Old-growth forests buffer climate-sensitive bird populations from warming
Abstract
Aim
Habitat loss and climate change constitute two of the greatest threats to biodiversity worldwide, and theory predicts that these factors may act synergistically to affect population trajectories. Recent evidence indicates that structurally complex old-growth forest can be cooler than other forest types during spring and summer months, thereby offering potential to buffer populations from negative effects of warming. Old growth may also have higher food and nest-site availability for certain species, which could have disproportionate fitness benefits as species approach their thermal limits.
Location
Pacific Northwestern United States.
Methods
We predicted that negative effects of climate change on 30-year population trends of old-growth-associated birds should be dampened in landscapes with high proportions of old-growth forest. We modelled population trends from Breeding Bird Survey data for 13 species as a function of temperature change and proportion old-growth forest.
Results
We found a significant negative effect of summer warming on only two species. However, in both of these species, this relationship between warming and population decline was not only reduced but reversed, in old-growth-dominated landscapes. Across all 13 species, evidence for a buffering effect of old-growth forest increased with the degree to which species were negatively influenced by summer warming.
Main conclusions
These findings suggest that old-growth forests may buffer the negative effects of climate change for those species that are most sensitive to temperature increases. Our study highlights a mechanism whereby management strategies to curb degradation and loss of old-growth forests—in addition to protecting habitat—could enhance biodiversity persistence in the face of climate warming.
1 INTRODUCTION
Projections of biodiversity in a changing climate suggest widespread declines and distributional shifts as the rate of warming overtakes species’ capacity to adapt (Huey & Tewksbury, 2009; Pecl et al., 2017; Thomas et al., 2004). Empirical data support the general observation that distributions and abundance of many species are not keeping pace with climate change (Chen, Hill, Ohlemuller, Roy, & Thomas, 2011; Van Mantgem et al., 2009). However, climate responses are highly variable across species and systems; empirical validations of climate–biodiversity projections reveal important gaps between predicted and observed population changes (Gutiérrez-Illán et al., 2014; Tingley, Koo, Moritz, Rush, & Beissinger, 2012) that are not necessarily well explained by variation in life history traits (Wogan, 2016). One possible reason for this mismatch is that the spatial resolution of climate data rarely matches the scales of habitat use experienced by organisms (Frey, Hadley, Johnson, et al., 2016; Hannah et al., 2014); most projections neglect to consider the local- and landscape-scale vegetation conditions experienced by species (Sirami et al., 2017). Indeed, most climate data are collected at spatial scales 103 times greater than focal species territories (Potter, Arthur Woods, & Pincebourde, 2013). However, organisms may be able to buffer themselves from stressful environmental conditions by selecting particular vegetation conditions (Suggitt et al., 2011).
A variety of mechanisms could contribute to such vegetation-mediated climate buffering effects. First, individuals may be able to inhabit microsites that are cooler than the surrounding landscape. The potential for this “microrefugia” effect seems particularly high for forest-associated species, which constitute the majority of terrestrial biodiversity (Mora, Tittensor, Adl, Simpson, & Worm, 2011). It is well known that thermal conditions beneath forest canopies are typically less extreme (cooler in hot climates and warmer in cold climates) than those in open habitats, where weather stations are located (Ewers & Banks-Leite, 2013; Mora et al., 2011). Furthermore, recent work has shown that even subtle changes in forest structure can have substantial influences on undercanopy conditions; recent studies have found that structures associated with old-growth forest (e.g., high biomass, vertical canopy layers) result in cooler spring and summer temperatures in relation to mature plantations with simplified structure (Frey, Hadley, Johnson, et al., 2016; Suggitt et al., 2011). Second, certain vegetation types—particularly old forest with complex structure—may have higher food and nest-site availability (Braunisch et al., 2014; Sillett, Holmes, & Sherry, 2000) which could have disproportionate fitness benefits as species approach their thermal limits (Rodenhouse et al., 2008). Further, if animals follow an ideal free distribution (Fretwell & Lucas, 1970), vegetation types of higher habitat quality should also have greater population sizes, which would inherently confer greater resistance to environmental perturbations (Gill et al., 2001). Given these potential differences in population-level effects of climate across forest types, there is potential for forest management to influence the effects of warming at finer spatial scales and enhance the capacity of forests to buffer species from warming.
