Hide-and-seek: Neurotropic squamous cell carcinoma of the periorbital region – a series of five cases and review of the literature
Summary
Squamous cell carcinoma is the second most common malignancy of the skin after basal cell carcinoma and mainly found in sun-exposed areas such as the face. This mostly locally destructive malignancy may show invasive growth and insidious mechanisms of dissemination such as perineural invasion. Periorbital squamous cell carcinoma is associated with perineural invasion in up to 14 % of cases. Specifically in this region, the proximity to cranial nerves and therefore the associated risk of progression to the central nervous system are associated with poor prognosis. The clinically concealed character of this entity often leads to a delay in diagnosis and consequently makes complete resection and reconstruction demanding. Careful clinical evaluation often hints at perineural invasion before obtaining histology.
Aside from presenting five challenging cases, this work analyzes risk factors, clinical as well as histological features, and treatment options for periorbital squamous cell carcinoma with perineural invasion.
Introduction
Squamous cell carcinoma (SCC) is the second most common malignancy of the skin after basal cell carcinoma. SCC of the skin commonly affects sun-exposed areas such as the face due to chronic UV exposure [1, 2]. Cutaneous SCC is often a locally destructive malignancy, but it can also show invasive behavior leading to more extended growth than clinically expected [3]. A special mechanism of SCC dissemination is perineural invasion (PNI). Perineural invasion itself was first described in 1985 [4] and has been defined as neoplastic invasion along planes of least resistance within connective tissue around a nerve or lymphatic vessels in the epineurium or perineurium [5]. Nowadays, the histological definition of Liebig et al. requires neoplastic cells “in close proximity to the nerve” with a circumferential involvement of at least one third (33 %), regardless of the nerve sheath layer in which the neoplastic cells are found [6]. The heterogeneous group of squamous cell cancers has a varying rate of PNI ranging from 5.2 % to 90 % [7], with advanced stages showing the highest incidence of PNI regardless of their location [8]. Histological PNI has to be assessed using immunohistochemistry [9] and is distinguished from clinical PNI, which is characterized by neurological symptoms such as numbness, motor impairment or neuralgia. Clinical PNI represents a late stage of neoplastic invasion [10], but might be the first clinical hint for neural involvement in some cases. Especially in the face, PNI affecting branches of cranial nerves can even result in neoplastic extension to the central nervous system as cranial nerves enter the bony skull via cranial foramina after only short courses through soft tissue. Invasive growth and the high recurrence rate of such lesions have prompted the suggestion for multimodal treatment [11]. Nevertheless, diagnostic and therapeutic guidelines are still lacking.
We here present a review of the current literature on SCC with PNI to create awareness for this special type of dissemination mechanism. We discuss risk factors, clinical and histological features and treatment options also supported by our experience with five recent cases of periorbital SCC with PNI.
Our experience and recent cases
Between 2012 and 2019, five (2.11 %) out of 237 cases of non-melanoma skin cancer located in the periorbital region were diagnosed with PNI at our department.
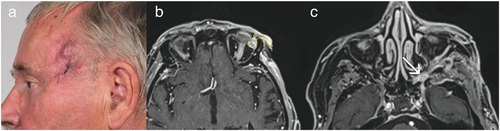
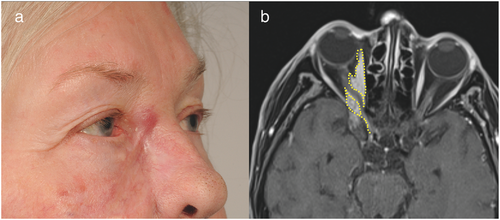
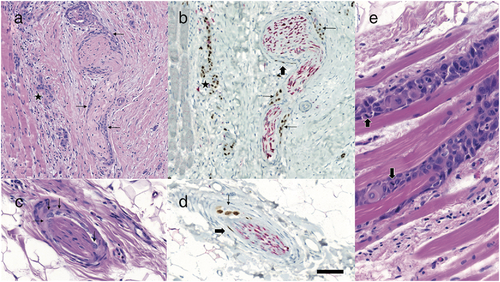
Four (Cases 1–4) of these five patients were referred to us after at least one attempt at incomplete excision of a SCC in the periorbital region (Figures 1, 2). The fifth patient (Case 5) was referred to us for scar correction after presumed prior complete excision of a SCC (Figure 3). However, histological examination of all five surgical specimens showed SCC with PNI confirmed by immunohistochemistry (Figures 4, 5).
