon), right colon (transverse colon, ascending colon, and cecum), and junctions. Tumors ranging over the dentate line, sigmoid-descending (SD) junction, splenic flexure, hepatic flexure, or ileocecal valve were defined as tumors at junctions. Macroscopic type of tumors was classified as granular laterally spreading tumor (LST-G), LST-NG, protruding, and depressed.16 We also assessed factors reflecting fibrosis in the submucosal layer as follows: lesions with a definite scar as a result of biopsy; lesions with three or more folds, sporadic localized lesions with ulcerative colitis, and local residual tumors after EMR.17 These factors were judged by other endoscopists independently using only preoperative endoscopic findings.
We evaluated the endoscopic en bloc resection rate, en bloc resection rate with tumor-free margins (R0 resection rate), and dissection speed. We also evaluated the rate of adverse events: perforation, postoperative hemorrhage, and others. Primary outcome was R0 resection rate. In order to assess the learning curve, we divided a total of 180 cases into three learning phases: the early phase (consisting of the first 60 cases), the middle phase (consisting of the middle 60 cases) and the late phase (consisting of the last 60 cases).
Next, we carried out multivariate analysis to identify factors predicting the treatment outcome (R0 resection failure) using clinical characteristics available before the ESD procedure. Possible factors included were as follows: disease site (rectum, left colon, right colon, or junctions), macroscopic type (LST-G, LST-NG, depressed, or protruding), factors reflecting fibrosis (present or absent), tumor size (≥40 mm or <40 mm), and learning phases (early, middle and late phases). Histological type (adenocarcinoma or adenoma) and existence of actual fibrosis in the submucosal layer were excluded.
Statistical analysis
Quantitative data are presented as the mean ± standard deviation (SD). All statistical analyses were done using JMP version 11 (SAS Institute Inc., Cary, NC, USA). Differences among groups were evaluated using the chi-squared test or Fisher's exact probability test, as appropriate. Among the clinical characteristics, factors influencing the main outcome were identified using a multiple logistic regression method. The level of significance was set at P < 0.05.
Results
Clinical characteristics
A total of 180 cases reviewed included 122 males (67.8%) and 58 females (32.2%). Overall mean age was 69.2 ± 11.2 years. Disease site was divided into the rectum, left colon, right colon, and junctions in 36 (20.0%), 32 (17.8%), 76 (42.2%), and 36 (20.0%) cases, respectively. Macroscopic type was divided into LST-G, LST-NG, depressed, and protruding in 87 (48.3%), 67 (37.2%), eight (4.5%), and 18 (10.0%) cases, respectively. Twenty four of 180 cases (13.3%) were judged to have factors reflecting fibrosis in the submucosal layer based on endoscopic findings before ESD (Table 1).
| N = 180 | |
|---|---|
| Gender | |
| Male | 122 (67.8%) |
| Female | 58 (32.2%) |
| Age | |
| Mean age, years (SD) | 69.2 (11.2) |
| Disease site | |
| Rectum | 36 (20.0%) |
| Left colon | 32 (17.8%) |
| Right colon | 76 (42.2%) |
| Junctiona | 36 (20.0%) |
| Macroscopic type | |
| LST-G | 87 (48.3%) |
| LST-NG | 67 (37.2%) |
| Depressed | 8 (4.5%) |
| Protruding | 18 (10.0%) |
| Factors reflecting fibrosis in the submucosal layerb | |
| Present | 24 (13.3%) |
| Absent | 156 (86.7%) |
- a Junction means tumors range over dentate line, sigmoid-descending junction, splenic flexure, hepatic flexure, or ileocecal valve.
- b Factors reflecting fibrosis in the submucosal layer are those thought to be accompanied by fibrosis in the submucosal layer: lesions with a definite scar as a result of biopsy, lesions with three or more folds, sporadic localized lesions with ulcerative colitis, and local residual tumors after endoscopic mucosal resection.
- LST-G, laterally spreading tumor granular type; LST-NG, laterally spreading tumor non-granular type; SD, standard deviation.