Although recent simulation studies have projected the interacting effects of land cover and climate on plant and animal populations (Maggini et al., 2014; Travis, 2003), there are still very few empirical tests of the degree to which such interactions occur in nature (Sirami et al., 2017). This research gap is due to the fact that vegetation data must be at sufficiently fine resolutions to match the spatial scales experienced by species (Wiens, 1989) but such data are rarely available at the broad spatial scales associated with species’ ranges and climate data. Second, climatic conditions and land cover tend to be highly correlated at broad spatial scales; climate is well known to influence vegetation (Staver, Archibald, & Levin, 2011), which makes it challenging to attribute proximate cause of species distributions to either climate change or land cover (Pearson & Dawson, 2003). It is only when climate and land cover vary independently that the opportunity exists to identify interactive or additive effects; space-for-time studies that sample climate and land cover within a single time period (Sohl, 2014), and then infer the effects of climate and land use change, assume that stationarity exists in animal–environment relationships—an assumption that does not necessarily hold (Bonthoux, Barnagaud, Goulard, & Balent, 2013; Gutiérrez-Illán et al., 2014).
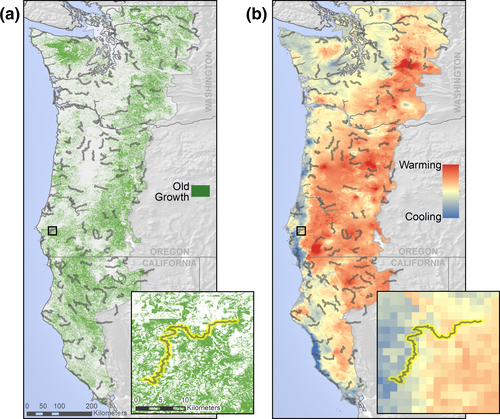
In this study, we tested the a priori hypothesis that landscapes with old-growth forest can buffer bird populations against potential negative effects of warming. We selected old growth as a focal forest type because recent findings indicate that it tends to have cooler microclimates during the avian breeding season (Frey, Hadley, Johnson, et al., 2016). This forest type is also of high conservation significance globally (Betts et al., 2017; Lindenmayer & Laurance, 2017; Morales-Hidalgo, Oswalt, & Somanathan, 2015) and in the Pacific Northwestern USA (Spies et al., 2007). We relied upon 30-year population trends of old-growth forest-associated birds in 138 landscapes distributed across the Pacific Northwestern United States (Figure 1); globally, this region is one of the few temperate forests with substantial remaining old growth (>20% of forest area; Davis et al., 2015; Thomas, Franklin, Gordon, & Johnson, 2006) and high-resolution data on its distribution (Ohmann & Gregory, 2002). We tested the hypothesis that old growth has buffered bird populations from warming by modelling bird population trends as a function of the interaction between temperature change and old growth; if old-growth forest provides refugia in the face of a warming climate, negative effects of route-level warming should be dampened in the presence of high amounts of old growth.