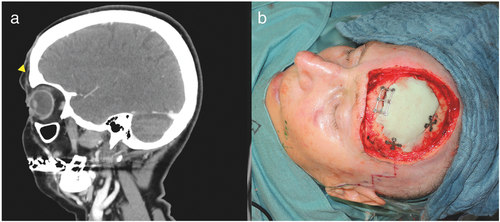
Mean patient age at the time of first diagnosis was 69 years (range 63–79 years). All patients were classified as Fitzpatrick skin type 2. Four cases had a history of malignant disease; two patients were taking immunosuppressants at diagnosis. Patient characteristics are presented in Table 1.
| Case # | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age at diagnosis | 69 | 66 | 63 | 79 | 71 |
| Gender | Female | Male | Female | Male | Female |
| History of malignant disease | Polycythemia rubra vera | Multiple trichoblastic / basal cell carcinomas | Breast cancer | None | Parotid gland cancer |
| Immunosuppression | Ruxolitinib | Mycophenolic acid, sirolimus (renal transplantation) | None | None | None |
| Number of resections (at our department) | 4 | 2 | 3 | 4 | 5 |
| Anatomical site of onset | Forehead | Medial cheek | Medial corner of the eye | Frontotemporal | Medial cheek |
| Involved nerve/s and muscle/s | Supraorbital nerve, supratrochlear nerve | Infraorbital nerve, mandibular nerve, oculomotor nerve buccal branch of facial nerve | Infraorbital nerve | Auriculotemporal nerve, superior rectus muscle/ oculomotor nerve | Optic nerve, infraorbital nerve |
| Clinical clues for PNI at first presentation | Paresthesia, numbness | Pain | Numbness | Paresthesia of temporal area | Painful mass mistaken for dacryocystitis |
| Involvement of bone | Yes | No | Yes | No | No |
| Orbital exenteration | Not performed | Not performed | Not performed | Not performed | Not performed |
| Reconstruction after excision | Gracilis free flap | Lateral orbital transposition (McGregor) flap, full-thickness skin graft | Forehead flap, lateral orbital transposition (McGregor) flap | Local flap, full-thickness skin graft | Forehead flap, lateral orbital transposition (McGregor) flap |
| Clinical symptoms of recurrence | Skin erosions near edges of free flap | Pain, feeling of pressure | – | Painful pressure behind the eye, oculomotor palsy | Pressure behind the eye, double images, partial loss of visual field |
| Irradiation therapy | No | Photons 60 Gy | No | Photons 60 Gy | Photons 60 Gy |
| Months to recurrence | 11 | 4 | – | 7 | 7 |
| Last follow-up (months after first diagnosis) | 24 | 14 | 17 | 15 | 47 |
| Current status | Disease-free after complete resection of recurrent SCC | Best supportive care; lost to follow up at 14 months | Disease-free | Stable disease after radiotherapy; no lymph node or systemic metastases | Stable disease after radiotherapy; no lymph node or systemic metastases |
Prior to surgical resection, clinical appearance of PNI was documented in four cases, presenting as paralysis or neuralgia of the involved nerves. In one case (Case 4), the patient showed painful dysesthesia associated with ocular motor palsy, indicating that nerves including the oculomotor nerve along the cavernous sinus were affected (Figure 2a). Neoplastic growth around the orbital rim and spreading along the superior rectus muscle was found, revealing direct orbital invasion beyond the orbital septum along with perineural manifestation (Figures 2b, c and 4e). In another case (Case 5), the patient did not present with paralysis, but showed painful swelling in the medial aspect of the upper cheek when referred to us for scar correction (Figure 3a). She was misdiagnosed with dacryocystitis and initially treated with antibiotics. Histology of the excised tissue showed local recurrence of SCC with PNI of the infraorbital nerve, explaining the initial symptoms.