Colorectal ESD performance
Colorectal ESD was carried out under sufficient bowel preparation in all cases. Tumors were endoscopically resected en bloc in 168 cases; therefore, the endoscopic en bloc resection rate was 93.3% (168/180). Mean procedural time was 89.2 ± 63.7 min. Procedural time was ≥90 min in 75 cases (41.7%) and <90 min in the remaining 105 cases (58.3%). Mean tumor diameter was 35.6 ± 17.3 mm. Tumor diameter was ≥40 mm in 53 cases (29.4%) and <40 mm in the remaining 127 cases (70.6%) (Table 2).
| N = 180 | |
|---|---|
| Procedural time | |
| Mean procedural time, min (SD) | 89.2 (63.7) |
| (<90 min) | (105) |
| (≥90 min) | (75) |
| Tumor diameter | |
| Mean tumor diameter, mm (SD) | 35.6 (17.3) |
| (<40 mm) | (127) |
| (≥40 mm) | (53) |
| Histological type | |
| Adenocarcinoma | 90 (50.0%) |
| (Non-invasive intramucosal tumor) | (76) |
| (Submucosal invasive carcinoma) | (14) |
| Adenoma | 90 (50.0%) |
| Endoscopic en bloc resection | |
| En bloc resection | 168 (93.3%) |
| Partial resection | 12 (6.7%) |
| R0 resectiona | |
| R0 resection | 148 (82.2%) |
| R1 or Rx resection | 32 (17.8%) |
- a If the tumor was resected en bloc endoscopically and the lateral and basal margins were free of tumor cells, it was defined as R0 (complete) resection. Other cases were defined as R1 (incomplete) or Rx (not evaluable) resection.
- ESD, endoscopic submucosal dissection; SD, standard deviation.
Perforation and postoperative hemorrhage occurred in 11 (6.1%) and five (2.7%) cases, respectively. Eleven cases with perforation had one rectal lesion and 10 colonic lesions (left colon: 1, right colon: 5, and junction: 4), and were managed by endoscopic closure and conservative medical treatment with bowel rest and i.v. antibiotics. Five patients with delayed postoperative hemorrhage (within 0 to 14 days after the procedure) had two rectal lesions and three colonic lesions (right colon: 2, and junction: 1), and were treated by endoscopic hemostasis. One patient developed bowel obstruction 2 days after ESD, which was managed by bowel rest only. Rate of all ESD-induced adverse events was 8.9% (16/180). No patient underwent surgery as a result of adverse events.
Histological analysis
Histological analysis showed that 90 (50.0%) and 90 (50.0%) cases had adenocarcinomas and adenomas, respectively. Of the 90 cases with adenocarcinomas, 76 had intramucosal carcinomas and 14 had submucosal invasions. Four cases with submucosal invasive adenocarcinomas underwent surgical resection with lymph node dissection; it revealed one case having lymph node metastasis (Table 2).
Although the lateral margins were tumor cell-free in all slices, tumor was present in the first or end slice in 20 of the 168 cases resected en bloc endoscopically. We judged lateral margins of these cases to be positive. Therefore, the histological R0 resection rate was 82.2% (148/180) (Table 2).
Clinical learning curve
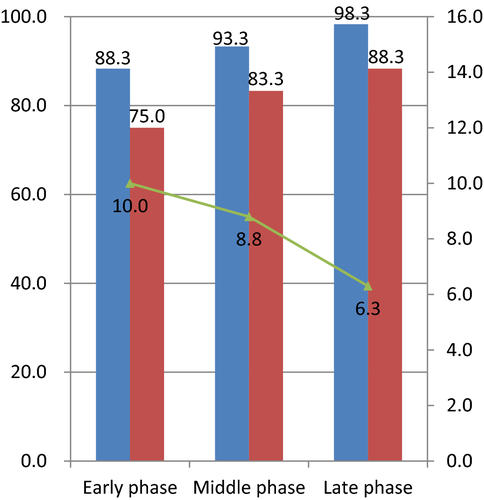
Dividing a total of 180 cases into three learning phases, the en bloc resection rate was 88.3% (53/60), 93.3% (56/60), and 98.3% (59/60) in the early, middle and late phases, respectively. Similarly, the R0 resection rate was 75.0% (45/60), 83.3% (50/60), and 88.3% (53/60), respectively (Fig. 1). Both rates improved from the early to the late phase. In terms of the speed of dissection, average speed was 10.0 ± 7.0, 8.8 ± 9.3, and 6.3 ± 3.6 min/cm2, respectively (Fig. 1). Dissection speed in the late phase was significantly faster than in the other two phases. In contrast, the rate of perforation decreased from 10.0% (6/60) in the early phase to 5.0% (3/60) in the middle phase and 3.3% (2/60) in the late phase.
 , en bloc resection rate (%);
, en bloc resection rate (%);  , R0 resection rate (%);
, R0 resection rate (%);  , average speed of dissection (min/cm2).