2 METHODS
Bird population data were collected as part of the USGS Breeding Bird Survey (BBS, www.pwrc.usgs.gov/bbs; Sauer, Link, Fallon, Pardieck, & Ziolkowski, 2013), which has been used widely in studies of bird distributions (Gutiérrez-Illán et al., 2014; Robbins, Bystrak, & Geissler, 1986). The BBS survey system consists of 39.4 km linear routes that are located along secondary roads throughout the coterminous United States and Canada. BBS data have been collected every May or June since 1966 by trained surveyors who recorded every species observed during 3-min counts at 50 point locations spaced at 0.8-km intervals along the route (Sauer & Link, 2011). We used the population trends at the scale of individual BBS routes calculated using hierarchical models (estimating equations; Sauer & Link, 2011). Estimating equations allow year and observer effects to be modelled as random effects. Duration of population trend data varied across routes (mean start year 1982), so to stabilize estimates, we did not include routes sampled <7 years. We defined old-growth-associated species as those listed in appendix 5-D of Thomas (1993). From this list, we selected species with ≥10% of their population in the Northern Pacific Rain forest region (Partners in Flight Committee, 2015). Finally, we refined the list to include only those with adequate data, defined as those in the highest category of the BBS “Regional Credibility Measure.” This resulted in a list of 13 target species for which route-level population trends were derived (Tables 1 and S1).
| Species | Month | Temperature change 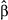 (95% CI) (95% CI) |
p | Old growth 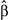 (95% CI) (95% CI) |
p | Old growth × Temperature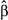 (95% CI) (95% CI) |
p | FDR p |
|---|---|---|---|---|---|---|---|---|
| Brown Creeper | July | 0.82 (−0.10, 1.74) | .083 | −0.04 (−0.98, 0.91) | .94 | −0.23 (−1.15, 0.69) | .62 | .73 |
| Chestnut-backed Chickadee | June | −0.65 (−1.41, 0.11) | .10 | 1.05 (0.29, 1.82) | .01 | 0.26 (−0.52, 1.04) | .52 | .73 |
| Golden-crowned Kinglet | July | −0.43 (−1.34, 0.47) | .35 | 0.5 (−0.55, 1.54) | .35 | −0.14 (−0.93, 0.65) | .74 | .73 |
| Hammond's Flycatcher | June | 1.59 (0.13, 3.06) | .04 | −0.45 (−1.65, 0.75) | .47 | −0.65 (−1.81, 0.51) | .28 | .67 |
| Hermit Thrush | June | 0.21 (−1.03, 1.45) | .74 | 0.76 (−0.12, 1.64) | .09 | 1.09 (−0.41, 2.58) | .16 | .48 |
| Hermit Warbler | July | −1.86 (−2.99, −0.72) | .002 | 0.86 (0.08, 1.63) | .03 | 1.3 (0.48, 2.12) | .003 | .03 |
| Pacific Wren | July | −0.24 (−0.83, 0.35) | .43 | 0.03 (−0.75, 0.81) | .94 | −0.09 (−0.68, 0.49) | .76 | .73 |
| Pacific-slope Flycatcher | June | −0.43 (−1.79, 0.94) | .54 | 0.34 (−1.08, 1.76) | .64 | −0.19 (−1.57, 1.2) | .79 | .73 |
| Red-breasted Sapsucker | June | 0.94 (−0.52, 2.4) | .21 | −1.51 (−2.78, −0.24) | .02 | 0.43 (−0.91, 1.77) | .53 | .73 |
| Townsend's Warbler | June | 3.53 (1.55, 5.52) | <.001 | 1.06 (−1.05, 3.17) | .33 | −1.78 (−3.45, −0.12) | .04 | .16 |
| Varied Thrush | July | −0.87 (−1.89, 0.16) | .10 | −0.62 (−1.79, 0.56) | .31 | −0.43 (−1.45, 0.59) | .41 | .73 |
| Vaux's Swift | July | 1.46 (0.02, 2.9) | .05 | −1.54 (−3.12, 0.05) | .06 | 0.45 (−1.01, 1.9) | .55 | .73 |
| Wilson's Warbler | June | −0.73 (−1.18, −0.27) | .002 | 0.01 (−0.6, 0.62) | .98 | 0.62 (0.18, 1.06) | .007 | .04 |
We quantified the amount of old-growth forest within 400 m of each BBS route (Figure 1a). Spatial data on old growth were quantified using the gradient nearest neighbour method (GNN; Ohmann & Gregory, 2002). The GNN method integrates vegetation measurements from regional grids of field plots (Forest Inventory Analysis [FIA] plots), mapped environmental data and Landsat Thematic Mapper (TM) imagery to predict vegetation structure and composition at a 30-m pixel resolution every year since 1984. At broader spatial scales, vegetation composition predictions from this method closely match those measured in ground-based forest inventory plots (Ohmann & Gregory, 2002) and have been used previously to predict bird distributions (Betts et al., 2010). We defined old-growth forest using the Old Growth Structure Index (after Spies et al., 2007). Old growth is quantified as the average of four separate indices representing structural features: number of large trees (>100 cm diameter at breast height [dbh]), large snags (>50 cm dbh and >15 m tall), the volume of large snags and tree size diversity. As policy in the northwestern USA has precluded cutting old-growth forest on Federal land, there has been little human disturbance in this forest since the early 1990s (mean change on BBS routes from 1984 = −0.3% [3.3% SD]). Our final metric of old-growth forest was therefore the average amount over the period of BBS sampling.