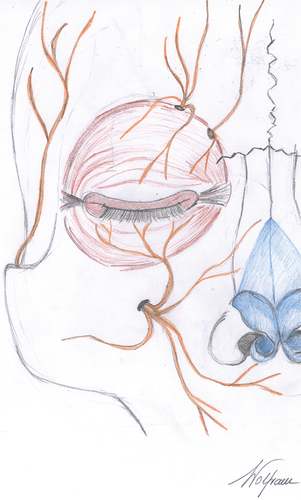
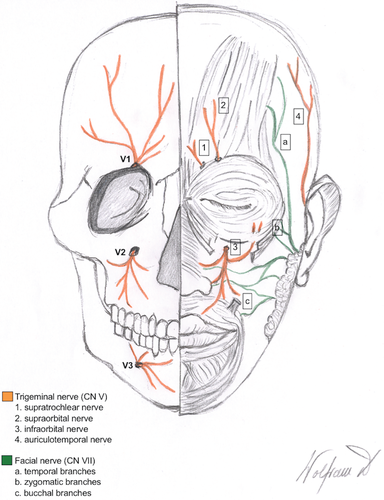
In most cases, branches of the trigeminal nerve were affected (n = 4), causing symptoms in the affected regions such as pain or numbness. The supraorbital nerve was involved once and the infraorbital nerve was involved three times, also affecting the mandibular nerve in one case. Invasion of the supratrochlear and the auriculotemporal nerve, being smaller branches of the trigeminal nerve, was also observed in two cases. Further cranial nerves involved were the optical nerve, the oculomotor nerve, and a buccal branch of the facial nerve. An anatomical depiction of the trigeminal and the facial nerve in the periorbital region is given in Figures 6 and 7.
All cases were routinely discussed by an interdisciplinary tumor board. Staging included preoperative ultrasound of regional lymph nodes to rule out disseminated malignancy, and computed tomography as well as magnetic resonance imaging to determine local neoplastic spread.
Lymph node metastases were not found in any patient in this series. Mean number of operations required before achieving complete resection was three (range 2–5). In two of the five cases resection required removal of bone segments. After achieving free resection margins, reconstruction was performed with local or distant flaps, in one case a free gracilis muscle flap was required. Despite former complete resection, recurrence occurred in four patients and mean time until recurrence was 7.25 months (range 4–11 months). One patient remained without local recurrence at a follow-up of 17 months. In total, photon irradiation radiotherapy was indicated in three patients. In the first one of these patients, indication for radiotherapy was given due to a lack of alternative adjuvant therapy as immunomodulatory therapy was not an option in an organ transplant recipient. This patient received best supportive care after radiotherapy and was lost to follow-up at 14 months. The other two of these patients showed stable disease following radiotherapy with no metastases detected at a follow-up of 15 and 47 months, respectively. The second patient received radiotherapy for a recurrent SCC with PNI that had already reached the central nervous system and, thus, was no longer surgically resectable. In the third patient, radiotherapy was indicated as histology implied that the neoplasm reached resection margins after already having performed resection of the infiltrated nerve down to the skull foramen. Radiotherapy was not performed in the remaining two patients. One of these patients showed complete resection after resection of bone segments. The other patient did not receive radiotherapy as adjuvant radiotherapy would have possibly resulted in major radiation-associated complications such as vision loss.