, average speed of dissection (min/cm2).Possible predictors of R0 resection failure
Next, we attempted to identify factors associated with R0 resection failure. On univariate analyses using clinical characteristics available prior to the ESD procedure (disease site, macroscopic type, factors reflecting fibrosis in the submucosal layer, tumor size, and learning phase), lesions at junctions and those with factors reflecting fibrosis had a significantly lower R0 resection rate. There was no significant difference in the macroscopic type, tumor size, or learning phase.
Multivariate analysis revealed that disease site at junctions, preoperative factors reflecting fibrosis, and late phase of the learning curve were significantly associated with R0 resection failure, with adjusted odds ratios of 6.8 (95% CI: 1.9–27.5), 5.8 (1.9–19.0), and 0.2 (0.1–0.7), respectively. Other factors showed no significant correlation with the R0 resection failure (Table 3).
| R0 resection failure | P-valuea | Multivariateb | ||||
|---|---|---|---|---|---|---|
| Odds ratio | P-value | 95% CI | ||||
| Disease site | ||||||
| Rectum | 6/36 (16.7%) | <0.01 | Reference | |||
| Left colon | 3/32 (9.4%) | 0.8 | 0.83 | 0.1 | 4.2 | |
| Right colon | 8/76 (10.5%) | 0.7 | 0.61 | 0.2 | 2.6 | |
| Junction | 15/36 (41.7%) | 6.8 | < 0.01 | 1.9 | 27.5 | |
| Macroscopic type | ||||||
| LST-G | 18/87 (20.7%) | 0.72 | Reference | |||
| LST-NG | 11/67 (16.4%) | 0.5 | 0.26 | 0.2 | 1.6 | |
| Depressed | 1/8 (12.5%) | 0.8 | 0.84 | 0.1 | 6.2 | |
| Protruding | 2/18 (11.1%) | 0.5 | 0.44 | 0.1 | 2.5 | |
| Factors reflecting fibrosis in the submucosal layer | ||||||
| Present | 22/156 (14.1%) | <0.01 | Reference | |||
| Absent | 10/24 (41.7%) | 5.8 | <0.01 | 1.9 | 19.0 | |
| Tumor size | ||||||
| <40 mm | 20/127 (15.7%) | 0.29 | Reference | |||
| ≥40 mm | 12/53 (22.6%) | 1.0 | 0.97 | 0.4 | 2.7 | |
| Learning phasec | ||||||
| Early phase | 15/60 (25.0%) | 0.16 | Reference | |||
| Middle phase | 10/60 (16.7%) | 0.3 | 0.048 | 0.1 | 0.99 | |
| Late phase | 7/60 (11.7%) | 0.2 | <0.01 | 0.1 | 0.7 | |
- a Chi-squared test or Fisher's exact probability test was used.
- b Multiple logistic regression method was used.
- c A total of 180 cases were divided into three learning phases: the early phase (consisting of the first 60 cases), the middle phase (consisting of the middle 60 cases) and the late phase (consisting of the last 60 cases).
- CI, confidence interval; LST-G, laterally spreading tumor granular type; LST-NG, laterally spreading tumor non-granular type.
Discussion
Endoscopic mucosal resection is the standard endoscopic treatment for colorectal tumors in both Western countries and Japan. However, in tumors larger than 20 mm in size and those that are difficult to resect using EMR as a result of fibrosis, colorectal ESD is useful. However, colorectal ESD requires a higher level of endoscopic skill, which is a barrier to the spread of colorectal ESD. Endoscopists are recommended to start their training with gastric ESD. In spite of these recommendations, we have reported that endoscopists with little experience in gastric ESD are capable of carrying out colorectal ESD.16, 17 In the present study, we aimed to re-evaluate this finding with more endoscopists and cases, and to suggest an appropriate program for becoming safely proficient in colorectal ESD. The overall en bloc resection rate was 93.3% and the R0 resection rate was 82.2%, which are not inferior to those reported by endoscopists with substantial experience in gastric ESD before they began to carry out colorectal ESD.6-8 As for the learning curve, the en bloc resection rate improved from 88.3% in the early phase (first 20 cases treated by each endoscopist) to 98.3% in the late phase (41st to 60th cases). The R0 resection rate similarly improved significantly from 75.0% to 88.3% over the same period. In addition, we found several factors associated with R0 resection failure, which should be avoided during the first 40 cases.