We obtained historical climate data generated by the Parameter Regression of Independent Slope Model (PRISM; Oregon Climate Service, Corvallis, Oregon, USA) for the continental United States (Daly, Gibson, Taylor, Johnson, & Pasteris, 2002; Figure 1b). This data set was created using point meteorological station data, digital elevation models and other spatial data sets to generate interpolated gridded estimates of monthly climatic temperature at a scale of 1 km2 (Daly et al., 2000). We selected only two climate variables—June and July maximum temperature—that we expected to influence breeding bird populations during the peak breeding period (Gutiérrez-Illán et al., 2014) and are moderated by old-growth structure (Frey, Hadley, & Betts, 2016; Frey, Hadley, Johnson, et al., 2016b). We calculated the 5-year average maximum temperatures from 1983 to 1987 (T1), and 2005 to 2010 (T2) at the 1-km scale and then quantified route-level temperature change as T2–T1 (after Gutiérrez-Illán et al., 2014). Summarizing climate data using such temporal windows has been used to successfully model changes in bird distributions of the northwestern USA (Gutiérrez-Illán et al., 2014) and has the advantage of smoothing short-term interannual fluctuations in climate. Importantly, at the scale of BBS routes, temperature changes in neither June (r = −.08) nor July (r = .25) were strongly correlated with old-growth forest amount, which enables a strong test of synergistic effects of climate change and land cover.
We used generalized linear models with a Gaussian distribution to model population trends of each species as a function of long-term change in maximum June or July temperatures, and the statistical interaction between these climate variables and old-growth forest amount. If old growth buffers populations against the effects of warming, we expected to see a positive interaction between temperature and old growth (i.e., population trends are more likely to be positive under warming climates when old growth is prevalent).
As the precision of population trends varied across routes, we down-weighted regressions using 1/variance2, conservatively reducing the influence of routes with high variance. We took the following steps in model building. First, for each species, we tested both climate variables (June or July) individually and retained the one that most reduced Akaike's information criterion (AIC). When the difference in AIC between the two models was less than 2, we selected the model with the most precise estimate of the effect of the temperature variable (i.e., the smallest standard error of the slope coefficient). Second, we tested for an interaction between this climate variable and the average amount of old growth in the surrounding landscape. The presence of a statistical interaction between temperature change and old growth constitutes support for our central hypothesis; if old-growth forest provides refugia in the face of a warming climate, negative effects of route-level warming should be dampened with high amounts of old growth (a positive interaction between temperature change and old growth). In contrast, support for a simple additive model of the effect of old growth and temperature on population trends (i.e., “population trend ~ old growth + temperature change”) would indicate that the effect of temperature on populations is not dependent upon the amount of old growth in landscapes.