Perineural Invasion of squamous cell carcinoma in the periorbital region
About 10 % of cutaneous neoplasms in the face involve the periorbital region, the anatomical area surrounding the orbit (Figure 6) [12]. The majority of neoplasms in this region are basal cell carcinomas, also referred to as trichoblastic carcinomas, followed by SCC as the second most common malignant lesion, accounting for 9–14 % [12]. The anatomical peculiarities make this region therapeutically demanding when diagnosed with SCC showing PNI. Most nerves in this area have a short course through the soft tissue before entering the skull base via cranial foramina. The orbit can be invaded along branches of the trigeminal nerve (cranial nerve V) (Figure 7) by neoplasms spreading in a centripetal manner along the supratrochlear or the supraorbital nerve (branches of the frontal nerve/V-1), the infraorbital nerve (branch of the maxillary nerve/V-2) or the auriculotemporal nerve (branch of the mandibular nerve/V-3), eventually reaching the main trunk of the trigeminal nerve [13] as well as other cranial nerves such as the oculomotor nerve (cranial nerve III). Moreover, growth along the eye muscles has been described following invasion of the orbit in a perineural fashion eventually reaching the optic nerve (cranial nerve II) [13]. Terminal branches of the facial nerve (cranial nerve VII) (Figure 7) such as the temporal, zygomatic or buccal branches can also be affected by PNI in SCC of the periorbital region. Peculiarities of facial anatomy, foremost the periorbital region, should always be considered on clinical examination. Clinical symptoms, such as paresthesia, numbness or pain, may already be present and hint at PNI before obtaining histology.
Risk factors for perineural invasion
Risk factors for non-melanoma skin cancer have been well documented and include Fitzpatrick skin type 1 or 2, advanced age and chronic UV damage. Regarding SCC with PNI, immunosuppression and desmoplastic growth are known to be specific risk factors. Immunosuppressant medication additionally exaggerates the risk for developing SCC, which is 65-fold higher after solid organ transplantation [2, 14]. This risk increases with the duration of immunosuppression [15, 16]. While organ rejection is prevented by general suppression of immune surveillance, the body becomes susceptible for the development of neoplasms. Recent work suggested that immunosuppressive drugs trigger mutations in tumor suppressor genes, resulting in carcinogenesis [17]. Furthermore, PNI has been reported in 39 % of SCC in solid organ transplant recipients [18] compared to 2.5 – 14 % of SCC in non-transplant patients [19].
Another important risk factor for PNI is desmoplastic growth, also referred to as scirrhous or sclerodermiform growth [20, 21]. Histopathologically, desmoplasia was already described in 1997 [22]. It is associated with fibrosis by increased number of fibroblasts, with sclerosis by decreased number of fibroblasts and homogenization of collagen fibers with presence of neoplastic cells in a strand-like growth pattern, known as Indian filing. While recent literature considers desmoplasia to be a risk factor for PNI [23], in 2020 Haug et al. published the first study suggesting that PNI is exclusively associated with desmoplastic SCC, which remains to be proven by further research [24].
Diagnosis of perineural invasion
Clinical features
Symptoms of PNI are often misinterpreted and thus misdiagnosed. Emphasizing this fact, clinical symptoms mistaken for dacryocystitis masquerading as a metastatic hepatocellular carcinoma of the periorbital region [25] have been described. If PNI in the periorbital region is suspected, a detailed ophthalmologic evaluation might be indicated for differential diagnosis [13]. Infiltration of various nerves induces a wide variety of symptoms, such as upper lip numbness by invasion of the infraorbital nerve and weakness of distal facial nerve branches, which result in the so-called “numb cheek – limp lower lid” syndrome [26]. Clinical symptoms such as trigeminal neuralgia have been described on invasion of the trigeminal nerve, often leading to diagnostic delay [27]. Invasion of the optic nerve itself (Figure 3b) is rare, yet not uncommon in cases that are diagnosed late or even neglected by the patient. Invasion of the optic nerve stroma already represents neoplastic extension to the central nervous system. Clinically, it might appear as a feeling of strong pressure behind the eye, double images and partial loss of visual field. Oculomotor palsy might hint at invasion of the cavernous sinus as described in the literature [28]. Neoplastic formations that invade the branches of the facial nerve might mimic facial palsy, leading to eyelid dysfunction, lagophthalmos, decreased blink frequency and, eventually, neuropathic keratopathy [13]. Cases mistaken for microvascular events have been described and seem initially plausible considering the high mean age of affected patients [29]. If non-characteristic symptoms are present, PNI should be considered.
Radiological features
Preoperative staging for regional lymph node involvement should be a routine matter using ultrasound according to the current literature and the AWMF (Association of Scientific Medical Societies in Germany) guidelines on cutaneous SCC [20, 21, 30]. High-risk lesions with PNI have not only shown regional or distant metastases in 47.3 % of cases, but also local recurrence rates of 47.2 % [31]. This indicates rapid progression and incoherent spreading patterns in neoplasms with PNI. Generally, regional lymph node invasion is found in only about 5 % of patients with cutaneous SCC [32].