As we have mentioned, early gastric carcinoma is less common in Western countries than in Japan.10, 11 This means that even if Western endoscopists attempt to learn how to carry out colorectal ESD, it takes a longer time to obtain sufficient experience in gastric ESD, making it difficult to step up to colorectal ESD. In addition, as endoscopic maneuverability greatly varies between the stomach and the colorectum, further experience in colorectal ESD is required even after some degree of experience in gastric ESD has been obtained. In fact, the aforementioned studies found that experts or endoscopists with sufficient experience in gastric ESD required experience in 30–100 colorectal ESD to become proficient in it.6-8, 19 In the present study, even with no or little experience in gastric ESD, endoscopists became proficient in colorectal ESD quickly and safely. Experience in a few cases of gastric ESD was sufficient to understand techniques generally used in ESD; the use of a training model based on resected pig stomachs may be a possible alternative. Yang et al. also reported the feasibility of colorectal ESD carried out by an endoscopist without experience in gastric ESD.20 However, different from our study, more than 100 colorectal ESD were needed to become proficient. As Ohata et al. have emphasized,21 supervision by experts might contribute to the present better results.
We identified the following three factors associated with R0 resection failure: disease site at junctions, preoperative factors reflecting fibrosis, and late phase of the learning curve. The colorectum has numerous folds and flexures; therefore, the devices often face the muscle layer perpendicularly, making dissection very difficult and leading to a high possibility of perforation. Several reports included this endoscopic maneuverability in the analysis of ESD failure.22-24 However, it is difficult to assess endoscopic maneuverability objectively. We therefore put emphasis on the disease site, and divided it into the following four groups: the rectum, left colon, right colon, and junctions. Usually, endoscopic maneuverability becomes worse as the endoscope is inserted more deeply. ESD in the colon has been reported to be more difficult than in the rectum; and in the right colon (transverse colon, ascending colon, and cecum) than in the left colon (sigmoid and descending colon).22, 25 In terms of the location at junctions, we included not only major flexures of the colon (consisting of SD junction, splenic flexure, and hepatic flexure) but also the anal canal and ileocecal valve. Endoscopic maneuverability in these sites has been described to be poor owing to the winding and narrowing intestinal tract.22, 26 Multivariate analysis revealed that location at junctions was significantly correlated with R0 resection failure. These lesions should be attempted after a certain amount of experience has been acquired.
Factors that indicate fibrosis on the basis of preoperative findings significantly influence R0 resection failure. Many studies on colorectal ESD have described that fibrosis of the submucosal layer increased the degree of difficulty of resection.22, 23, 25, 27 However, submucosal fibrosis should be evaluated histologically after ESD, and thus this factor should not be used to predict clinical difficulty. In a case which requires a long procedural time owing to flexures or other factors, local injection into the submucosal layer becomes difficult, and low elevation of the tumor might be mistaken as fibrosis. Only a few retrospective studies have investigated the presence of submucosal fibrosis histologically.28 We therefore used factors reflecting fibrosis based on findings available before the ESD procedure.
The endoscopists who participated in the present study had undergone training using resected pig stomachs to get used to basic ESD procedures. Resected pig stomachs have become widely used for gastric ESD training. However, it is inadequate as training for colorectal ESD. A training model using resected pig rectums has also been reported to be useful29; however, it is also inadequate as training for the human colon containing many folds and flexures. Ohata et al. described the usefulness of an ex vivo porcine proximal colon model, in which the rectum suitable for training is upended so that it is placed on the proximal colon side.21 This model contributed to reduced time for becoming proficient in colorectal ESD even without experience in gastric ESD. Needless to say, unlike living bodies, this model cannot provide experience of respiratory movement or hemorrhage.
In our present study, endoscopists without experience in gastric ESD carried out colorectal ESD safely. Taking our results in conjunction with those of other studies, the recommended strategy for endoscopists without experience in gastric ESD is to treat mainly rectal lesions during the early and middle phases of the learning phase (≤40 cases). However, because of the narrow lumen and rich vessels in the submucosal layer, rectal lesions around the anal canal should be avoided. In contrast, colonic lesions need not be avoided during the early and middle phases, except for lesions at flexures, which were classified as junctions in the present study. The final step is to attempt resection of lesions at flexures, or those with factors reflecting fibrosis based on preoperative findings. As these lesions are most difficult, ESD should only be attempted after around 40 procedures have been completed. Obtaining experience in gastric ESD is the best option if it is possible. Effective training using animal models also shortens the time to acquire proficiency in colorectal ESD.
Conflicts of Interest
Authors declare no conflicts of interest for this article.