Third, for species showing a statistically significant interaction term, we tested the alternative hypothesis that a temperature change × old-growth interaction could be driven by confounding effects of total forest cover, elevation, latitude or topographic complexity. Warming effects could be influenced by the baseline climate of individual routes; routes that are cool at the beginning of the survey (due to high elevation or latitude) should have dampened effects of warming (environmental conditions would be less likely to shift outside of species’ thermal niches). Because of the logistical constraints of timber harvest, old-growth forest might be more likely to remain in locations that are topographically complex. Such locations could be thermally buffered due to effects of microtopography (Dobrowski, 2011) rather than the effects of old growth per se. Further, the basic shading effects provided by forest cover (rather than old growth) could result in thermal buffering. Therefore, we tested the interactions “elevation × temperature change.” “latitude × temperature change,” “total forest cover × temperature change” and “topographic complexity × temperature change” along with “old growth × temperature change” in a global model and used AICc model averaging (Burnham & Anderson, 2003; Grueber, Nakagawa, Laws, & Jamieson, 2011) to calculate average parameter estimates from the full model set containing each interaction term (Barton, 2013; see Tables S2 and S3).
Finally, statistical tests were conducted across all 13 species, which risks inflating Type I error rate. Sequential Bonferroni-type multiple comparisons are often used to account for such error inflation but are highly conservative (Pike, 2011). Therefore, we used a false discovery rate (FDR) procedure (the “graphically sharpened method” [Pike, 2011]) which does not suffer from the same loss of power but corrects for multiple comparisons across species. Climate change and land cover variables used in analysis were not collinear (r < .2), and all variables were standardized (z-transformed) prior to analysis to allow comparison of effect sizes.
3 RESULTS
We detected effects of summer warming on population trends in only four of the 13 mature forest-associated birds examined. Two species (Hammond's flycatcher, Townsend's warbler) were positively associated with warming, and two species (Wilson's warbler, hermit warbler) were negatively influenced (Table 1). At the landscape scale (BBS route), for each 1°C increase in maximum June temperature, Wilson's warblers declined −1.50%/year (95% CI: −2.38 to −0.63). On average, hermit warblers declined −2.72%/year (−4.34 to −1.10) for each 1°C increase in maximum July temperature.
Both species showing negative effects of warming had reduced population declines in landscapes with high proportions of old-growth forest; in both cases, we detected a statistical interaction between warming and old-growth amount (Table 1, Figure 2). In landscapes with warming and high proportions of old growth, populations were more stable (i.e., showed a reduced tendency to decline). Indeed, where old-growth was most prevalent the relationship between population trends and warming tended to reverse (Figure 2). Across all species considered, stronger negative effects of summer warming on populations were associated with apparent buffering effects (Figure 3). Interestingly, for some species, positive influences of warming appeared to be dampened by high amounts of old growth in the landscape (e.g., Townsend's warbler), although these results were not statistically significant after correcting for multiple comparisons (Table 1, Figure 3).