In cases of suspected or diagnosed PNI, further imaging is advised. Both computed tomography (CT) and magnetic resonance imaging (MRI) are considered feasible [33] in preoperative diagnosis. MRI is the modality of choice for perineural spreading because of its multiplanar imaging and excellent soft-tissue contrast. In general, spin-echo sequences (for example standard T1-weighted sequences without fat saturation) are the best choice for the evaluation of soft tissue [34]. Further, gadolinium-based contrast agents help evaluate a possible perineural spread by achieving better demarcation. However, for bone involvement CT scans might be more accurate [35]. Computed tomography scans are mostly preferred in primary diagnosis, but preoperative MRI is advised in the case of suspected PNI as the clinical picture may not delineate the actual expansion of the neoplasm (Figures 1-3). However, the most sensitive and accurate method for evaluating neoplastic spread remains intraoperative assessment and exploration in combination with postoperative histological results provided by the excised specimen [16, 35].
Histological features
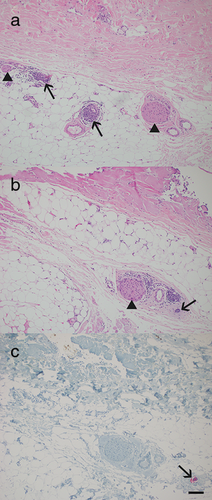
Perineural invasion is diagnosed by histological examination [35]. In addition to standard hematoxylin and eosin (HE) staining, specific immunohistochemical stainings need to be considered [36]. Criteria for malignancy by HE staining include dense cellularity accompanied by multiple mitoses, nuclear pleomorphism and hyperchromasia, per definition of PNI, along hyperplastic nerves. Immunohistochemically, S100 protein is used as a cytoplasmic marker for neural Schwann cells and an epithelial membrane antigen for perineural cells. P40 is a nuclear marker of normal keratinocytes as well as neoplastic SCC and may act as positive control also staining nuclei of perineural malignant cells (Figure 4). Cytokeratin marker AE1/AE3, CAM5.2, CK5/6 and p63 may also be used on immunohistochemistry to detect cytoplasmic and nuclear staining of neoplastic cells, respectively.
The investigation of intraoperative frozen sections is problematic regarding PNI and usually not recommended. Accurate histologic interpretation of PNI during Mohs micrographic surgery may also be complicated [9]. Discohesive strand-like growth, known as Indian filing, and perineural invasion may be erroneously interpreted in both directions as the preparation of frozen sections can cause a non-representative alignment of cells or tears within a sample: on the one hand, vessels, mostly capillaries, may imitate Indian filing of scirrhous SCC; on the other hand, the neoplasm might go unrecognized due to misinterpretation of Indian filing as vessels.
An important clue for distinguishing PNI and identifying scirrhous SCC as well as sclerodermiform basal cell carcinoma and desmoplastic melanoma is an inflammatory response of lymphocytes with follicle-like formation at the periphery of the lesion in an otherwise seemingly normal tissue (Figure 5).
Therapy of squamous cell carcinoma with perineural invasion
Radical surgical resection is considered first-line treatment in SCC with PNI. However, invasive subtypes in the facial region increase the risk that surgical excision will result in positive resection margins [37]. This fact may be partially attributed to the fear of functional deficits after extensive resections [38]. Particularly, in anatomically challenging areas such as the periorbital region, the extent of surgical excision may be restrained. In cases of suspected and/or diagnosed PNI, radical resection should be performed by an experienced surgeon skilled in reconstruction to minimize concern for large defects and challenging reconstruction. The surgeon should not fear defect size but should prioritize complete resection. A discohesive spreading pattern may complicate surgical planning and cause excision margins to be chosen incorrectly [29]. Intraoperative frozen sections are of limited informative value as is Mohs micrographic surgery in these instances [9]. However, the current literature is inconsistent with regard to the surgical approach to cutaneous SCC [39]. An interdisciplinary approach, preoperative planning and imaging are key to optimizing surgical treatment in order to reduce the number of surgeries and prevent delay in adjuvant therapy [40].