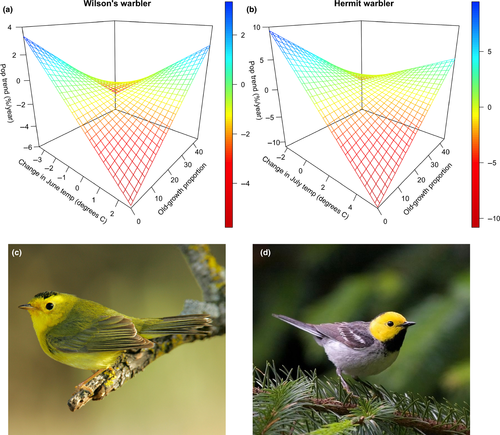
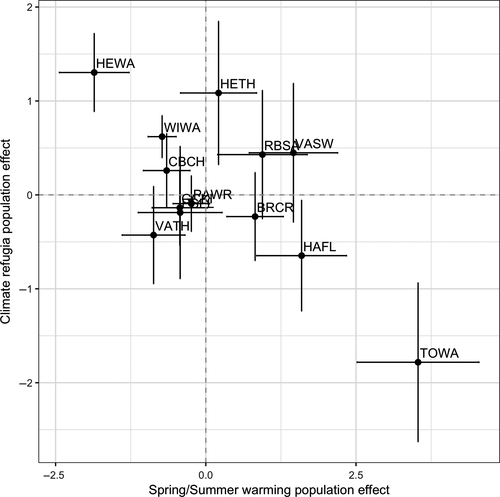
 ] ± SE) and the degree to which old-growth forest buffers populations from warming (old growth x temperature change interaction;
] ± SE) and the degree to which old-growth forest buffers populations from warming (old growth x temperature change interaction; 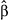 ± SE). Species with the strongest negative effects of warming on populations (upper left quadrant) tended to show the greatest buffering effects of old growth. Species that were neutral or positive in their response to spring or summer warming were not significantly influenced by the effect of old growth in the landscape. See Table S1 for species code definitions
± SE). Species with the strongest negative effects of warming on populations (upper left quadrant) tended to show the greatest buffering effects of old growth. Species that were neutral or positive in their response to spring or summer warming were not significantly influenced by the effect of old growth in the landscape. See Table S1 for species code definitionsOld-growth buffering effects remained for Wilson's and hermit warbler after statistically controlling for the influence of latitude, elevation, forest amount and topographic complexity (Tables S2 and S3). Elevation negatively interacted with summer maximum temperatures to influence Wilson's warbler population trends with higher-elevation populations more likely to be in decline as a result of warming (Table S2). For hermit warbler, the interactive effect of elevation with temperature change was the opposite; lower elevation populations were more likely to decline with warming. We detected no substantial spatial autocorrelation in model residuals for either species (Moran's I < 0.2, for all spatial lags; Figure S1).
4 DISCUSSION
Our most striking findings were that: (1) population declines of both species for which significant negative effects of summer warming were detected were reduced and even reversed in landscapes with high proportions of old-growth forest, and (2) there was a positive correlation between the strength of climate warming effects on bird population trends and the degree to which old-growth forest buffers populations from warming (Figure 3). Taken together, we interpret these findings as providing strong support to our hypothesis that old-growth forest has the capacity to buffer populations of the most climate-sensitive species against the negative effects of warming.
At face value, our finding of a significant negative effect of climate warming on only two of the 13 species we investigated might seem to indicate that any climate buffering effect is idiosyncratic and limited to a few special cases. However, there are several reasons not to dismiss this result. First, the relationship between climate sensitivity and the strength of the buffering effect was consistent across all 13 species. The occurrence of old-growth forests tended to moderate population increases in species that responded positively to warming, as well as buffering declines in those that responded negatively. Although the majority of species did not show an old-growth buffering effect, we would not expect them to, given that contemporary warming has yet to negatively influence their populations. Second, our FDR corrections for multiple comparisons across species substantially limit the chance that results for these species are due to Type I error. Third, it is clear from other studies that the species most sensitive to climate change are often rare and poorly represented in the subset of species with adequate data. For example, a study of the 101 most common bird species in Sweden (those with sufficient data for certain analyses) found a temperature response in only 20%, but a later study including 247 species, including those with sparse data, found that >50% responded to changes in temperature (Tayleur et al., 2016). We would not expect all species to be equally affected by warming; instead, warming is likely to selectively deplete populations of specialist species associated with cooler conditions. These species are then expected to be replaced with generalist species associated with warmer conditions (Davey, Chamberlain, Newson, Noble, & Johnston, 2012; Tayleur et al., 2016).