In cases of optical nerve invasion, orbital exenteration is the treatment of choice in a curative surgical setting [41]. If complete excision is technically not possible, considered too mutilating or declined by the patient, irradiation therapy can offer a valuable alternative. Adjuvant irradiation therapy is considered an option to decrease local recurrence rates [42] and significantly prolong disease-free survival when histological findings show narrow or even positive resection margins [43]. However, PNI of large-caliber nerves such as cranial nerves and their branches should generally be managed by an interdisciplinary tumor board and only then be considered for radiotherapy [44]. In elderly patients with reduced general condition, brachytherapy might be an alternative to external beam radiotherapy [45]. However, radiation therapy may result in radiation-induced damage. In the periorbital region, this may even cause loss of vision, as in radiation-induced optic neuropathy, in 10–30 % of patients treated with conventional radiation techniques. A recent comparative review of intensity-modulated radiotherapy versus proton beam therapy applied in the periorbital region showed no significant difference in the incidence of vision loss. Nevertheless, modern modalities show reduced radiation doses reaching the optic apparatus [46].
If surgical resection and radiation therapy prove insufficient, systemic therapy should be considered. Novel therapies, such as inhibitors of the transmembrane protein programmed cell death protein 1 (PD1), were approved in 2018 for the treatment of advanced cutaneous SCC [47]. To date, these have shown promising results in patients with poor prognosis, but only a few case reports have described PD-1 inhibitors in the treatment of SCC with PNI [48-50]. Stevenson et al. investigated PD-1 and PD-L2 (PD ligand) expression in biopsy specimens of cutaneous SCC by immunohistochemistry. The strongest PD-1 and PD-L2 expression was seen for SCC with PNI as compared to infiltrative SCC, superficial SCC and transplant-associated SCC [48].
Moreover, the significance of sentinel lymph node biopsy in facial SCC remains unclear, as there is insufficient evidence in the literature regarding survival outcome [51]. It is therefore not considered a standard procedure in staging of facial cutaneous SCC. Although sentinel lymph node biopsy has been considered in the case of particularly invasive neoplasms such as SCC with PNI [52], adequate data and studies are not available. Consequently, prophylactic lymph node dissection is not recommended for cutaneous SCC of the face [53]. Lymph node dissection is supported by the literature only in manifest lymph node metastasis [54] to achieve local neoplastic control.
Prognosis of squamous cell carcinoma with perineural invasion
Studies have shown that tumor diameter > 2 cm, tumor depth > 6 mm, and hyperplastic nerves ≥ 0.1 mm in diameter are associated with poor outcome [55]. Brantsch et al. previously proposed tumor depth of > 6 mm and desmoplastic tumor growth as independent risk factors for recurrence and metastasis [3], while a recent study [24] reported a significantly higher risk for neoplastic progression in desmoplasia with PNI in comparison to desmoplasia without PNI. Additionally, the presence of PNI was also associated with an increased tumor diameter and depth of invasion [24]. Thus, PNI should be considered a feature with poor prognostic outcome that is often accompanied by other risk factors.
Conclusions
PNI in cutaneous SCC of the periorbital region is a surgical challenge. Careful clinical evaluation may indicate PNI even before obtaining histology. Preoperative imaging by MRI or CT scan should be considered if PNI is suspected. Cases should be discussed by an interdisciplinary tumor board and the procedure should be planned precisely. In case of discohesive spreading patterns increased safety margins must be considered. Hence, the periorbital region should be treated by surgeons skilled in reconstruction, followed by close postoperative monitoring over an extended period. Re-biopsy of suspicious skin alterations should be generously indicated. Further prospective observational studies are needed to establish clear treatment guidelines for such complex lesions.
Acknowledgment
We thank Inge Jehart of the Department of Pathology of the Medical University of Innsbruck for technical assistance with immunohistochemical double stainings and photographic scanning of the slides.
Conflict of interest
None.