Synergistic effects of climate and land cover change are hypothesized to result from the spatial structure of landscapes; if landscapes are characterized by low amounts of habitat cover and high fragmentation (i.e., reduced connectivity), species may be less able to adapt to increasing temperatures by shifting upslope or poleward (Hill et al., 2002; Opdam & Wascher, 2004) but see: Jarzyna, Porter, Maurer, Zuckerberg, and Finley (2015). Our analysis suggests two additional potential mechanisms for synergistic climate–land cover effects; first, particular vegetation types (in our case, old-growth forest) may provide microclimates that are uncoupled from regional thermal regimes (Frey, Hadley, Johnson, et al., 2016), thereby mitigating the effects of climate change on cold-associated species. However, it is important to emphasize that our study was necessarily correlative; experimentally varying climate and forest cover at landscape scales would be challenging, if not impossible. Unlike many previous studies, we relied on long-term climate and bird population data to make inference about climate change effects. Nevertheless, because old-growth forest did not change substantively over the course of our study, it was necessary to substitute space for time (Bonthoux et al., 2013). Thus, we must acknowledge that unmeasured variables that are correlated with the spatial distribution of old growth may constitute the true drivers of the patterns we observed (Lindenmayer et al., 1999). Also, bird populations may be responding to climate features that change concurrently with temperature; for instance, in the Pacific Northwest, summer temperatures are known to correlate negatively with precipitation (Nott, Desante, Siegel, & Pyle, 2002) which is an important driver of bird distributions (Gutiérrez-Illán et al., 2014).
Further, certain forest types, such as old growth, may simply constitute higher quality habitat (e.g., greater food availability, reduced predation); such characteristics may help to buffer populations as climate warms towards the boundaries of a species’ fundamental niche. For this scenario to be supported, one would expect that landscapes with greater amounts of high-quality habitat should contain more individuals (assuming these species follow an ideal free distribution; Fretwell & Lucas, 1970). This was in fact the case for hermit warbler; the amount of old growth in the landscape was strongly associated with average population size (GLM: 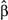 = 48.41 [95% CI: 33.08, 63.75], t = 6.19, p < .0001). However, Wilson's warbler abundance followed no such pattern (GLM:
= 48.41 [95% CI: 33.08, 63.75], t = 6.19, p < .0001). However, Wilson's warbler abundance followed no such pattern (GLM: 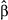 = −2.70 [95% CI: −16.64, 11.23], t = −0.38, p = .70). Thus, it seems more likely that the synergistic effects of climate and old growth on Wilson's warblers were driven by microclimate than the intrinsic habitat quality of old growth.
= −2.70 [95% CI: −16.64, 11.23], t = −0.38, p = .70). Thus, it seems more likely that the synergistic effects of climate and old growth on Wilson's warblers were driven by microclimate than the intrinsic habitat quality of old growth.
Previous work has indicated the potential for microtopography (i.e., fine scale differences in terrain) to provide microrefugia (Dobrowski, 2011). Indeed, such topographic refugia have been implicated in the mismatch between expected and observed distributions of species under changing historical climates (Stewart, Lister, Barnes, & Dalén, 2010). Although vegetation often tends to be dynamic over relatively short temporal scales, the longevity of many coniferous tree species in the Pacific Northwestern USA (>500 years for Douglas fir) and elsewhere (Lindenmayer, Laurance, & Franklin, 2012) suggests that the capacity of old-growth forest to buffer species from long-term climate change could be analogous to that of microtopography.
The synergistic effects of habitat and climate that we observed may be driven by direct physiological effects of microclimate; however, as endotherms, temperate forest birds seem to have quite high tolerances for thermal stress—as adults at least (Jiguet et al., 2006). Temperature effects in the Pacific Northwest are more likely to be mediated by changes in food availability (Both, Bouwhuis, Lessells, & Visser, 2006) or possibly competition with expanding warm-associated species (Gross & Price, 2000). Elsewhere, climate and land cover have synergistic effects on songbird productivity through their influence on the behaviour of predators and brood parasites (Gross & Price, 2000). Regardless of the mechanism, our findings suggest that landscapes with complex habitats may serve as refugia offer the potential for vagile animals to adapt to variable climate regimes through short- or long-term movements to those refugia (Frey, Hadley, & Betts, 2016).
Understanding the mechanisms for the patterns we observed will require knowledge about the scales at which organisms are influenced by climate changes (likely <1 ha; Briscoe et al., 2014). Future models of species response to climate change would benefit greatly from accounting for differences in thermal regimes across vegetation types; given the proliferation of undercanopy microsensors, high-resolution data on thermal regimes across major land cover types will soon be possible. Such data could also help to identify the particular structural or landscape features that are most beneficial to temperature-sensitive species as the climate warms.
For old-growth forest to buffer population trends, climatic conditions within regional micorefugia must continue to be uncoupled from the regional climate (Hannah et al., 2014); if temperature in thermally buffered locations rises outside species’ niches, one would expect populations to eventually decline at similar rates to those in the surrounding landscape. Also, species not associated with old growth are unlikely to benefit from thermal buffering. For instance, species associated with early successional stages are becoming a conservation concern in the Pacific Northwest, largely as a result of habitat loss (Betts et al., 2010); given that early successional forests may not have equivalent microclimate refugia, the synergistic negative effects on these species of land use and climate change will be unmoderated. Interestingly, Wilson's warbler—which occurs in early successional as well as old forest (Betts, Verschuyl, Giovanini, Stokely, & Kroll, 2013)—is declining significantly across the Pacific Northwest (BBS trend = 1.84%/year (CI: −2.5 to −1.3), whereas hermit warbler, a mature forest obligate is not declining significantly (BBS trend = −0.04%/year (CI: −0.85 to 0.82) (Sauer & Link, 2011)). It is possible that Wilson's warblers in early successional habitats are more exposed to temperature changes, and that old-growth forest-associated populations are not sufficiently large or productive to serve as sources for early successional forest sinks.
The degree to which old-growth structure continues to buffer species against rising temperatures will also depend upon the timing and severity of disturbance; climate change may already be increasing the prevalence of stand-replacing fires (Westerling, Hidalgo, Cayan, & Swetnam, 2006). Further, in many parts of the world, primary old-growth forests continue to decline rapidly due to timber harvesting (Hansen et al., 2013). Recent research indicates that species that benefit from formally protected forests appear to be at reduced risk of negative climate effects (Gauzere, Jiguet, & Devictor, 2016). Our findings suggest that one important reason for this may be through the buffering effect of structurally complex forests. Continued efforts worldwide towards protection of primary and old-growth forests (Butchart et al., 2010; Mackey et al., 2015) may provide additional benefits for mitigating effects of climate change on biodiversity.
ACKNOWLEDGEMENTS
The project described in this publication was supported by grants to M.G.B. from the US National Science Foundation (NSF) LTER at the HJ Andrews Experimental Forest (NSF DEB-0823380) and NSF-ARC-0941748. Additional funding was provided by the Department of the Interior Northwest Climate Science Center through Cooperative Agreement No. G11AC20255 from the United States Geological Survey. Its contents are solely the responsibility of the authors and do not necessarily represent the views of the Northwest Climate Science Center or the USGS. The authors are grateful to Julia Jones and Tom Spies who provided comments on earlier versions of his manuscript as well as Thomas Albright, David Lindenmayer and anonymous referees for their constructive reviews.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA ACCESSIBILITY
Data and code reported in this manuscript are available online at http://datadryad.org.
REFERENCES
BIOSKETCH
Matthew Betts is a professor of Landscape Ecology in the Department of Forest Ecosystems and Society at Oregon State University and the Director of the Forest Biodiversity Research Network (https://www.forestbiodiversity.org). His group studies drivers and consequences of species diversity and distributions in tropical and temperate systems.
Author contributions: M.G.B conceived the study; M.G.B., S.F., J.R. and Z.Y. contributed to the analysis; M.G.B. wrote the first draft and B.P., S.F., J.R. and Z.Y. contributed substantially to the final version of the manuscript.




